 Found 37 hits of ic50 data for polymerid = 50007027
Found 37 hits of ic50 data for polymerid = 50007027 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50316226
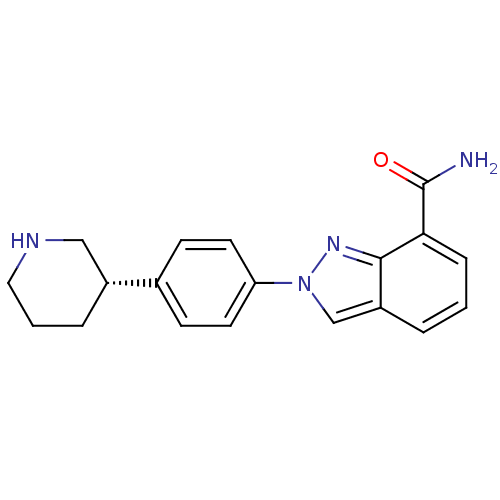
((S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-car...)Show SMILES NC(=O)c1cccc2cn(nc12)-c1ccc(cc1)[C@@H]1CCCNC1 |r| Show InChI InChI=1S/C19H20N4O/c20-19(24)17-5-1-3-15-12-23(22-18(15)17)16-8-6-13(7-9-16)14-4-2-10-21-11-14/h1,3,5-9,12,14,21H,2,4,10-11H2,(H2,20,24)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PARP12 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins fol... |
J Med Chem 60: 1262-1271 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00990
BindingDB Entry DOI: 10.7270/Q28054VV |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50316226

((S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-car...)Show SMILES NC(=O)c1cccc2cn(nc12)-c1ccc(cc1)[C@@H]1CCCNC1 |r| Show InChI InChI=1S/C19H20N4O/c20-19(24)17-5-1-3-15-12-23(22-18(15)17)16-8-6-13(7-9-16)14-4-2-10-21-11-14/h1,3,5-9,12,14,21H,2,4,10-11H2,(H2,20,24)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PARP12 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins fol... |
J Med Chem 60: 1262-1271 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00990
BindingDB Entry DOI: 10.7270/Q28054VV |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM27497
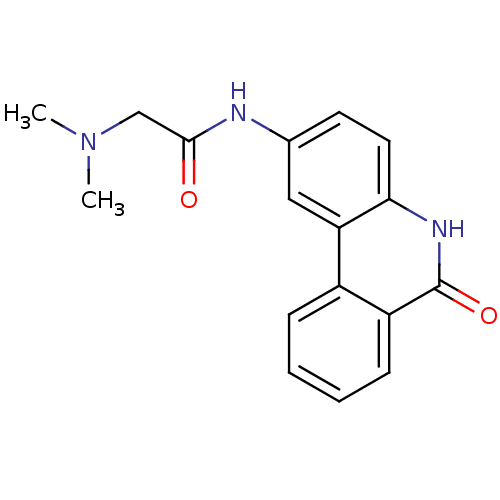
(2-(dimethylamino)-N-(6-oxo-5,6-dihydrophenanthridi...)Show InChI InChI=1S/C17H17N3O2/c1-20(2)10-16(21)18-11-7-8-15-14(9-11)12-5-3-4-6-13(12)17(22)19-15/h3-9H,10H2,1-2H3,(H,18,21)(H,19,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PARP12 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins fol... |
J Med Chem 60: 1262-1271 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00990
BindingDB Entry DOI: 10.7270/Q28054VV |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM27497

(2-(dimethylamino)-N-(6-oxo-5,6-dihydrophenanthridi...)Show InChI InChI=1S/C17H17N3O2/c1-20(2)10-16(21)18-11-7-8-15-14(9-11)12-5-3-4-6-13(12)17(22)19-15/h3-9H,10H2,1-2H3,(H,18,21)(H,19,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PARP12 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins fol... |
J Med Chem 60: 1262-1271 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00990
BindingDB Entry DOI: 10.7270/Q28054VV |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50594028
