

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50265871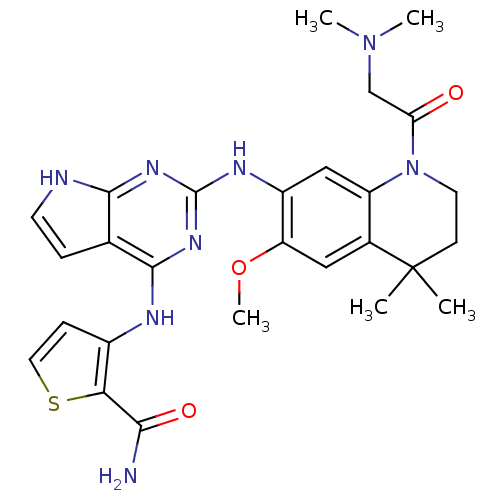 (3-(2-(1-(2-(dimethylamino)acetyl)-6-methoxy-4,4-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of BODIPY TR-ADP from bovine GRK1 (1 to 535 residues) preincubated for 10 mins followed by compound addition and measured after 10 to 15... | ACS Med Chem Lett 10: 1628-1634 (2019) Article DOI: 10.1021/acsmedchemlett.9b00365 BindingDB Entry DOI: 10.7270/Q28W3HR8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50529469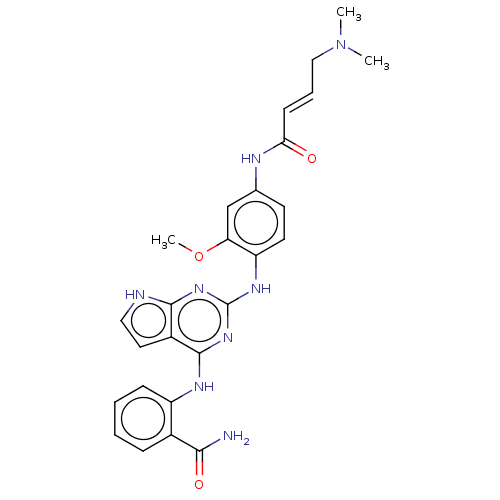 (CHEMBL4456539) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 using tubulin as substrate measured after 4 hrs by [gamma-32P]-ATP assay | ACS Med Chem Lett 10: 1628-1634 (2019) Article DOI: 10.1021/acsmedchemlett.9b00365 BindingDB Entry DOI: 10.7270/Q28W3HR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50529470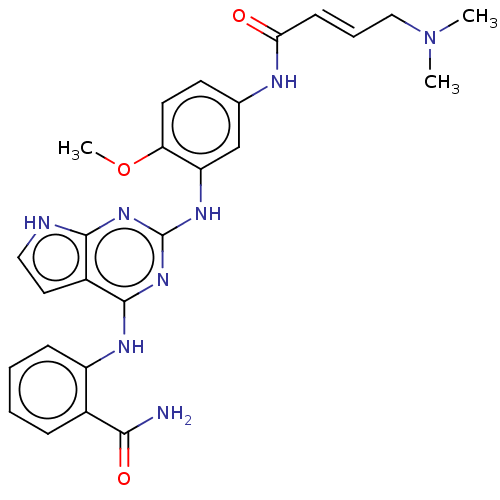 (CHEMBL4475305) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 using tubulin as substrate measured after 4 hrs by [gamma-32P]-ATP assay | ACS Med Chem Lett 10: 1628-1634 (2019) Article DOI: 10.1021/acsmedchemlett.9b00365 BindingDB Entry DOI: 10.7270/Q28W3HR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM3149 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of bovine C-terminal truncated GRK1 (535 residues) assessed as decrease in phosphorylation of urea-washed bovine rod outer segments preinc... | J Med Chem 59: 9277-9294 (2016) BindingDB Entry DOI: 10.7270/Q2348NB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50173313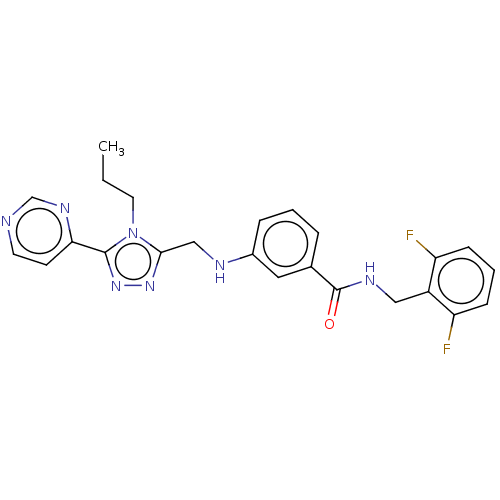 (CHEMBL1738878) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM27791 (2-{[2-({4-chloro-2-methoxy-5-[(1-propylpiperidin-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of BODIPY TR-ADP from bovine GRK1 (1 to 535 residues) preincubated for 10 mins followed by compound addition and measured after 10 to 15... | ACS Med Chem Lett 10: 1628-1634 (2019) Article DOI: 10.1021/acsmedchemlett.9b00365 BindingDB Entry DOI: 10.7270/Q28W3HR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260129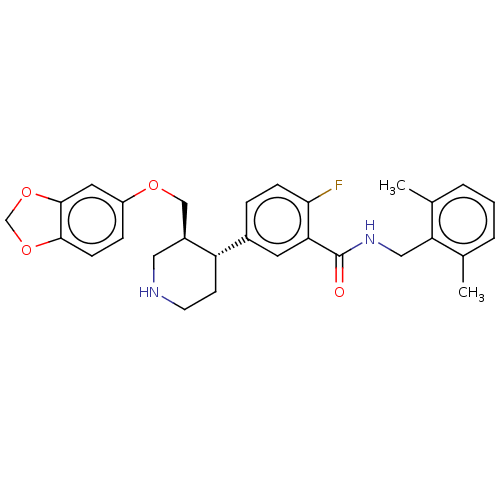 (CHEMBL4098656) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260147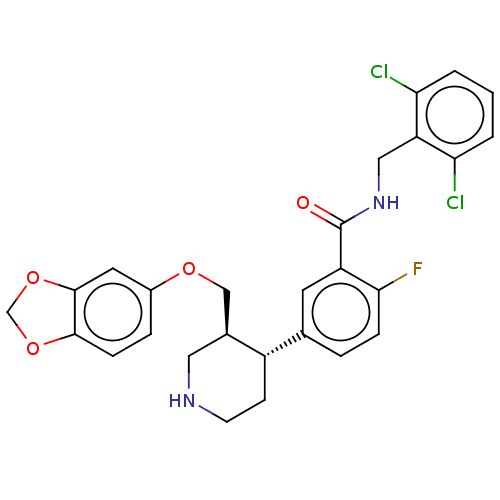 (CHEMBL4063014) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Binding affinity towards serotonin transporter determined using [3H]paroxetine as radioligand | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260130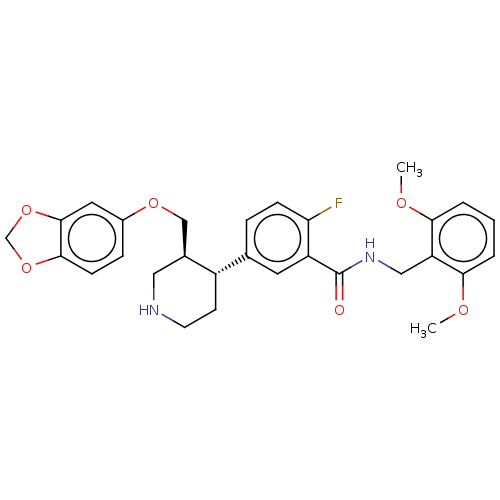 (CHEMBL4090923) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibitory concentration against human alpha-L-fucosidase | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50257350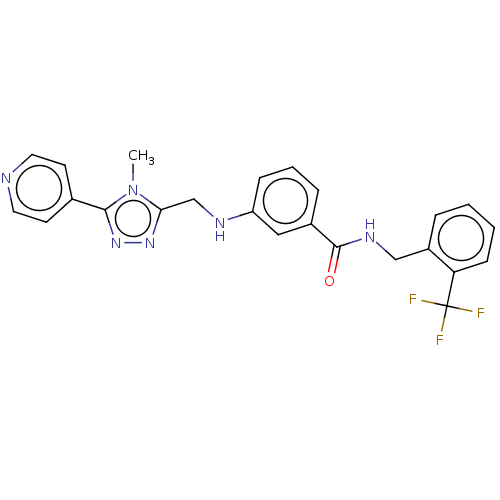 (CHEMBL1738877) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 5.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibitory concentration against alpha-galactosidase of coffee bean | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260137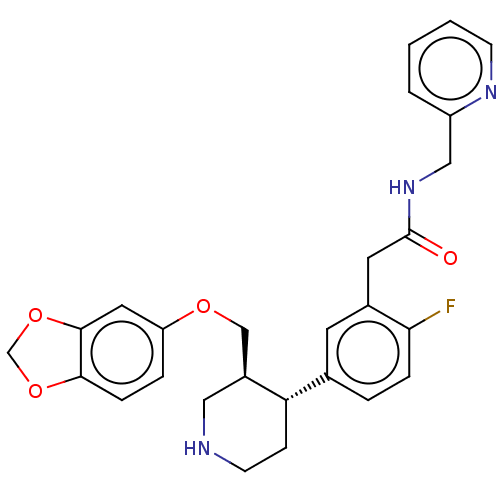 (CHEMBL4062790) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260141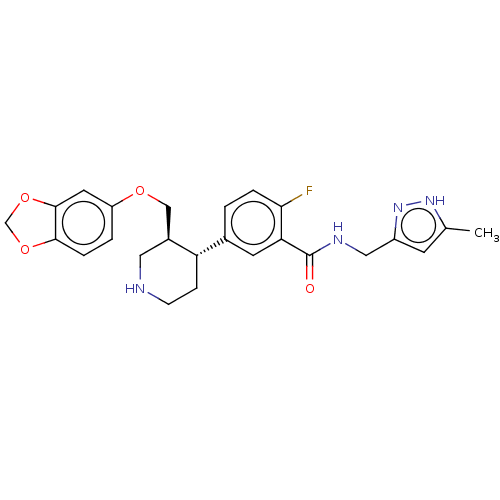 (CHEMBL4097393) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260140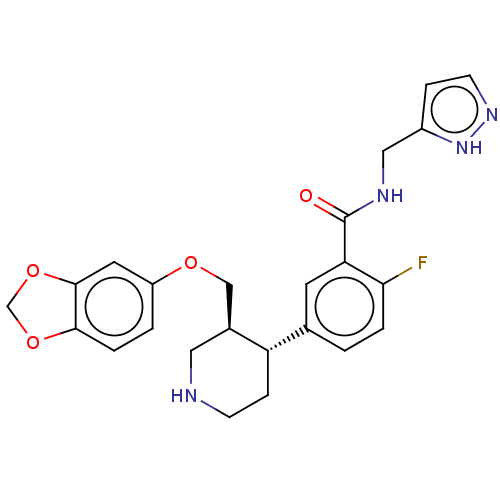 (CHEMBL4090144) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260136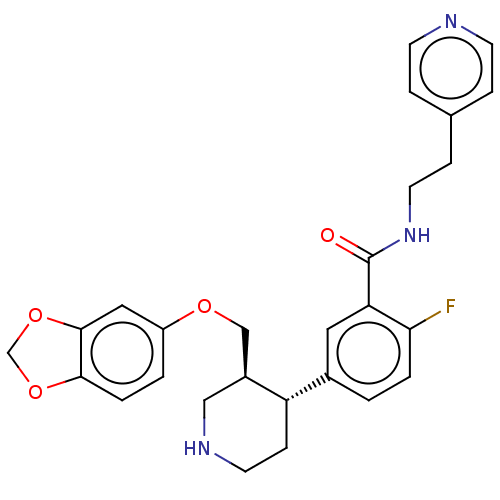 (CHEMBL4077459) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibitory concentration against alpha-galactosidase of coffee bean | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260135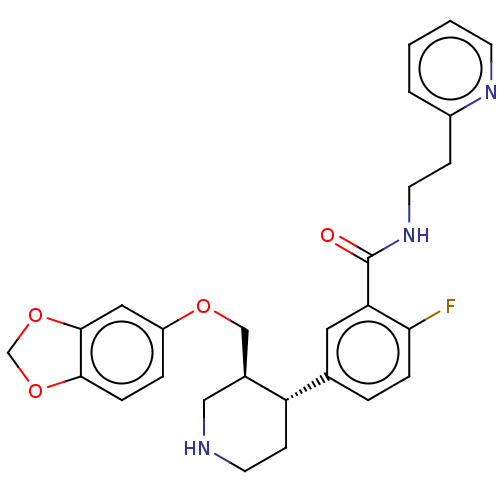 (CHEMBL4076131) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibitory concentration against alpha-galactosidase of coffee bean | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260134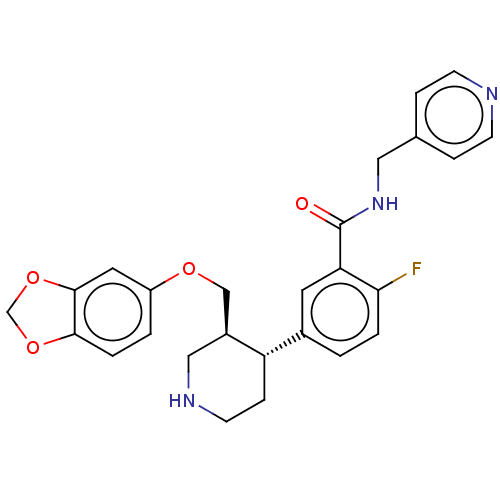 (CHEMBL4072898) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibitory concentration against alpha-galactosidase of coffee bean | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260133 (CHEMBL4077197) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Ki value against bovine alpha-L-fucosidase | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260132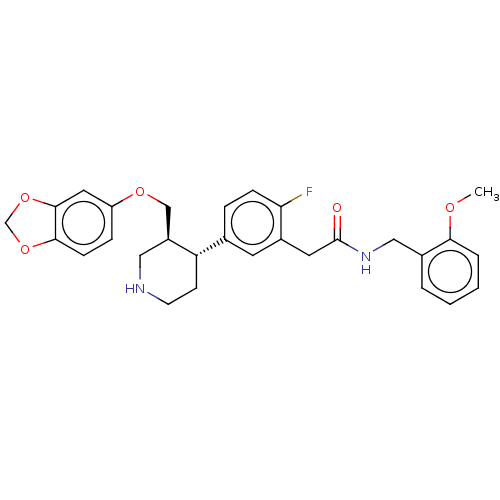 (CHEMBL4083142) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibitory concentration against alpha-galactosidase of coffee bean | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260131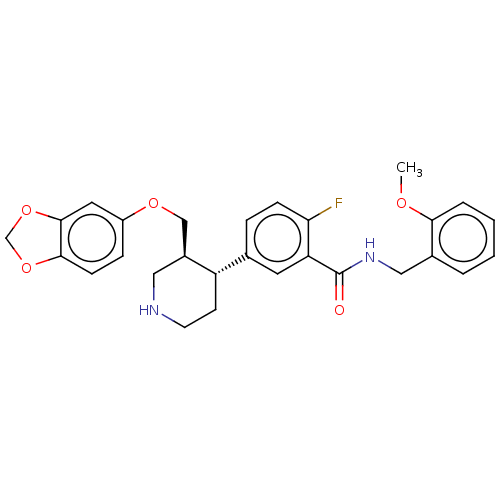 (CHEMBL4101136) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibitory concentration against bovine alpha-L-fucosidase | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260128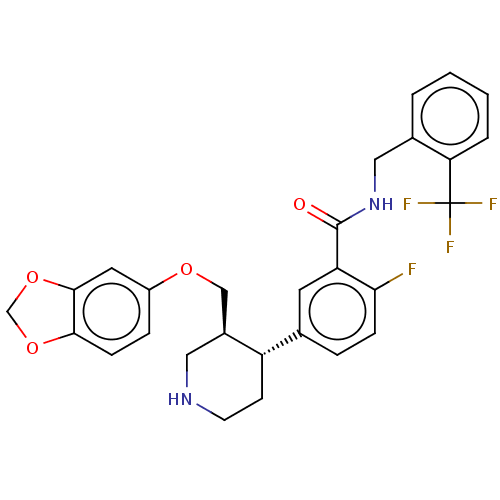 (CHEMBL4071638) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibitory concentration against beta-glucosidase | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260127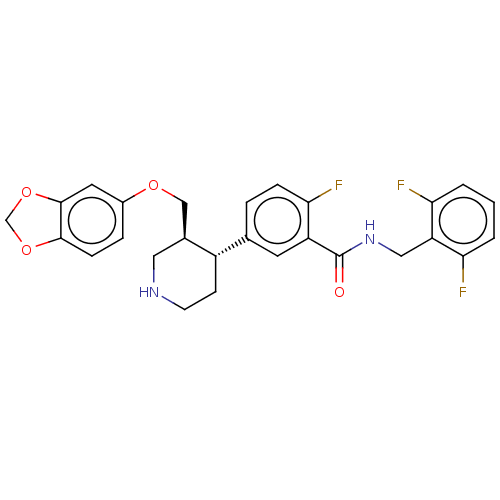 (CHEMBL4091281) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Ki value against bovine alpha-L-fucosidase | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260126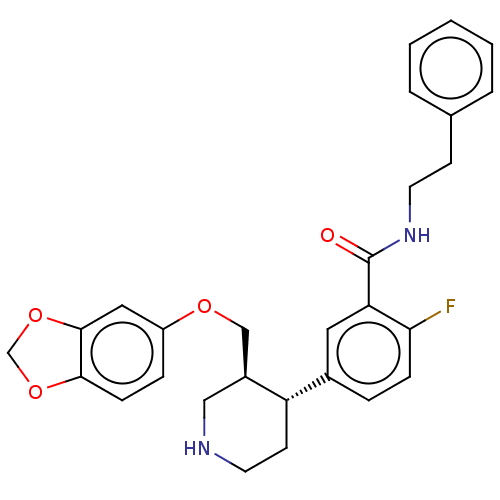 (CHEMBL4073639) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibitory concentration against alpha-galactosidase of coffee bean | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260125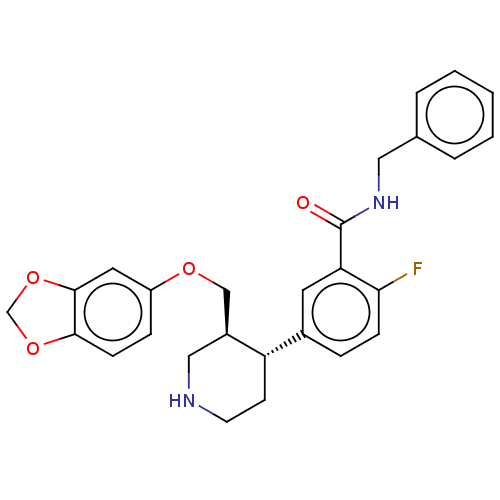 (CHEMBL4099398) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibitory concentration against human beta-glucosidase | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50173319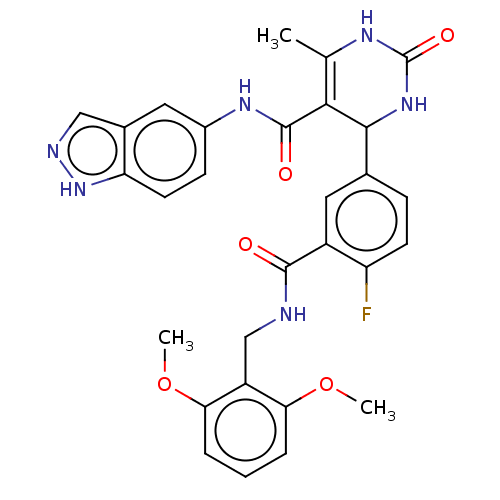 (CHEMBL3809020 | US10023564, Example 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibitory concentration against alpha-galactosidase of coffee bean | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM22416 ((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50529463 (CHEMBL4449730) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 using tubulin as substrate measured after 4 hrs by [gamma-32P]-ATP assay | ACS Med Chem Lett 10: 1628-1634 (2019) Article DOI: 10.1021/acsmedchemlett.9b00365 BindingDB Entry DOI: 10.7270/Q28W3HR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50529464 (CHEMBL4543927) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 using