

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50253810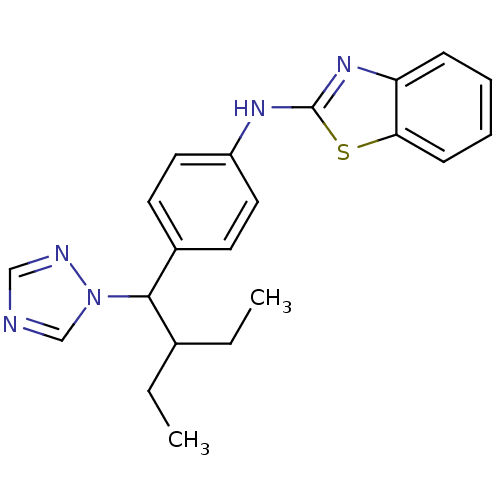 (CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50253810 (CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM393281 (US9963439, R116010) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157605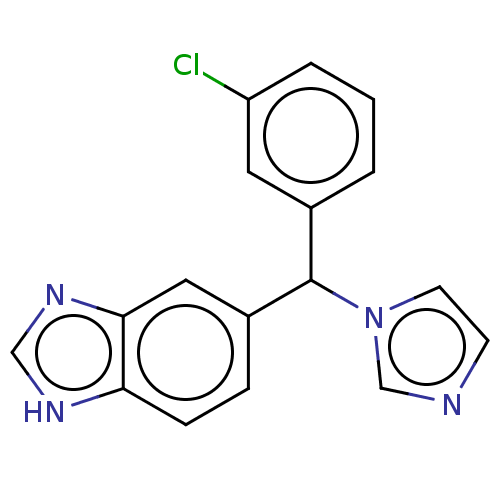 (Liarozole | Liazal | R-75251 | US9963439, Liarozol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157605 (Liarozole | Liazal | R-75251 | US9963439, Liarozol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM557292 (US11358933, Compound 026) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CYP26 inhibitory activities of various compounds. | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476235 (US10874634, Cmpd No. 17 | US11364220, Compound 17) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This retinoid activity assay measures the ability of test compounds to induce expression of a transiently transfected RA sensitive reporter construct... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QN6B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476235 (US10874634, Cmpd No. 17 | US11364220, Compound 17) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen''s University at Kingston US Patent | Assay Description CYP26A1 or CYP26B1 stably transfected HeLa cells were maintained in Minimum Essential Medium (MEM) containing 10% fetal bovine serum (FBS) and 100 &#... | US Patent US10874634 (2020) BindingDB Entry DOI: 10.7270/Q2Q52SP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM557299 (US11358933, Compound 034) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CYP26 