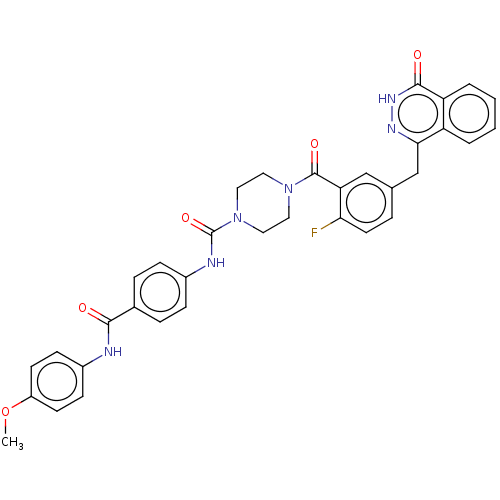
(CHEMBL5176874)Show SMILES COc1ccc(NC(=O)c2ccc(NC(=O)N3CCN(CC3)C(=O)c3cc(Cc4n[nH]c(=O)c5ccccc45)ccc3F)cc2)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 371 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114417
BindingDB Entry DOI: 10.7270/Q2RB78MX |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM207624
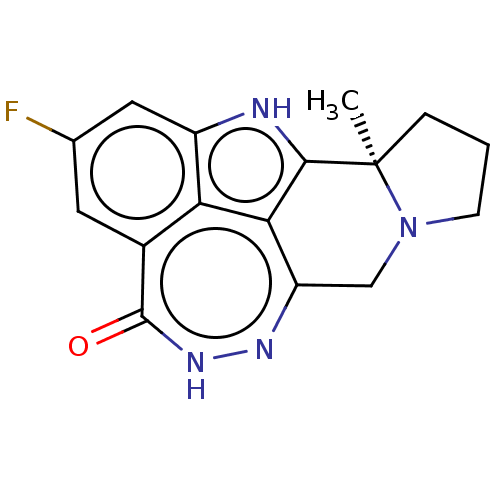
(US10501467, Example 69 | US9260440, 69 | US9617273...)Show SMILES C[C@]12CCCN1Cc1n[nH]c(=O)c3cc(F)cc4[nH]c2c1c34 |r| Show InChI InChI=1S/C16H13FN4O/c1-16-3-2-4-21(16)7-11-13-12-9(15(22)20-19-11)5-8(17)6-10(12)18-14(13)16/h5-6H,2-4,7H2,1H3/t16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PARP-12 (unknown origin) pre-incubated for 30 mins before addition of activated DNA and NAD by chemiluminescent assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01346
BindingDB Entry DOI: 10.7270/Q2474FGF |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50593627
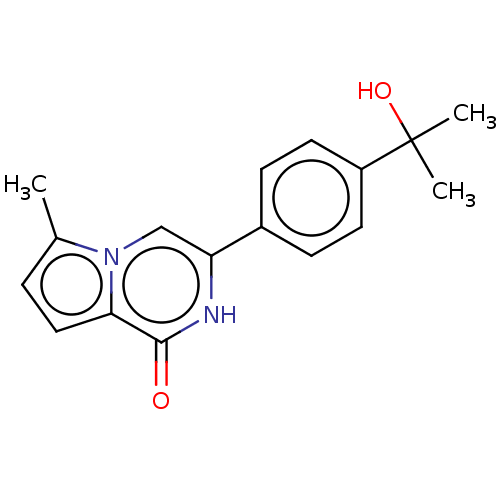
(CHEMBL5199558) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PARP12 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins fol... |
J Med Chem 60: 1262-1271 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00990
BindingDB Entry DOI: 10.7270/Q28054VV |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PARP12 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins fol... |
J Med Chem 60: 1262-1271 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00990
BindingDB Entry DOI: 10.7270/Q28054VV |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50242630
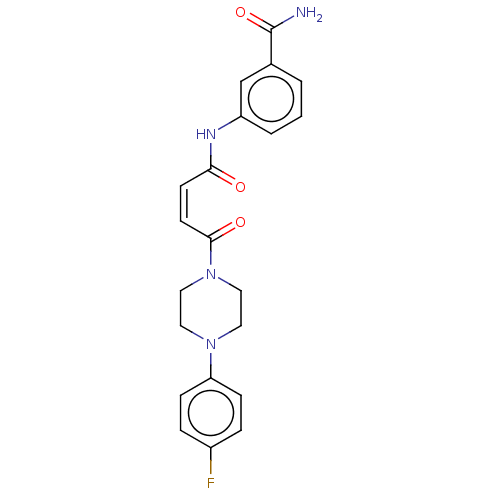
(CHEMBL4089522)Show SMILES NC(=O)c1cccc(NC(=O)\C=C/C(=O)N2CCN(CC2)c2ccc(F)cc2)c1 Show InChI InChI=1S/C21H21FN4O3/c22-16-4-6-18(7-5-16)25-10-12-26(13-11-25)20(28)9-8-19(27)24-17-3-1-2-15(14-17)21(23)29/h1-9,14H,10-13H2,(H2,23,29)(H,24,27)/b9-8- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McDaniel College
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged PARP12 (unknown origin) preincubated for 15 mins followed by biotinylated NAD+ addition by chemiluminescence assay |
Bioorg Med Chem Lett 27: 2907-2911 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.089
BindingDB Entry DOI: 10.7270/Q24X5B5F |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50136755
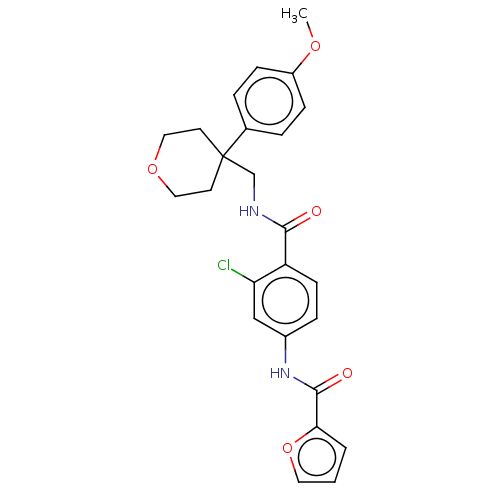
