

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50024092 (6-Iodomethylene-4-phenyl-tetrahydro-pyran-2-one | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding constant of the compound alpha-chymotrypsin was determined by competitive inhibition assay with BTEE as substrate | J Med Chem 29: 230-8 (1986) BindingDB Entry DOI: 10.7270/Q2NC6068 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50014743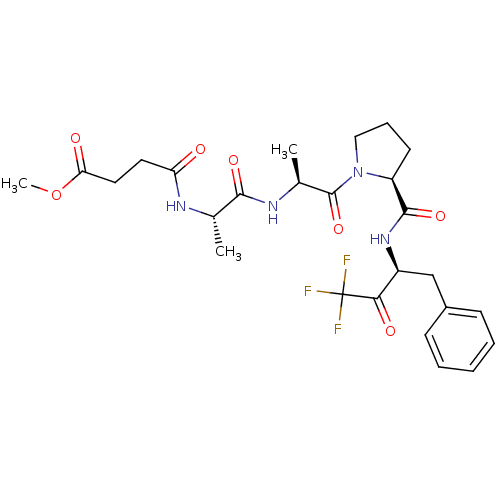 (CHEMBL132171 | N-(1-{2-[2-(1-Benzyl-3,3,3-trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha-chymotrypsin | J Med Chem 33: 394-407 (1990) BindingDB Entry DOI: 10.7270/Q26D5RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50014731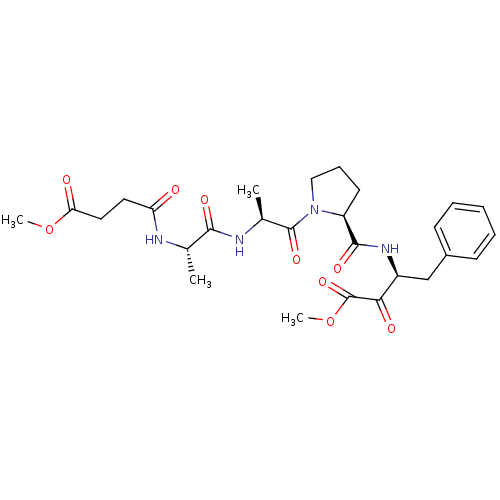 (3-[(1-{2-[2-(3-Methoxycarbonyl-propionylamino)-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha-chymotrypsin | J Med Chem 33: 394-407 (1990) BindingDB Entry DOI: 10.7270/Q26D5RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50025866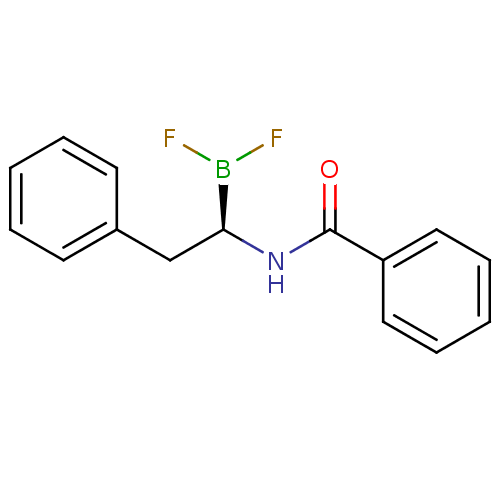 (CHEMBL60695 | N-[1R-(2-phenyl ethyl)]benzamide dif...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competetive inhibition of alpha-chymotrypsin | J Med Chem 28: 1917-25 (1986) BindingDB Entry DOI: 10.7270/Q22B8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50025877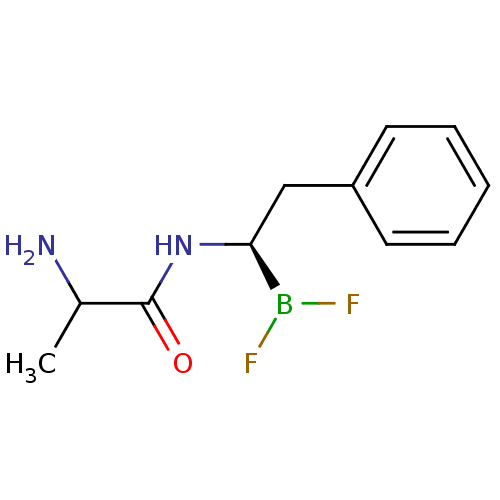 (CHEMBL58683 | N-[1R-(2-phenyl ethyl)]propanamide d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competetive inhibition of alpha-chymotrypsin | J Med Chem 28: 1917-25 (1986) BindingDB Entry DOI: 10.7270/Q22B8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human Chymotrypsinogen | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50025875 (2-Amino-N-[1R-(2-phenyl ethyl)]propanamide boronic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competetive