inhibitory activities of various compounds. | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM31886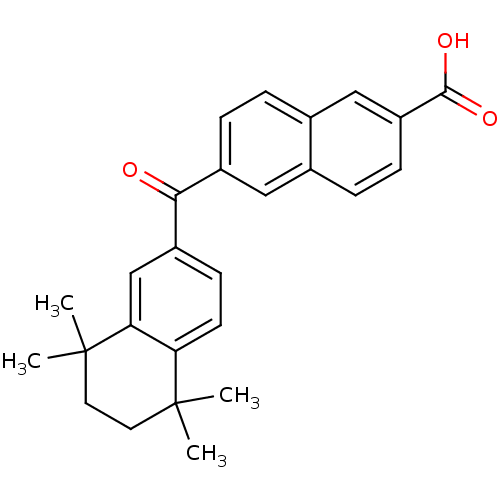 (CD564 | CHEMBL309282) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476228 (US10874634, Cmpd No. 10 | US11364220, Compound 10) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen''s University at Kingston US Patent | Assay Description CYP26A1 or CYP26B1 stably transfected HeLa cells were maintained in Minimum Essential Medium (MEM) containing 10% fetal bovine serum (FBS) and 100 &#... | US Patent US10874634 (2020) BindingDB Entry DOI: 10.7270/Q2Q52SP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476228 (US10874634, Cmpd No. 10 | US11364220, Compound 10) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This retinoid activity assay measures the ability of test compounds to induce expression of a transiently transfected RA sensitive reporter construct... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QN6B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157722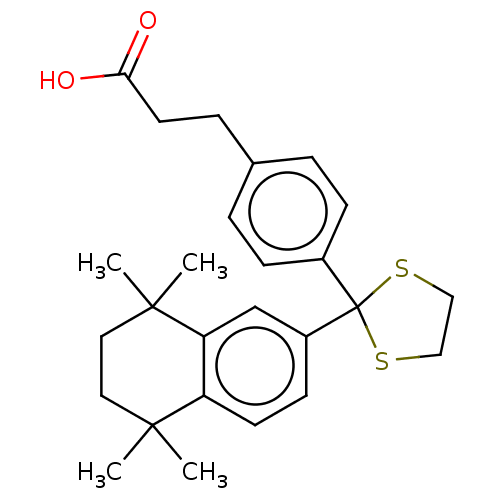 (CHEMBL3787323) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157724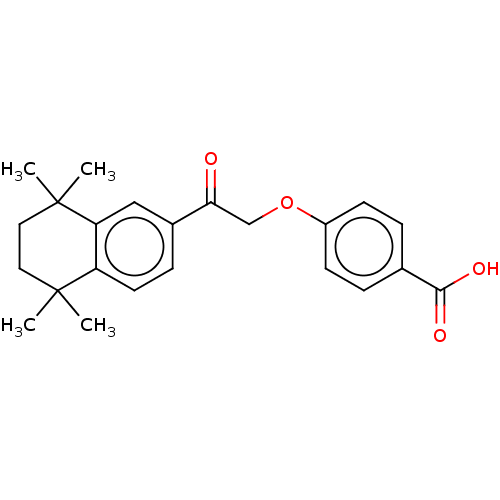 (CHEMBL3787564 | US9963439, Compound B) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157727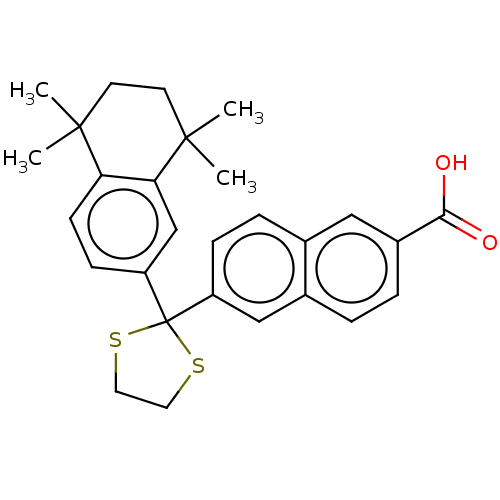 (CHEMBL3786184) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157721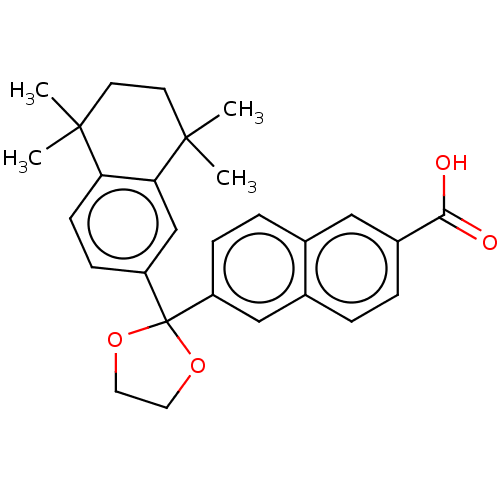 (CHEMBL3786620) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50033067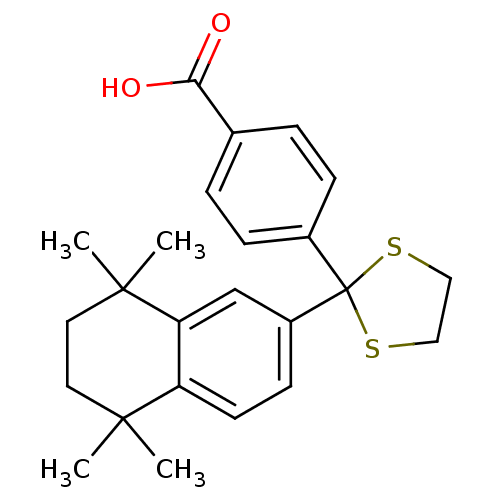 (4-[2-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157729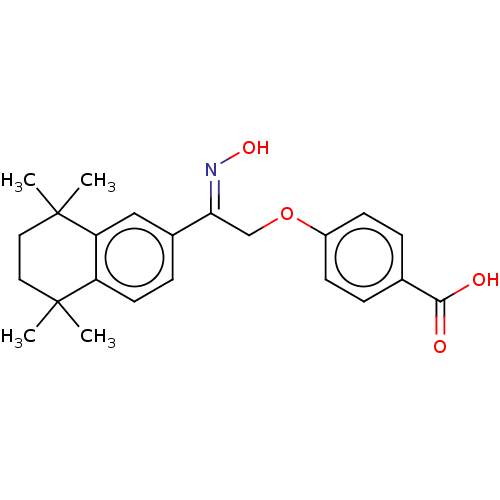 (CHEMBL3787691) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157731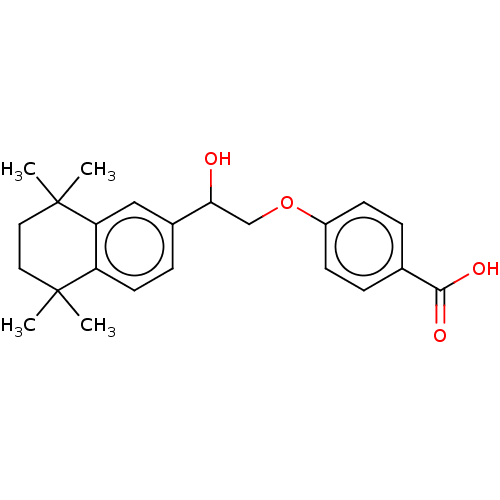 (CHEMBL3785511 | US9963439, Compound C) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157604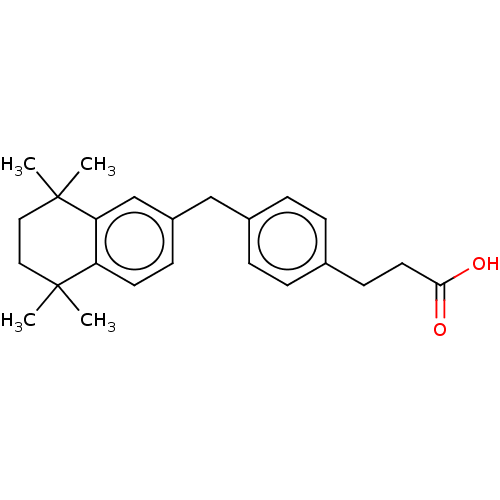 (CHEMBL3786324) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476180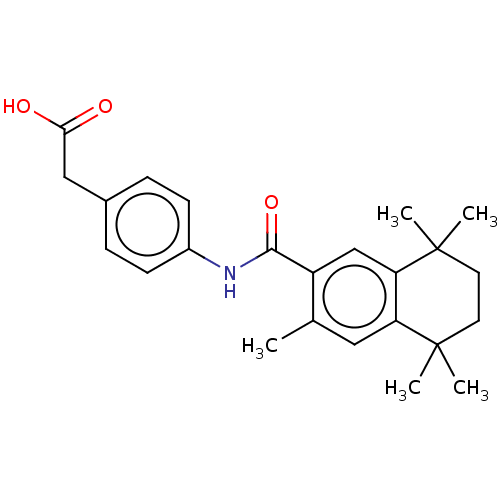 (US10874634, Cmpd No. 02 | US11364220, Compound 02) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen''s University at Kingston US Patent | Assay Description CYP26A1 or CYP26B1 stably transfected HeLa cells were maintained in Minimum Essential Medium (MEM) containing 10% fetal bovine serum (FBS) and 100 &#... | US Patent US10874634 (2020) BindingDB Entry DOI: 10.7270/Q2Q52SP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476180 (US10874634, Cmpd No. 02 | US11364220, Compound 02) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This retinoid activity assay measures the ability of test compounds to induce expression of a transiently transfected RA sensitive reporter construct... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QN6B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM557298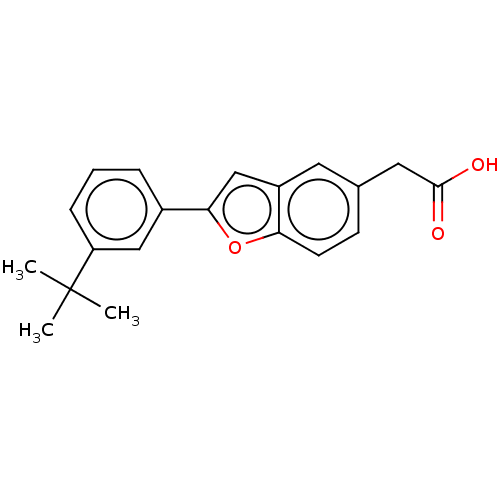 (US11358933, Compound 032) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CYP26 inhibitory activities of various compounds. | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157718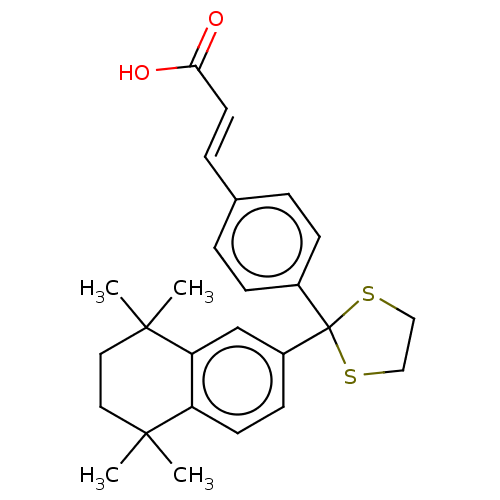 (CHEMBL3785701) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157719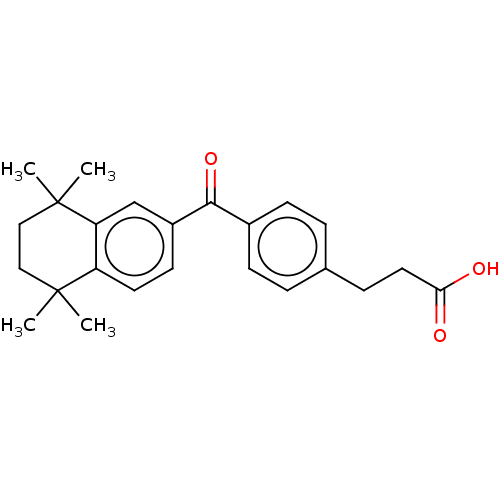 (CHEMBL3785624) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157723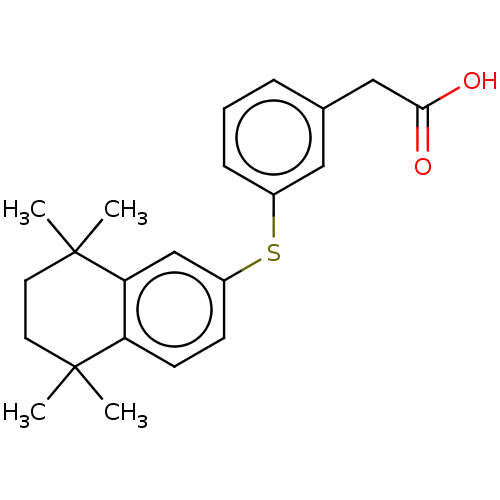 (CHEMBL3786927) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157726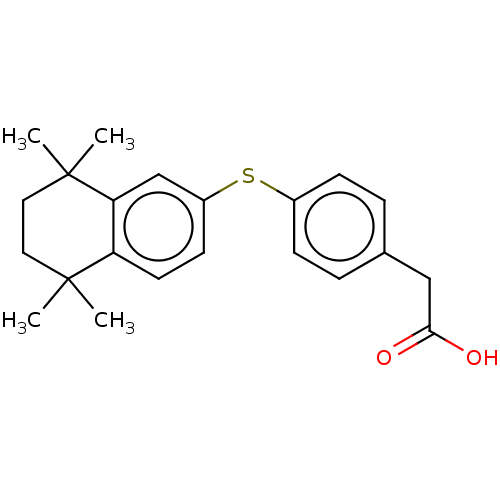 (CHEMBL3786043) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157608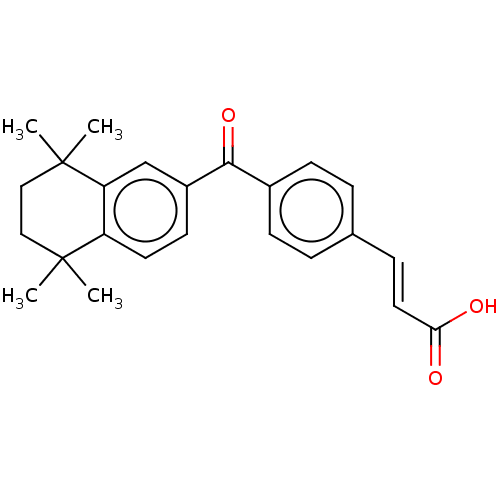 (CHEMBL95949) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157730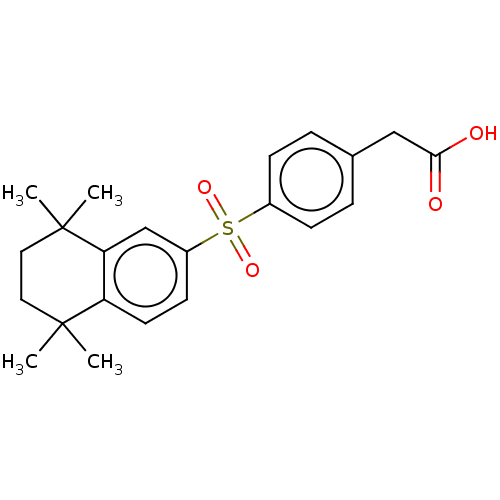 (CHEMBL3785557) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157728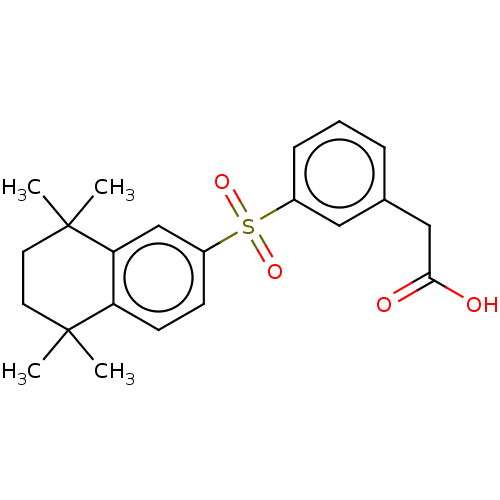 (CHEMBL3785761) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50032675 (4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157720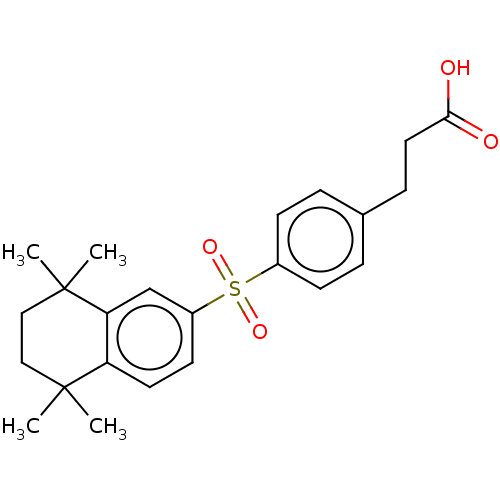 (CHEMBL3786340) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM557293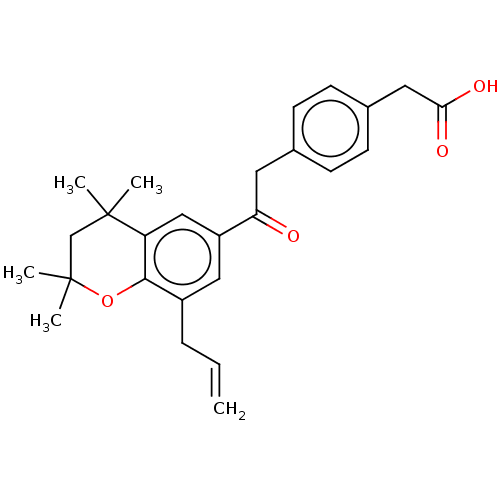 (US11358933, Compound 027) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CYP26 inhibitory activities of various compounds. | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476173 (US10874634, Cmpd No. 01 | US11364220, Compound 01) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 8.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This retinoid activity assay measures the ability of test compounds to induce expression of a transiently transfected RA sensitive reporter construct... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QN6B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476173 (US10874634, Cmpd No. 01 | US11364220, Compound 01) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 8.