tubulin as substrate measured after 4 hrs by [gamma-32P]-ATP assay | ACS Med Chem Lett 10: 1628-1634 (2019) Article DOI: 10.1021/acsmedchemlett.9b00365 BindingDB Entry DOI: 10.7270/Q28W3HR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM27826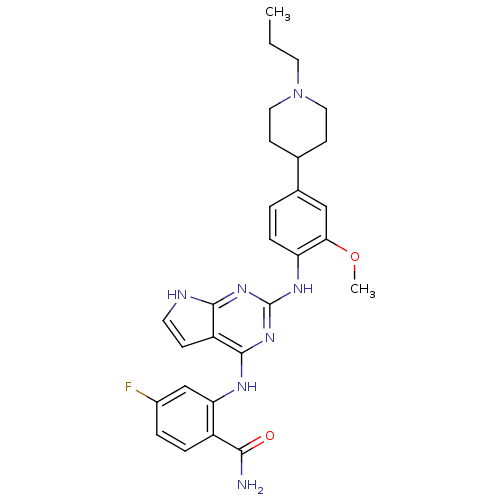 (4,6-bis-anilino-1H-pyrrolo[2,3-d]pyrimidine, 9 | 4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of BODIPY TR-ADP from bovine GRK1 (1 to 535 residues) preincubated for 10 mins followed by compound addition and measured after 10 to 15... | ACS Med Chem Lett 10: 1628-1634 (2019) Article DOI: 10.1021/acsmedchemlett.9b00365 BindingDB Entry DOI: 10.7270/Q28W3HR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50529465 (CHEMBL4464632) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 using tubulin as substrate measured after 4 hrs by [gamma-32P]-ATP assay | ACS Med Chem Lett 10: 1628-1634 (2019) Article DOI: 10.1021/acsmedchemlett.9b00365 BindingDB Entry DOI: 10.7270/Q28W3HR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50529460 (CHEMBL4450181) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 using tubulin as substrate measured after 4 hrs by [gamma-32P]-ATP assay | ACS Med Chem Lett 10: 1628-1634 (2019) Article DOI: 10.1021/acsmedchemlett.9b00365 BindingDB Entry DOI: 10.7270/Q28W3HR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260152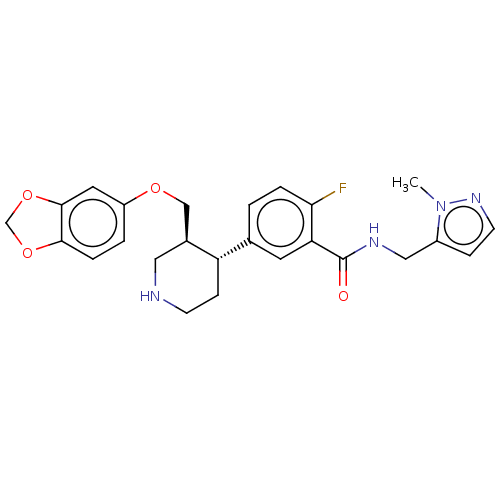 (CHEMBL4105100) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Binding affinity towards serotonin transporter determined using [3H]paroxetine as radioligand | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260151 (CHEMBL4076529) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Binding affinity towards norepinephrine transporter determined using [3H]nisoxetine as radioligand | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260150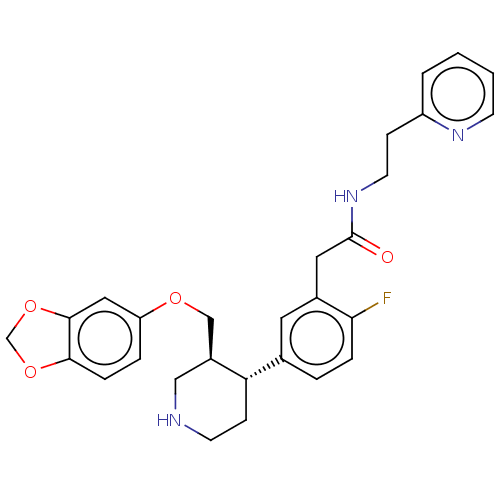 (CHEMBL4084465) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Binding affinity of compound towards dopamine transporter determined using [3H]WIN-35 428 as radioligand | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260138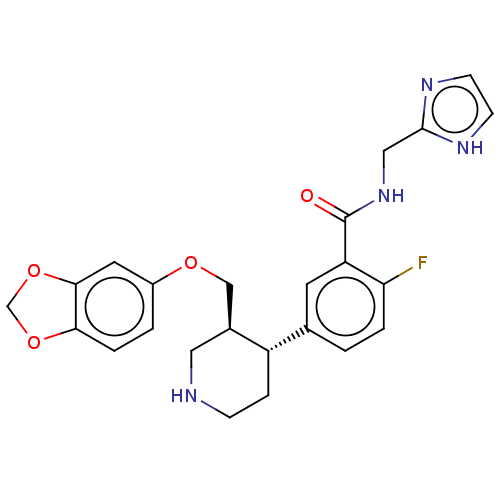 (CHEMBL4075475) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260139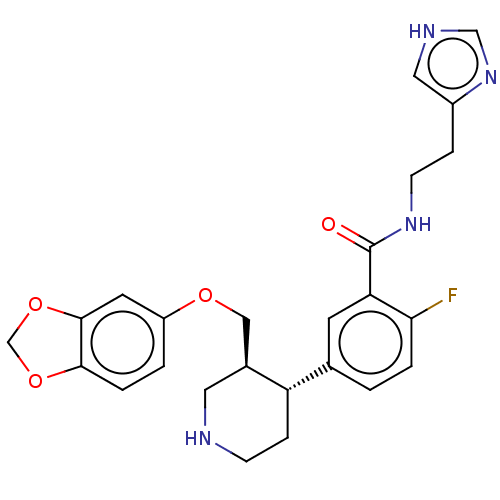 (CHEMBL4075712) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260142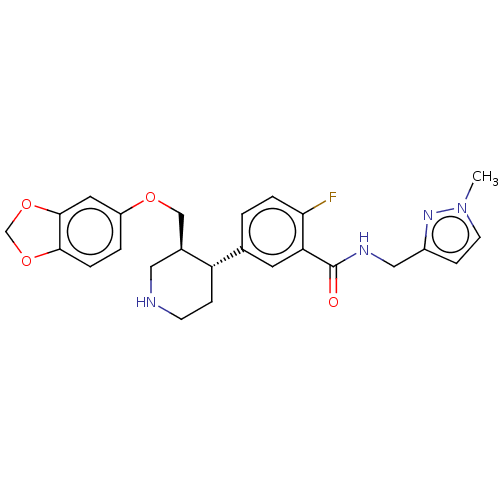 (CHEMBL4079362) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260143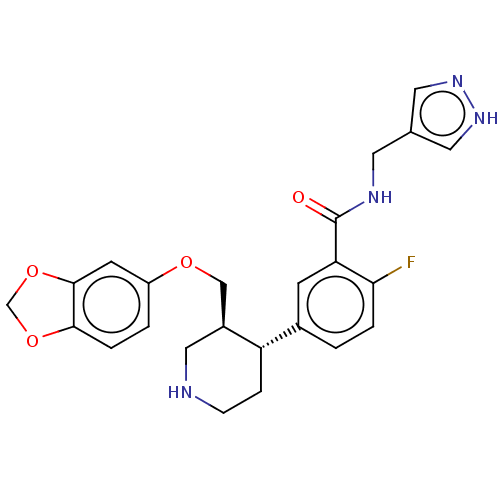 (CHEMBL4087244) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260144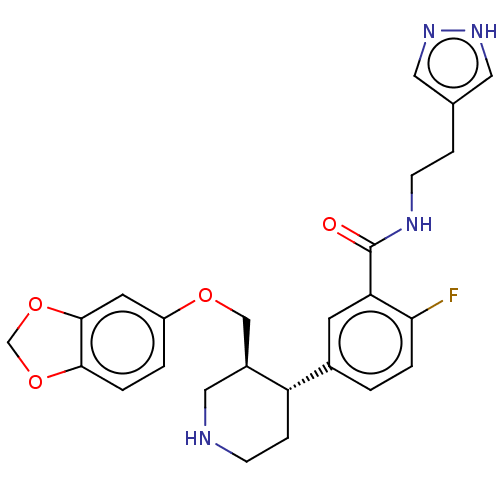 (CHEMBL4081802) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260145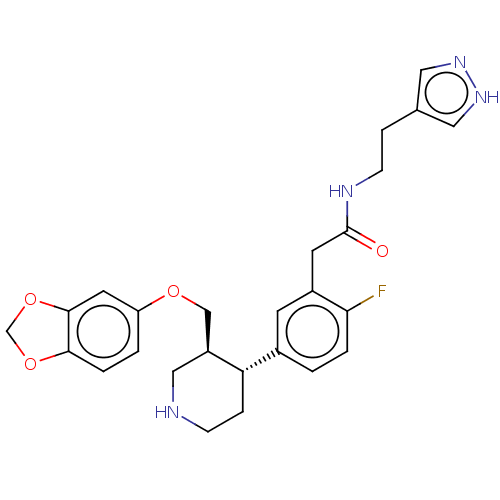 (CHEMBL4070290) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50173320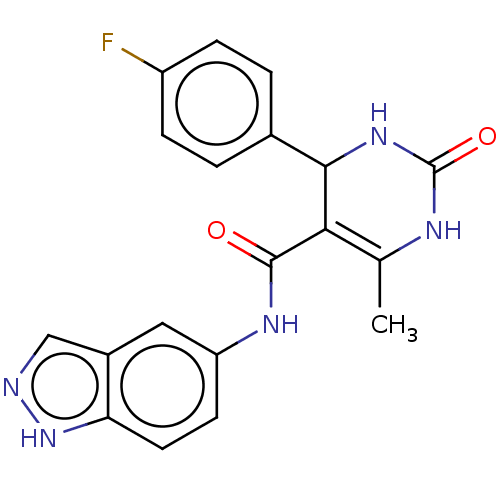 (GSK-180736A | GSK180736A | US10023564, Compound GS...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Binding affinity of compound towards dopamine transporter determined using [3H]WIN-35 428 as radioligand | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260154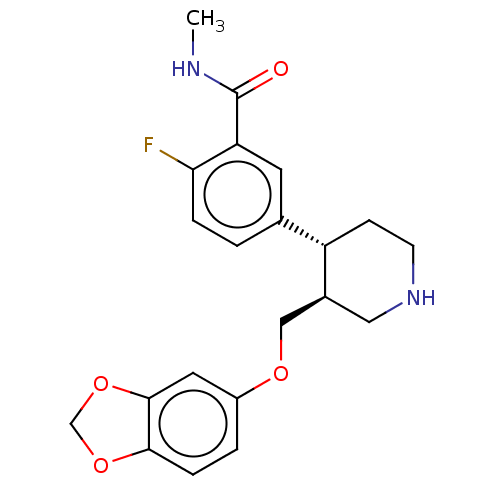 (CHEMBL4077133) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Binding affinity towards norepinephrine transporter determined using [3H]nisoxetine as radioligand | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260146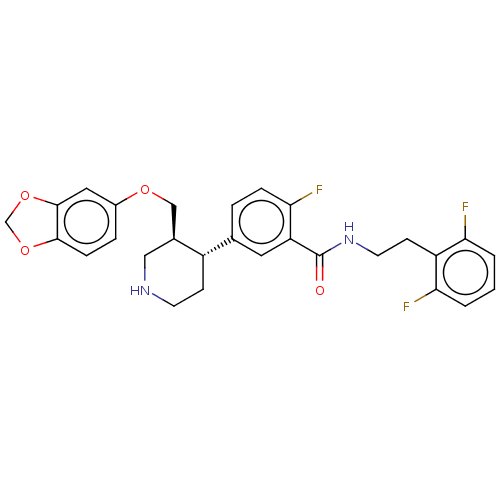 (CHEMBL4064274) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Binding affinity towards norepinephrine transporter determined using [3H]nisoxetine as radioligand | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260148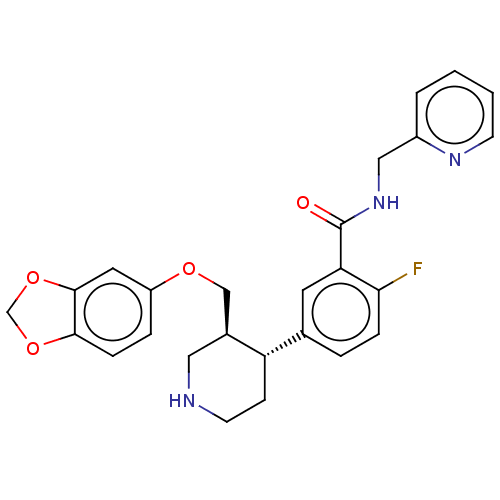 (CHEMBL4070885) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Binding affinity of compound towards dopamine transporter determined using [3H]WIN-35 428 as radioligand | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260149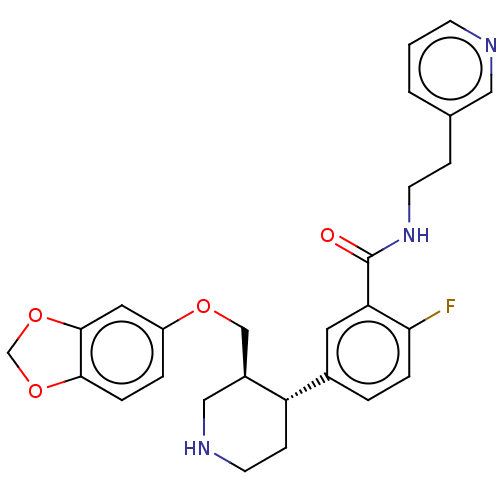 (CHEMBL4074476) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Binding affinity of compound towards dopamine transporter determined using [3H]WIN-35 428 as radioligand | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50331515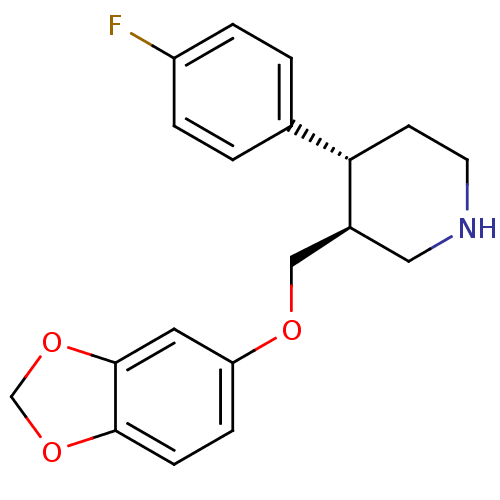 (CHEMBL1708 | PAROXETINE | Paroxetine hydrochloride...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 1.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of C-terminal hexahistidine tagged bovine GRK1 (535 residues) assessed as decrease in phosphorylation of tubulin preincubated for 30 mins ... | J Med Chem 59: 9277-9294 (2016) BindingDB Entry DOI: 10.7270/Q2348NB9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50331515 (CHEMBL1708 | PAROXETINE | Paroxetine hydrochloride...