(CHEMBL3752386)Show SMILES COc1ccc(cc1)C1(CNC(=O)c2ccc(NC(=O)c3ccco3)cc2Cl)CCOCC1 Show InChI InChI=1S/C25H25ClN2O5/c1-31-19-7-4-17(5-8-19)25(10-13-32-14-11-25)16-27-23(29)20-9-6-18(15-21(20)26)28-24(30)22-3-2-12-33-22/h2-9,12,15H,10-11,13-14,16H2,1H3,(H,27,29)(H,28,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu
Curated by ChEMBL
| Assay Description
Inhibition of human ARTD12 by fluorescence analysis |
Bioorg Med Chem Lett 26: 328-33 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.018
BindingDB Entry DOI: 10.7270/Q20003ZD |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50136772

(CHEMBL3752492)Show SMILES COc1ccc(cc1)C(=O)Nc1ccc(cc1)C(=O)N(c1ccncn1)c1ccccc1Cl Show InChI InChI=1S/C25H19ClN4O3/c1-33-20-12-8-17(9-13-20)24(31)29-19-10-6-18(7-11-19)25(32)30(23-14-15-27-16-28-23)22-5-3-2-4-21(22)26/h2-16H,1H3,(H,29,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu
Curated by ChEMBL
| Assay Description
Inhibition of human ARTD12 by fluorescence analysis |
Bioorg Med Chem Lett 26: 328-33 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.018
BindingDB Entry DOI: 10.7270/Q20003ZD |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50594011
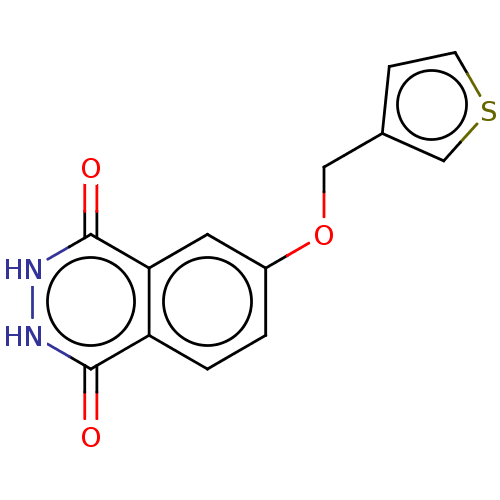
(CHEMBL5200521) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114362
BindingDB Entry DOI: 10.7270/Q20V8HSS |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50594010
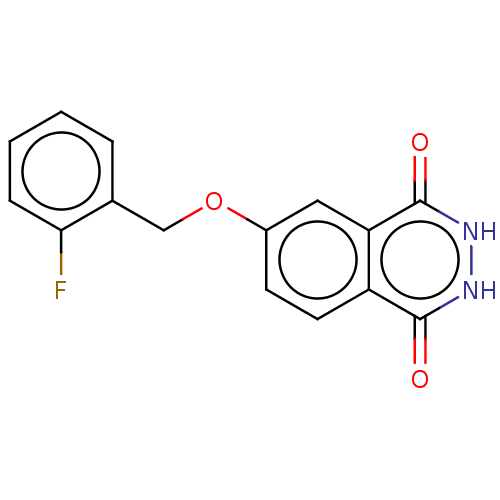
(CHEMBL5200358) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114362
BindingDB Entry DOI: 10.7270/Q20V8HSS |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50594005

(CHEMBL5181947) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114362
BindingDB Entry DOI: 10.7270/Q20V8HSS |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50593992
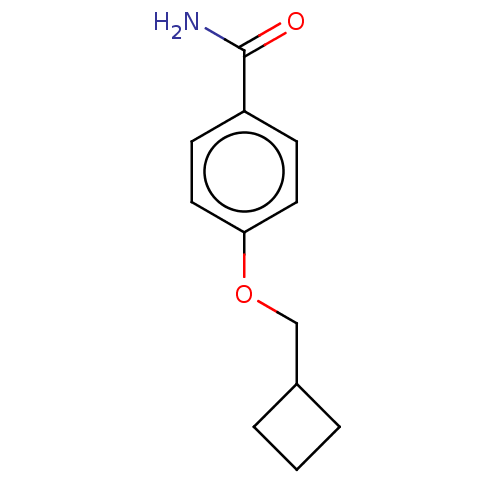
(CHEMBL5186967) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114362
BindingDB Entry DOI: 10.7270/Q20V8HSS |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50579974
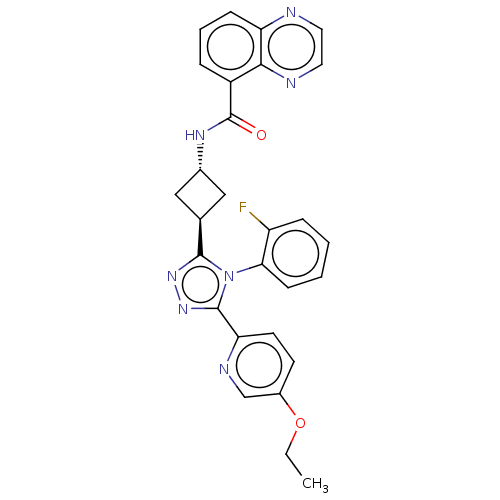
(CHEMBL5090849 | US20230322721, Example 154 of WO 2...)Show SMILES CCOc1ccc(nc1)-c1nnc([C@H]2C[C@@H](C2)NC(=O)c2cccc3nccnc23)n1-c1ccccc1F |r,wU:15.18,wD:13.13,(1.18,-57.78,;2.08,-56.54,;3.59,-56.7,;4.49,-55.47,;6.01,-55.63,;6.9,-54.39,;6.29,-53.01,;4.77,-52.84,;3.87,-54.07,;7.18,-51.78,;6.71,-50.31,;7.95,-49.39,;9.21,-50.31,;10.68,-49.82,;12.05,-50.53,;12.76,-49.16,;11.38,-48.45,;14.23,-48.68,;14.56,-47.17,;13.41,-46.13,;16.03,-46.68,;16.34,-45.17,;17.81,-44.69,;18.97,-45.72,;18.65,-47.24,;19.79,-48.27,;19.48,-49.77,;18,-50.26,;16.86,-49.22,;17.18,-47.72,;8.73,-51.78,;9.63,-53.02,;9,-54.41,;9.9,-55.64,;11.41,-55.48,;12.03,-54.08,;11.13,-52.85,;11.75,-51.45,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PARP12 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01264
BindingDB Entry DOI: 10.7270/Q21R6VDH |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50541697

(CHEMBL4633637)Show SMILES CCOc1ccc(nc1)-c1nnc([C@H]2C[C@@H](C2)NC(=O)c2ccccn2)n1-c1ccccc1F |r,wU:15.18,wD:13.13,(2.31,-18.95,;3.77,-18.47,;4.92,-19.5,;6.38,-19.02,;7.54,-20.05,;9,-19.57,;9.31,-18.07,;8.17,-17.04,;6.7,-17.51,;10.77,-17.6,;11.25,-16.13,;12.79,-16.13,;13.26,-17.6,;14.73,-18.07,;15.42,-19.45,;16.8,-18.75,;16.1,-17.38,;18.26,-19.23,;19.41,-18.2,;19.09,-16.69,;20.87,-18.67,;22,-17.64,;23.47,-18.11,;23.79,-19.62,;22.65,-20.65,;21.19,-20.18,;12.02,-18.5,;12.02,-20.04,;10.68,-20.81,;10.68,-22.34,;12.02,-23.12,;13.36,-22.34,;13.35,-20.8,;14.68,-20.03,)| Show InChI InChI=1S/C25H23FN6O2/c1-2-34-18-10-11-20(28-15-18)24-31-30-23(32(24)22-9-4-3-7-19(22)26)16-13-17(14-16)29-25(33)21-8-5-6-12-27-21/h3-12,15-17H,2,13-14H2,1H3,(H,29,33)/t16-,17- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PARP12 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01264
BindingDB Entry DOI: 10.7270/Q21R6VDH |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50250875
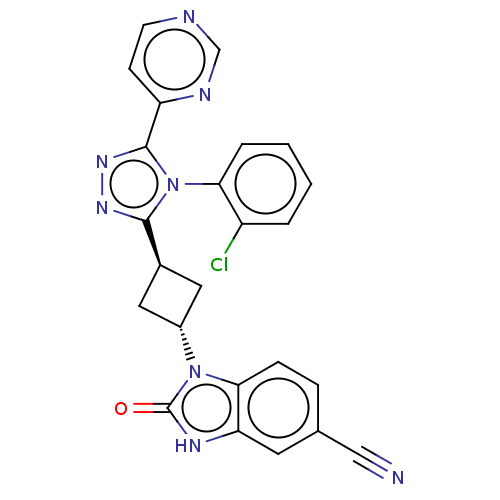
(CHEMBL4095003)Show SMILES Clc1ccccc1-n1c(nnc1-c1ccncn1)[C@H]1C[C@@H](C1)n1c2ccc(cc2[nH]c1=O)C#N |r,wU:20.25,wD:18.20,(25.39,-29.03,;24.05,-29.8,;24.05,-31.34,;22.72,-32.11,;21.39,-31.34,;21.39,-29.8,;22.72,-29.03,;22.72,-27.49,;23.97,-26.59,;23.49,-25.13,;21.95,-25.13,;21.48,-26.59,;20.01,-27.07,;19.69,-28.57,;18.22,-29.05,;17.08,-28.02,;17.4,-26.52,;18.87,-26.04,;25.43,-27.07,;26.13,-28.44,;27.5,-27.74,;26.8,-26.37,;28.97,-28.22,;29.44,-29.68,;28.67,-31.01,;29.44,-32.35,;30.98,-32.35,;31.75,-31.01,;30.98,-29.68,;31.46,-28.22,;30.21,-27.31,;30.21,-25.77,;31.75,-33.68,;32.52,-35.01,)| Show InChI InChI=1S/C24H17ClN8O/c25-17-3-1-2-4-20(17)33-22(30-31-23(33)18-7-8-27-13-28-18)15-10-16(11-15)32-21-6-5-14(12-26)9-19(21)29-24(32)34/h1-9,13,15-16H,10-11H2,(H,29,34)/t15-,16- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged thioredoxin-fused human ARTD12 (469 to 701 residues) expressed in Escherichia coli Rosetta2 (DE3) after 20 hrs in... |