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen''s University at Kingston US Patent | Assay Description CYP26A1 or CYP26B1 stably transfected HeLa cells were maintained in Minimum Essential Medium (MEM) containing 10% fetal bovine serum (FBS) and 100 &#... | US Patent US10874634 (2020) BindingDB Entry DOI: 10.7270/Q2Q52SP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476236 (US10874634, Cmpd No. 18 | US11364220, Compound 18) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This retinoid activity assay measures the ability of test compounds to induce expression of a transiently transfected RA sensitive reporter construct... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QN6B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476236 (US10874634, Cmpd No. 18 | US11364220, Compound 18) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen''s University at Kingston US Patent | Assay Description CYP26A1 or CYP26B1 stably transfected HeLa cells were maintained in Minimum Essential Medium (MEM) containing 10% fetal bovine serum (FBS) and 100 &#... | US Patent US10874634 (2020) BindingDB Entry DOI: 10.7270/Q2Q52SP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157717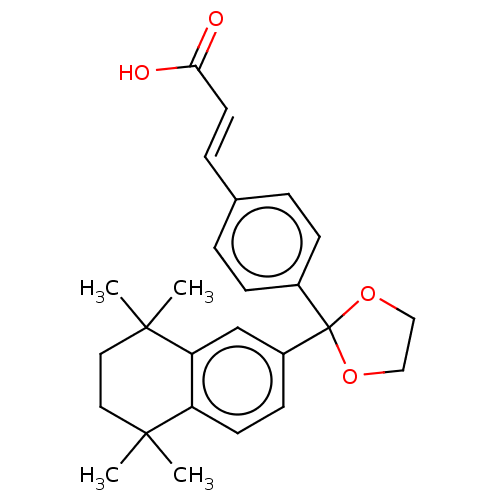 (CHEMBL3786581) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476222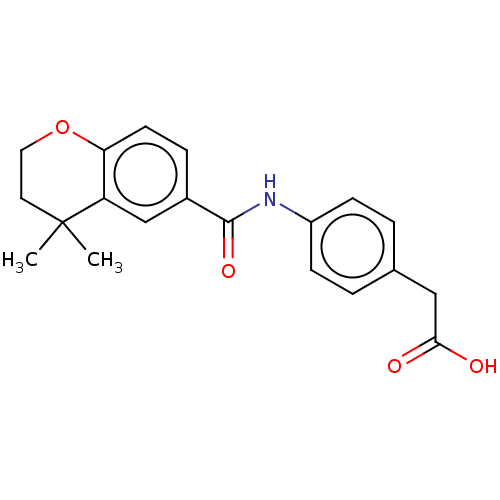 (US10874634, Cmpd No. 05 | US11364220, Compound 05) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen''s University at Kingston US Patent | Assay Description CYP26A1 or CYP26B1 stably transfected HeLa cells were maintained in Minimum Essential Medium (MEM) containing 10% fetal bovine serum (FBS) and 100 &#... | US Patent US10874634 (2020) BindingDB Entry