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 3.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of C-terminal hexahistidine tagged bovine GRK1 (535 residues) assessed as decrease in phosphorylation of light-activated bovine rod outer ... | J Med Chem 59: 9277-9294 (2016) BindingDB Entry DOI: 10.7270/Q2348NB9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50331515 (CHEMBL1708 | PAROXETINE | Paroxetine hydrochloride...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 3.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis | J Med Chem 59: 9277-9294 (2016) BindingDB Entry DOI: 10.7270/Q2348NB9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM14052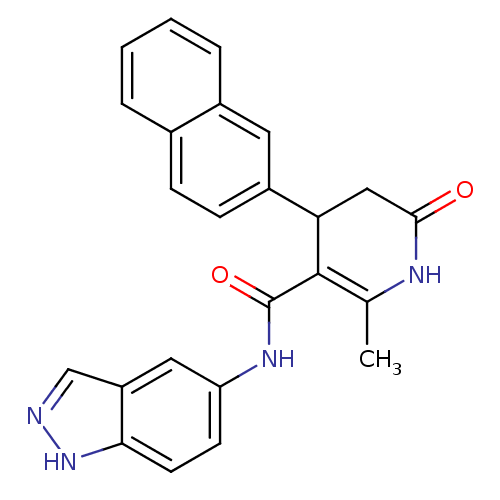 (N-(1H-indazol-5-yl)-2-methyl-4-(naphthalen-2-yl)-6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis | J Med Chem 59: 9277-9294 (2016) BindingDB Entry DOI: 10.7270/Q2348NB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM14056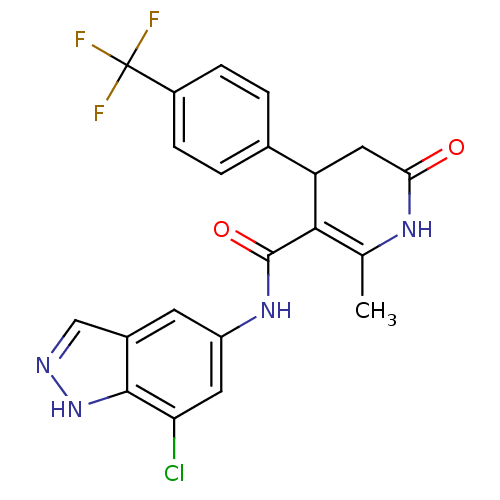 (N-(7-chloro-1H-indazol-5-yl)-2-methyl-6-oxo-4-[4-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis | J Med Chem 59: 9277-9294 (2016) BindingDB Entry DOI: 10.7270/Q2348NB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50173320 (GSK-180736A | GSK180736A | US10023564, Compound GS...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) assessed as decrease in phosphorylation of tubulin after 5 mins by SDS-PAGE analysis | J Med Chem 59: 9277-9294 (2016) BindingDB Entry DOI: 10.7270/Q2348NB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |