

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Botulinum neurotoxin type E (Clostridium botulinum) | BDBM50189879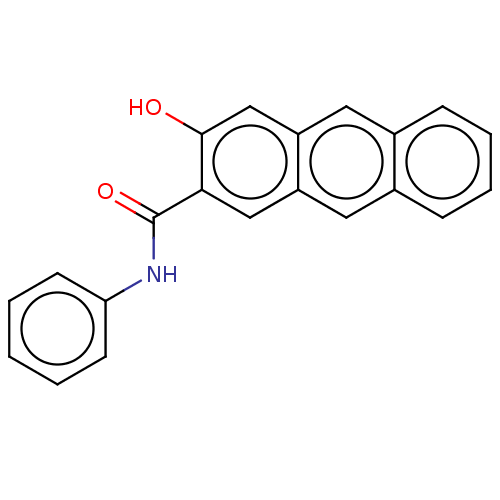 (CHEMBL3827999) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory Curated by ChEMBL | Assay Description Inhibition of endopeptidase activity of Clostridium botulinum BoNT/E light chain using SNAP-25 as substrate after 15 mins by HPLC analysis | Bioorg Med Chem 24: 3978-3985 (2016) Article DOI: 10.1016/j.bmc.2016.06.036 BindingDB Entry DOI: 10.7270/Q2VT1V28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type E (Clostridium botulinum) | BDBM50189880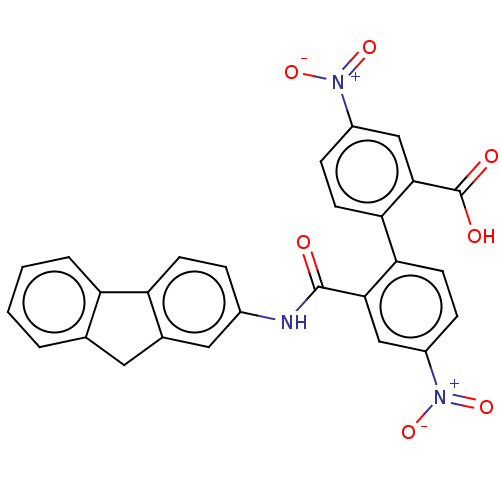 (CHEMBL3828454) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory Curated by ChEMBL | Assay Description Inhibition of endopeptidase activity of Clostridium botulinum BoNT/E light chain using SNAP-25 as substrate after 15 mins by HPLC analysis | Bioorg Med Chem 24: 3978-3985 (2016) Article DOI: 10.1016/j.bmc.2016.06.036 BindingDB Entry DOI: 10.7270/Q2VT1V28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type E (Clostridium botulinum) | BDBM50189881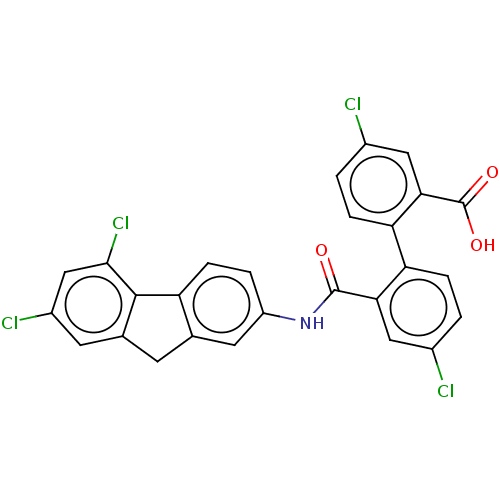 (CHEMBL3827632) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory Curated by ChEMBL | Assay Description Inhibition of endopeptidase activity of Clostridium botulinum BoNT/E light chain using SNAP-25 as substrate after 15 mins by HPLC analysis | Bioorg Med Chem 24: 3978-3985 (2016) Article DOI: 10.1016/j.bmc.2016.06.036 BindingDB Entry DOI: 10.7270/Q2VT1V28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type E (Clostridium botulinum) | BDBM50322673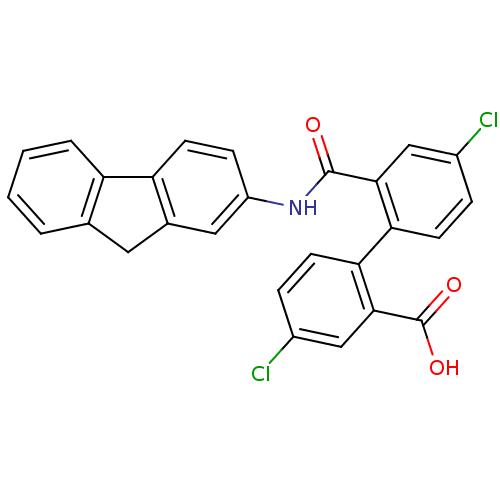 (2'-(9H-fluoren-2-ylcarbamoyl)-4,4'-dichlorobipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory Curated by ChEMBL | Assay Description Inhibition of endopeptidase activity of Clostridium botulinum BoNT/E light chain using SNAP-25 as substrate