DOI: 10.7270/Q2Q52SP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476222 (US10874634, Cmpd No. 05 | US11364220, Compound 05) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This retinoid activity assay measures the ability of test compounds to induce expression of a transiently transfected RA sensitive reporter construct... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QN6B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476234 (US10874634, Cmpd No. 16 | US11364220, Compound 16) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen''s University at Kingston US Patent | Assay Description CYP26A1 or CYP26B1 stably transfected HeLa cells were maintained in Minimum Essential Medium (MEM) containing 10% fetal bovine serum (FBS) and 100 &#... | US Patent US10874634 (2020) BindingDB Entry DOI: 10.7270/Q2Q52SP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476234 (US10874634, Cmpd No. 16 | US11364220, Compound 16) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This retinoid activity assay measures the ability of test compounds to induce expression of a transiently transfected RA sensitive reporter construct... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QN6B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50033077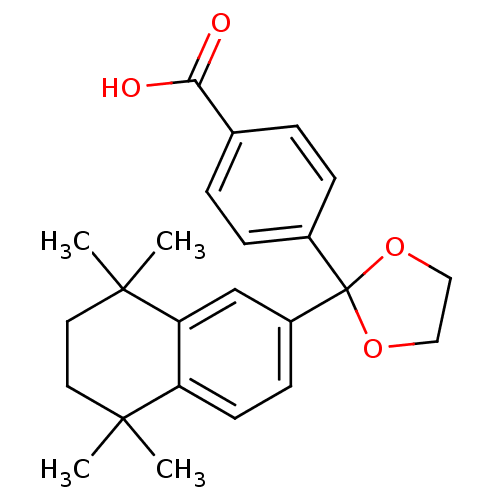 (4-[2-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476226 (US10874634, Cmpd No. 09 | US11364220, Compound 09) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This retinoid activity assay measures the ability of test compounds to induce expression of a transiently transfected RA sensitive reporter construct... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QN6B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476226 (US10874634, Cmpd No. 09 | US11364220, Compound 09) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen''s University at Kingston US Patent | Assay Description CYP26A1 or CYP26B1 stably transfected HeLa cells were maintained in Minimum Essential Medium (MEM) containing 10% fetal bovine serum (FBS) and 100 &#... | US Patent US10874634 (2020) BindingDB Entry DOI: 10.7270/Q2Q52SP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157611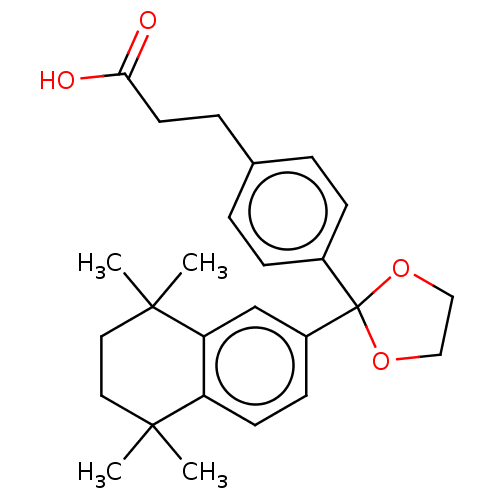 (CHEMBL3787627) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM557291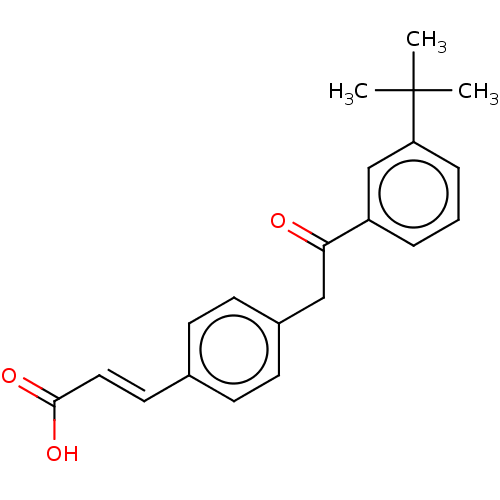 (US11358933, Compound 025) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CYP26 inhibitory activities of various compounds. | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM557297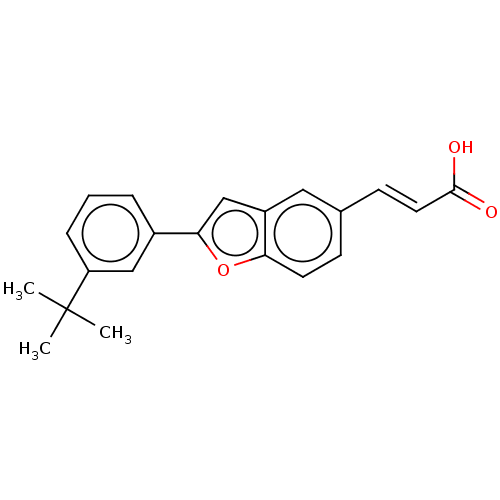 (US11358933, Compound 031) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CYP26 inhibitory activities of various compounds. | Citation and Details BindingDB Entry DOI: 10.7270/Q2CC13XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM476217 (US10874634, Cmpd No. 04 | US11364220, Compound 04) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen''s University at Kingston US Patent | Assay Description CYP26A1 or CYP26B1 stably transfected HeLa cells were maintained in Minimum Essential Medium (MEM) containing 10% fetal bovine serum (FBS) and 100 &#... | US Patent US10874634 (2020) BindingDB Entry DOI: 10.7270/Q2Q52SP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 77 total ) | Next | Last >> |