J Med Chem 63: 6834-6846 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00208
BindingDB Entry DOI: 10.7270/Q2RR22SX |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50541697

(CHEMBL4633637)Show SMILES CCOc1ccc(nc1)-c1nnc([C@H]2C[C@@H](C2)NC(=O)c2ccccn2)n1-c1ccccc1F |r,wU:15.18,wD:13.13,(2.31,-18.95,;3.77,-18.47,;4.92,-19.5,;6.38,-19.02,;7.54,-20.05,;9,-19.57,;9.31,-18.07,;8.17,-17.04,;6.7,-17.51,;10.77,-17.6,;11.25,-16.13,;12.79,-16.13,;13.26,-17.6,;14.73,-18.07,;15.42,-19.45,;16.8,-18.75,;16.1,-17.38,;18.26,-19.23,;19.41,-18.2,;19.09,-16.69,;20.87,-18.67,;22,-17.64,;23.47,-18.11,;23.79,-19.62,;22.65,-20.65,;21.19,-20.18,;12.02,-18.5,;12.02,-20.04,;10.68,-20.81,;10.68,-22.34,;12.02,-23.12,;13.36,-22.34,;13.35,-20.8,;14.68,-20.03,)| Show InChI InChI=1S/C25H23FN6O2/c1-2-34-18-10-11-20(28-15-18)24-31-30-23(32(24)22-9-4-3-7-19(22)26)16-13-17(14-16)29-25(33)21-8-5-6-12-27-21/h3-12,15-17H,2,13-14H2,1H3,(H,29,33)/t16-,17- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged thioredoxin-fused human ARTD12 (469 to 701 residues) expressed in Escherichia coli Rosetta2 (DE3) after 20 hrs in... |
J Med Chem 63: 6834-6846 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00208
BindingDB Entry DOI: 10.7270/Q2RR22SX |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50505333
(CHEMBL4437173)Show SMILES CC(C)(O)c1ccc(cc1)-c1nc2c(F)cc(F)cc2c(=O)[nH]1 Show InChI InChI=1S/C17H14F2N2O2/c1-17(2,23)10-5-3-9(4-6-10)15-20-14-12(16(22)21-15)7-11(18)8-13(14)19/h3-8,23H,1-2H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His/GST-tagged PARP12 (500 to 701 residues) expressed in baculovirus infected Sf9 insect cells using biotinylated subs... |
J Med Chem 62: 7897-7909 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00656
BindingDB Entry DOI: 10.7270/Q2FJ2M28 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50250875

(CHEMBL4095003)Show SMILES Clc1ccccc1-n1c(nnc1-c1ccncn1)[C@H]1C[C@@H](C1)n1c2ccc(cc2[nH]c1=O)C#N |r,wU:20.25,wD:18.20,(25.39,-29.03,;24.05,-29.8,;24.05,-31.34,;22.72,-32.11,;21.39,-31.34,;21.39,-29.8,;22.72,-29.03,;22.72,-27.49,;23.97,-26.59,;23.49,-25.13,;21.95,-25.13,;21.48,-26.59,;20.01,-27.07,;19.69,-28.57,;18.22,-29.05,;17.08,-28.02,;17.4,-26.52,;18.87,-26.04,;25.43,-27.07,;26.13,-28.44,;27.5,-27.74,;26.8,-26.37,;28.97,-28.22,;29.44,-29.68,;28.67,-31.01,;29.44,-32.35,;30.98,-32.35,;31.75,-31.01,;30.98,-29.68,;31.46,-28.22,;30.21,-27.31,;30.21,-25.77,;31.75,-33.68,;32.52,-35.01,)| Show