after 15 mins by HPLC analysis | Bioorg Med Chem 24: 3978-3985 (2016) Article DOI: 10.1016/j.bmc.2016.06.036 BindingDB Entry DOI: 10.7270/Q2VT1V28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type E (Clostridium botulinum) | BDBM50189882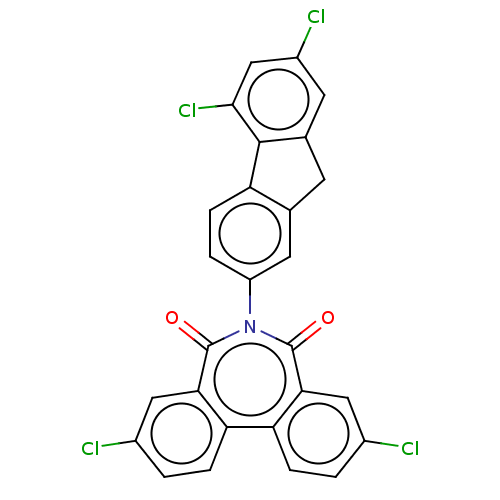 (CHEMBL3828595) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory Curated by ChEMBL | Assay Description Inhibition of endopeptidase activity of Clostridium botulinum BoNT/E light chain using SNAP-25 as substrate after 15 mins by HPLC analysis | Bioorg Med Chem 24: 3978-3985 (2016) Article DOI: 10.1016/j.bmc.2016.06.036 BindingDB Entry DOI: 10.7270/Q2VT1V28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type E (Clostridium botulinum) | BDBM50534915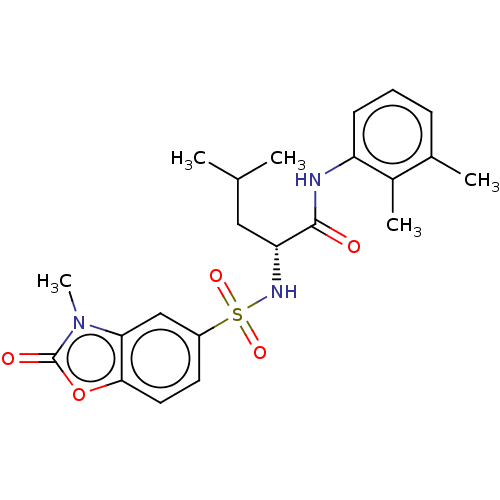 (CHEMBL4546947) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/E light chain using GFP-tagged VAMP2/SNAP-25 (Ala128 to Gly206 residues) as substrate after 15 mins by GFP b... | Bioorg Med Chem 24: 4875-4889 (2016) Article DOI: 10.1016/j.bmc.2016.07.031 BindingDB Entry DOI: 10.7270/Q2JH3QPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type E (Clostridium botulinum) | BDBM50534916 (CHEMBL4441787) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/E light chain using GFP-tagged VAMP2/SNAP-25 (Ala128 to Gly206 residues) as substrate after 15 mins by GFP b... | Bioorg Med Chem 24: 4875-4889 (2016) Article DOI: 10.1016/j.bmc.2016.07.031 BindingDB Entry DOI: 10.7270/Q2JH3QPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type E (Clostridium botulinum) | BDBM50189883 (CHEMBL1999371) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/E light chain using GFP-tagged VAMP2/SNAP-25 (Ala128 to Gly206 residues) as substrate after 15 mins by GFP b... | Bioorg Med Chem 24: 4875-4889 (2016) Article DOI: 10.1016/j.bmc.2016.07.031 BindingDB Entry DOI: 10.7270/Q2JH3QPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||