inhibition of alpha-chymotrypsin | J Med Chem 28: 1917-25 (1986) BindingDB Entry DOI: 10.7270/Q22B8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50025872 (CHEMBL293513 | N-[1R-(2-phenyl ethyl)]benzamide bo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competetive inhibition of alpha-chymotrypsin | J Med Chem 28: 1917-25 (1986) BindingDB Entry DOI: 10.7270/Q22B8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM521743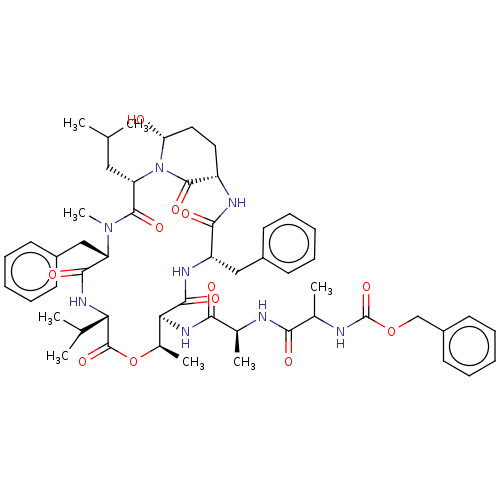 (US11149067, Compound Ahp2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50025870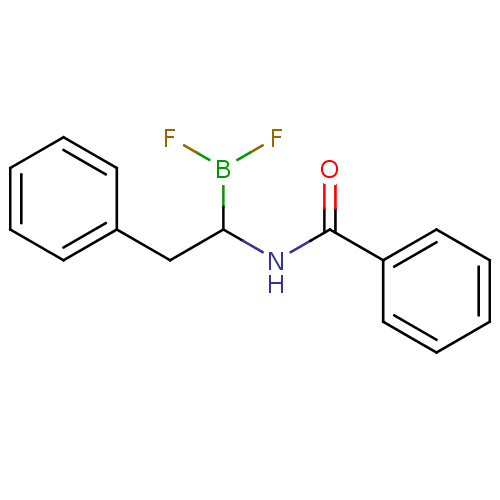 (CHEMBL57262 | N-(2-phenyl ethyl)benzamide difluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competetive inhibition of alpha-chymotrypsin | J Med Chem 28: 1917-25 (1986) BindingDB Entry DOI: 10.7270/Q22B8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50025860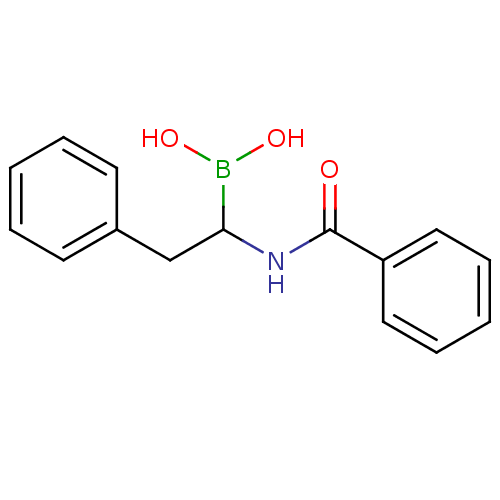 (CHEMBL292664 | N-(2-phenyl ethyl)benzamide boronic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competetive inhibition of alpha-chymotrypsin | J Med Chem 28: 1917-25 (1986) BindingDB Entry DOI: 10.7270/Q22B8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50025868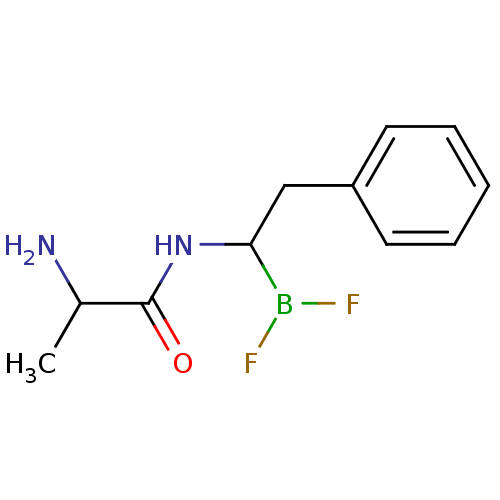 (2-Amino-N-(2-phenyl ethyl)propanamide difluorobora...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competetive inhibition of alpha-chymotrypsin | J Med Chem 28: 1917-25 (1986) BindingDB Entry DOI: 10.7270/Q22B8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50025865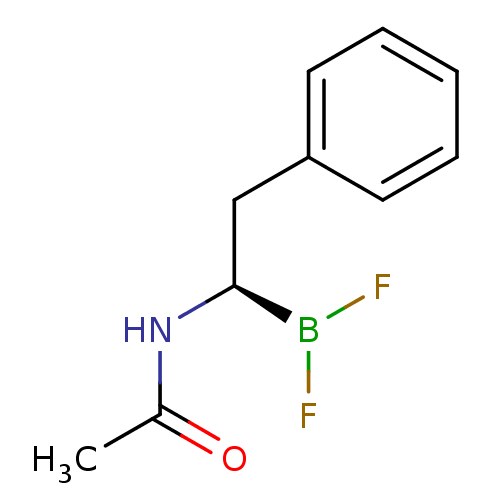 (CHEMBL292593 | N-[1R-(2-phenyl ethyl)]acetamide di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competetive inhibition of alpha-chymotrypsin | J Med Chem 28: 1917-25 (1986) BindingDB Entry DOI: 10.7270/Q22B8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50025874 (2-Amino-N-benzylpropanamide difluoroborane | CHEMB...