InChI InChI=1S/C24H17ClN8O/c25-17-3-1-2-4-20(17)33-22(30-31-23(33)18-7-8-27-13-28-18)15-10-16(11-15)32-21-6-5-14(12-26)9-19(21)29-24(32)34/h1-9,13,15-16H,10-11H2,(H,29,34)/t15-,16- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Leibniz-Forschungsinstitut f�r Molekulare Pharmakologie (FMP)
Curated by ChEMBL
| Assay Description
Inhibition of human PARP12 using NAD+ as substrate by fluorescence assay |
J Med Chem 60: 10013-10025 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00883
BindingDB Entry DOI: 10.7270/Q2CJ8GXG |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50468579
(CHEMBL4287262)Show InChI InChI=1S/C14H11NO4/c15-13(16)9-1-5-11(6-2-9)19-12-7-3-10(4-8-12)14(17)18/h1-8H,(H2,15,16)(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged thioredoxin-fused human ARTD12 (469 to 701 residues) expressed in Escherichia coli Rosetta2 (DE3) after 20 hrs in... |
Eur J Med Chem 156: 93-102 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.047
BindingDB Entry DOI: 10.7270/Q2P271SW |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50340090

(4-methoxybenzamide | CHEMBL449635)Show InChI InChI=1S/C8H9NO2/c1-11-7-4-2-6(3-5-7)8(9)10/h2-5H,1H3,(H2,9,10) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged thioredoxin-fused human ARTD12 (469 to 701 residues) expressed in Escherichia coli Rosetta2 (DE3) after 20 hrs in... |
Eur J Med Chem 156: 93-102 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.047
BindingDB Entry DOI: 10.7270/Q2P271SW |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50468581
(CHEMBL4287655)Show InChI InChI=1S/C14H11NO3/c15-14(17)11-3-7-13(8-4-11)18-12-5-1-10(9-16)2-6-12/h1-9H,(H2,15,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged thioredoxin-fused human ARTD12 (469 to 701 residues) expressed in Escherichia coli Rosetta2 (DE3) after 20 hrs in... |
Eur J Med Chem 156: 93-102 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.047
BindingDB Entry DOI: 10.7270/Q2P271SW |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50452662
(CHEMBL4208737)Show InChI InChI=1S/C14H12FNO2/c15-13-4-2-1-3-11(13)9-18-12-7-5-10(6-8-12)14(16)17/h1-8H,9H2,(H2,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged thioredoxin-fused human ARTD12 (469 to 701 residues) expressed in Escherichia coli Rosetta2 (DE3) after 20 hrs in... |
Eur J Med Chem 156: 93-102 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.047
BindingDB Entry DOI: 10.7270/Q2P271SW |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM199181
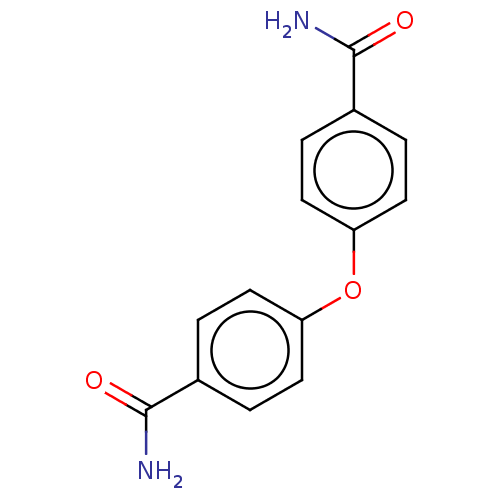
(4-[(4-Carbamoylcyclohexyl)oxy]cyclohexane-1-carbox...)Show InChI InChI=1S/C14H12N2O3/c15-13(17)9-1-5-11(6-2-9)19-12-7-3-10(4-8-12)14(16)18/h1-8H,(H2,15,17)(H2,16,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged thioredoxin-fused human ARTD12 (469 to 701 residues) expressed in Escherichia coli Rosetta2 (DE3) after 20 hrs in... |
Eur J Med Chem 156: 93-102 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.047
BindingDB Entry DOI: 10.7270/Q2P271SW |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PARP12 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins fol... |
J Med Chem 60: 1262-1271 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00990
BindingDB Entry DOI: 10.7270/Q28054VV |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PARP12 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins fol... |
J Med Chem 60: 1262-1271 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00990
BindingDB Entry DOI: 10.7270/Q28054VV |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50503853
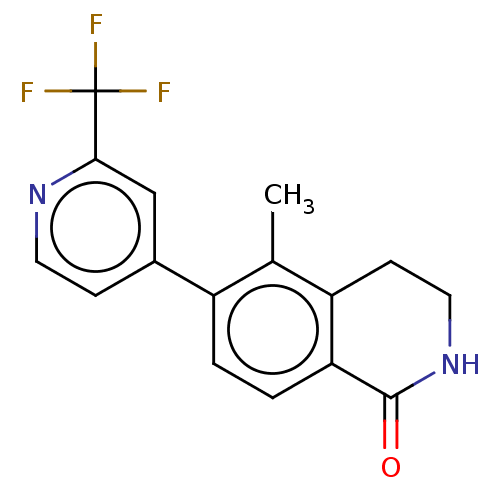
(CHEMBL4545565)Show InChI InChI=1S/C16H13F3N2O/c1-9-11(2-3-13-12(9)5-7-21-15(13)22)10-4-6-20-14(8-10)16(17,18)19/h2-4,6,8H,5,7H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon Health and Science University
Curated by ChEMBL
| Assay Description
Inhibition of GFP-tagged human PARP12 expressed in HEK293T cells assessed as reduction in auto-MARylation after 60 mins in presence of 6-a-NAD+ by ch... |
ACS Med Chem Lett 10: 74-79 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00429
BindingDB Entry DOI: 10.7270/Q2HX1GXV |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50594265
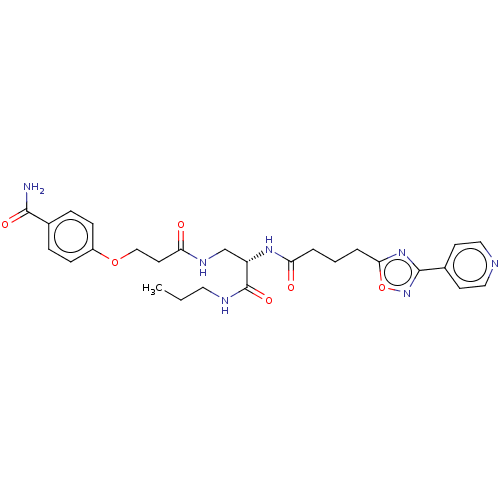
(CHEMBL5191886)Show SMILES CCCNC(=O)[C@H](CNC(=O)CCOc1ccc(cc1)C(N)=O)NC(=O)CCCc1nc(no1)-c1ccncc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00281
BindingDB Entry DOI: 10.7270/Q26D5Z03 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50594264
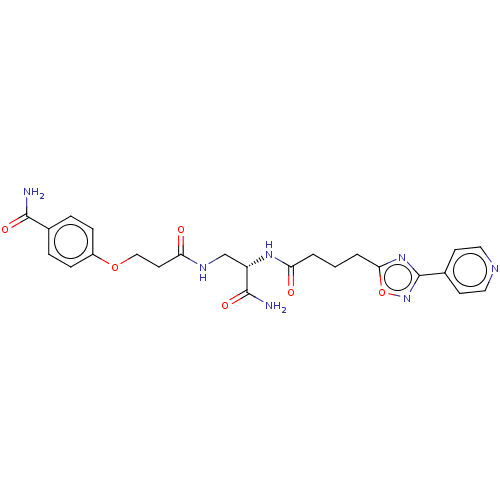
(CHEMBL5206632)Show SMILES NC(=O)[C@H](CNC(=O)CCOc1ccc(cc1)C(N)=O)NC(=O)CCCc1nc(no1)-c1ccncc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00281
BindingDB Entry DOI: 10.7270/Q26D5Z03 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM431646
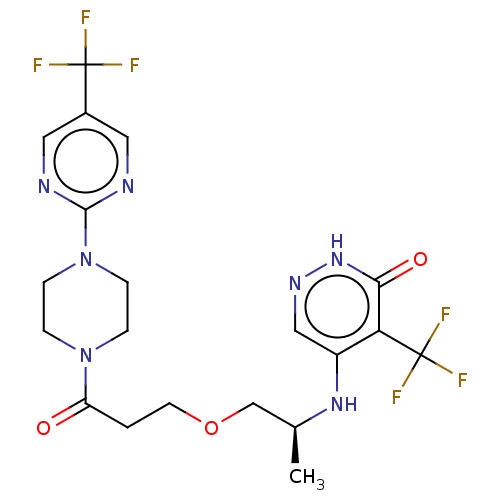
(5-[[(2S)-1-(3-Oxo-3-[4-[5-(trifluoromethyl)pyrimid...)Show SMILES C[C@@H](COCCC(=O)N1CCN(CC1)c1ncc(cn1)C(F)(F)F)Nc1cn[nH]c(=O)c1C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00281
BindingDB Entry DOI: 10.7270/Q26D5Z03 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM27135

(2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...)Show InChI InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PARP12 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins fol... |
J Med Chem 60: 1262-1271 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00990
BindingDB Entry DOI: 10.7270/Q28054VV |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM27135

(2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...)Show InChI InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-tagged PARP12 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins fol... |
J Med Chem 60: 1262-1271 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00990
BindingDB Entry DOI: 10.7270/Q28054VV |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50234916
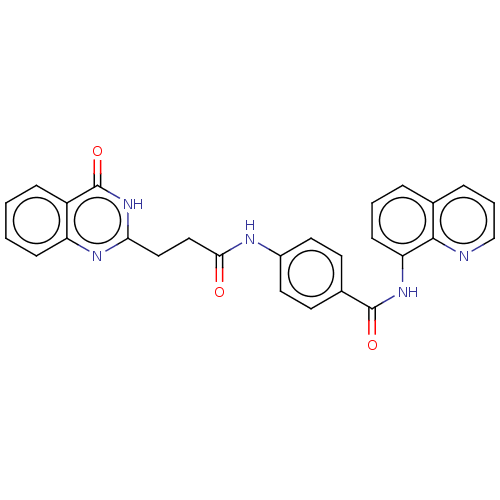
(CHEMBL4098188)Show SMILES O=C(CCc1nc2ccccc2c(=O)[nH]1)Nc1ccc(cc1)C(=O)Nc1cccc2cccnc12 Show InChI InChI=1S/C27H21N5O3/c33-24(15-14-23-30-21-8-2-1-7-20(21)27(35)32-23)29-19-12-10-18(11-13-19)26(34)31-22-9-3-5-17-6-4-16-28-25(17)22/h1-13,16H,14-15H2,(H,29,33)(H,31,34)(H,30,32,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of PARP12 (unknown origin) |
J Med Chem 60: 814-820 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01574
BindingDB Entry DOI: 10.7270/Q20R9RPP |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP12
(Homo sapiens (Human)) | BDBM50234915
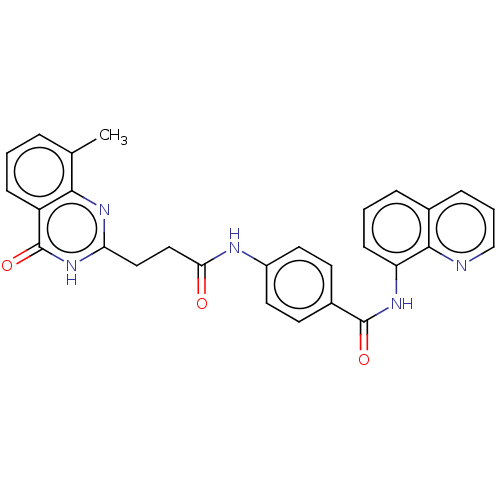
(CHEMBL4069447)Show SMILES Cc1cccc2c1nc(CCC(=O)Nc1ccc(cc1)C(=O)Nc1cccc3cccnc13)[nH]c2=O Show InChI InChI=1S/C28H23N5O3/c1-17-5-2-8-21-25(17)32-23(33-28(21)36)14-15-24(34)30-20-12-10-19(11-13-20)27(35)31-22-9-3-6-18-7-4-16-29-26(18)22/h2-13,16H,14-15H2,1H3,(H,30,34)(H,31,35)(H,32,33,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of PARP12 (unknown origin) |
J Med Chem 60: 814-820 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01574
BindingDB Entry DOI: 10.7270/Q20R9RPP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data