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competetive inhibition of alpha-chymotrypsin | J Med Chem 28: 1917-25 (1986) BindingDB Entry DOI: 10.7270/Q22B8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM521750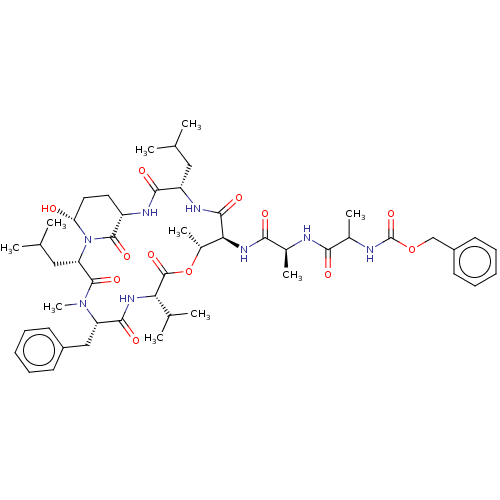 (US11149067, Compound Ahp9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50025862 (CHEMBL58706 | N-[1R-(2-phenyl ethyl)]acetamide bor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competetive inhibition of alpha-chymotrypsin | J Med Chem 28: 1917-25 (1986) BindingDB Entry DOI: 10.7270/Q22B8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50133629 (2,3-Dioxo-2,3-dihydro-indole-1-carboxylic acid ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant against alpha-chymotrypsin | Bioorg Med Chem Lett 5: 89-92 (1995) Article DOI: 10.1016/0960-894X(94)00464-Q BindingDB Entry DOI: 10.7270/Q2PZ58S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50025861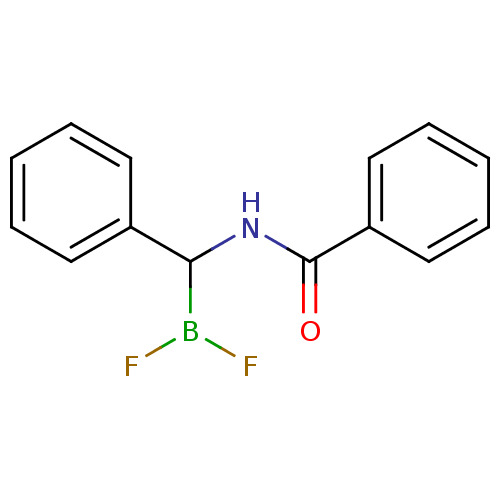 (CHEMBL56829 | N-benzylbenzamide difluoroborane) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competetive inhibition of alpha-chymotrypsin | J Med Chem 28: 1917-25 (1986) BindingDB Entry DOI: 10.7270/Q22B8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50025859 (CHEMBL61146 | N-(2-phenyl ethyl)acetamide boronic ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competetive inhibition of alpha-chymotrypsin | J Med Chem 28: 1917-25 (1986) BindingDB Entry DOI: 10.7270/Q22B8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50025863 (2-Amino-N-[(1R)-benzyl]propanamide difluoroborane ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competetive inhibition of alpha-chymotrypsin | J Med Chem 28: 1917-25 (1986) BindingDB Entry DOI: 10.7270/Q22B8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50025871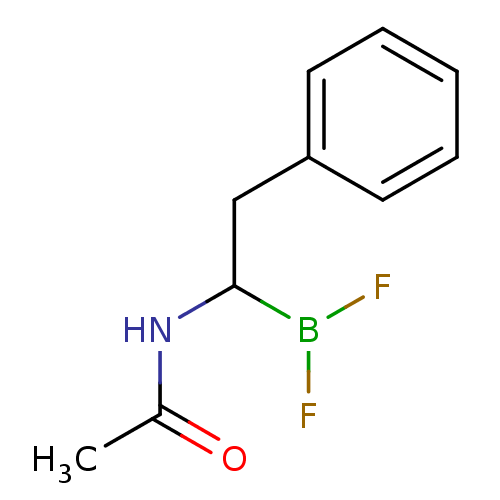 (CHEMBL430767 | N-(2-phenyl ethyl)acetamide difluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competetive inhibition of alpha-chymotrypsin | J Med Chem 28: 1917-25 (1986) BindingDB Entry DOI: 10.7270/Q22B8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50126635 (3-[3-({[(Z)-amino(imino)methyl]amino}oxy)ethoxy]-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound was tested against serine protease Chymotrypsin | Bioorg Med Chem Lett 13: 1495-8 (2003) BindingDB Entry DOI: 10.7270/Q2668CJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50287589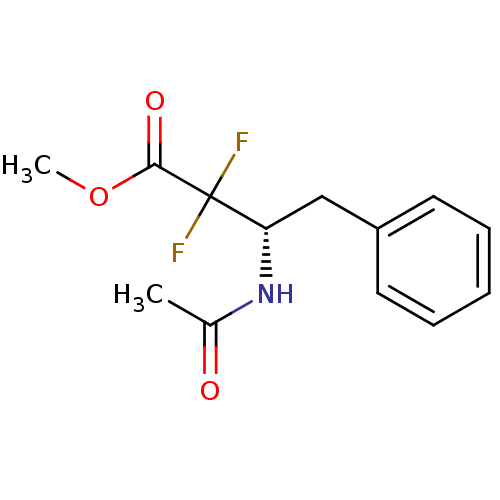 ((S)-3-Acetylamino-2,2-difluoro-4-phenyl-butyric ac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.8 | n/a |
TBA Curated by ChEMBL | Assay Description Reversible inhibitory activity of the compound toward alpha-chymotrypsin (5 nM concentration) at a pH of 7.8 by progress curve method. | Bioorg Med Chem Lett 6: 1875-1880 (1996) Article DOI: 10.1016/0960-894X(96)00342-3 BindingDB Entry DOI: 10.7270/Q2BG2P0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM521751 (US11149067, Compound Ahp10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM521749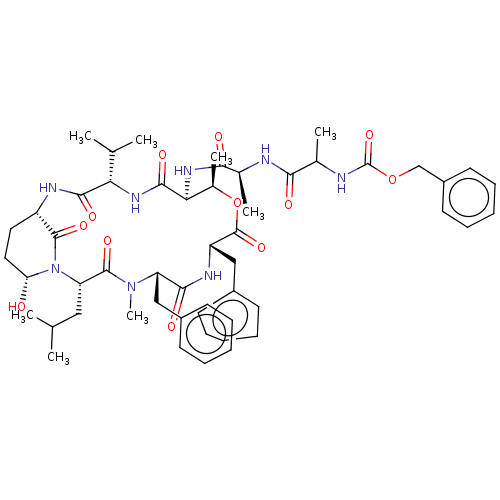 (US11149067, Compound Ahp8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM521748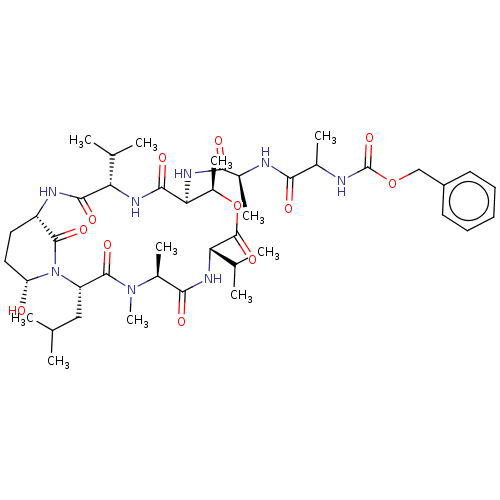 (US11149067, Compound Ahp7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM521747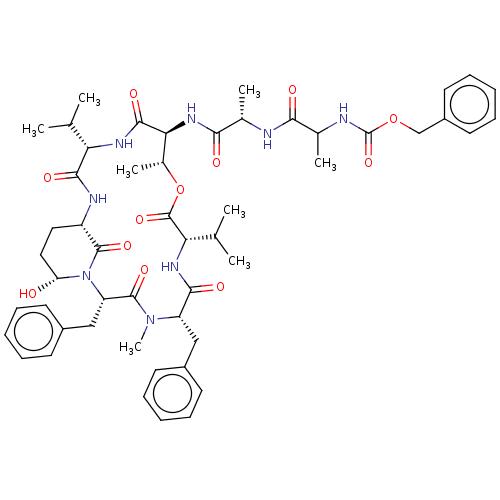 (US11149067, Compound Ahp6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM521746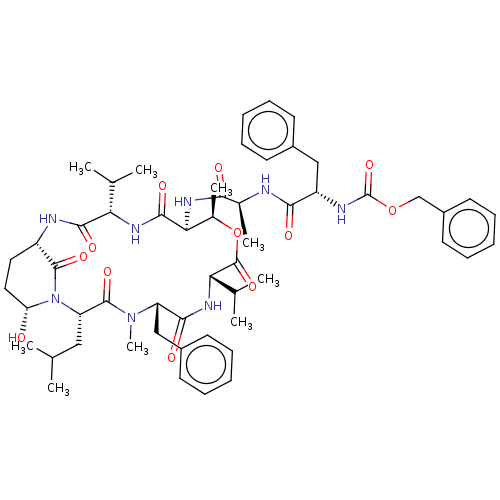 (US11149067, Compound Ahp5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM521745 (US11149067, Compound Ahp4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM521744 (US11149067, Compound Ahp3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM521742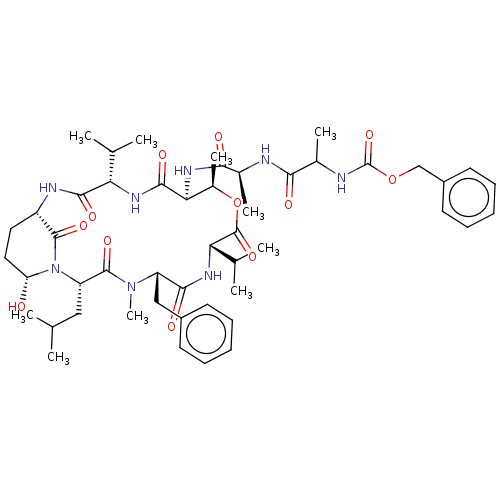 (US11149067, Compound Ahp1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In general, proteolytic activity was tested by monitoring the cleavage of the specific chromogenic substrate at 405 nm wavelength for 60 or 120 minut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50024093 (6-Iodomethylene-5-phenyl-tetrahydro-pyran-2-one | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding constant of the compound alpha-chymotrypsin was determined by competitive inhibition assay with Suc-Ala-Ala-Pro-Phe-pNA as substrate | J Med Chem 29: 230-8 (1986) BindingDB Entry DOI: 10.7270/Q2NC6068 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50076819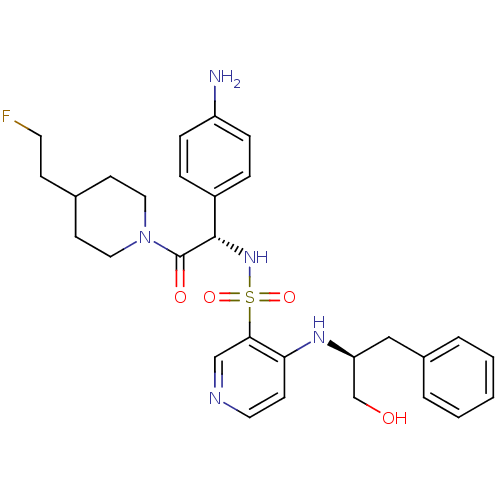 (4-((S)-1-Hydroxymethyl-2-phenyl-ethylamino)-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated against against human chymotrypsin | Bioorg Med Chem Lett 9: 1103-8 (1999) BindingDB Entry DOI: 10.7270/Q2SB44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50287590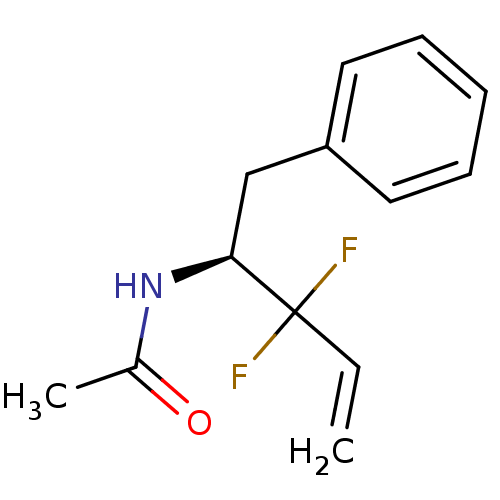 (CHEMBL291936 | N-((S)-1-Benzyl-2,2-difluoro-but-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 4.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.8 | n/a |
TBA Curated by ChEMBL | Assay Description Reversible inhibitory activity of the compound toward alpha-chymotrypsin (5 nM concentration) at a pH of 7.8 by progress curve method. | Bioorg Med Chem Lett 6: 1875-1880 (1996) Article DOI: 10.1016/0960-894X(96)00342-3 BindingDB Entry DOI: 10.7270/Q2BG2P0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50288336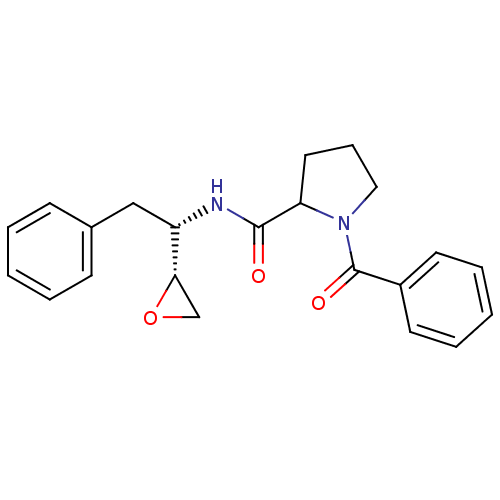 (1-Benzoyl-pyrrolidine-2-carboxylic acid ((S)-1-(R)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 8.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against alpha-chymotrypsin | Bioorg Med Chem Lett 6: 2837-2840 (1996) Article DOI: 10.1016/S0960-894X(96)00536-7 BindingDB Entry DOI: 10.7270/Q2C53KTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50284351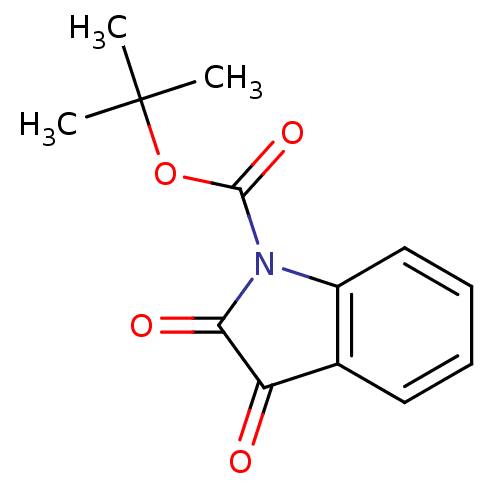 (2,3-Dioxo-2,3-dihydro-indole-1-carboxylic acid ter...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant of the compound against Alpha-chymotrypsin inhibitor was determined | Bioorg Med Chem Lett 5: 89-92 (1995) Article DOI: 10.1016/0960-894X(94)00464-Q BindingDB Entry DOI: 10.7270/Q2PZ58S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50287591 ((S)-3-Acetylamino-2-fluoro-4-phenyl-butyric acid m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.44E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.8 | n/a |
TBA Curated by ChEMBL | Assay Description Reversible inhibitory activity of the compound toward alpha-chymotrypsin (5 nM concentration) at a pH of 7.8 by progress curve method. | Bioorg Med Chem Lett 6: 1875-1880 (1996) Article DOI: 10.1016/0960-894X(96)00342-3 BindingDB Entry DOI: 10.7270/Q2BG2P0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50287591 ((S)-3-Acetylamino-2-fluoro-4-phenyl-butyric acid m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.44E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.8 | n/a |
TBA Curated by ChEMBL | Assay Description Reversible inhibitory activity of the compound toward alpha-chymotrypsin (5 nM concentration) at a pH of 7.8 by progress curve method. | Bioorg Med Chem Lett 6: 1875-1880 (1996) Article DOI: 10.1016/0960-894X(96)00342-3 BindingDB Entry DOI: 10.7270/Q2BG2P0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||