 Found 250 hits of kd data for polymerid = 5059
Found 250 hits of kd data for polymerid = 5059 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM81499
(CAS_155346 | CYANOPINDOLOL | CYANOPINDOLOL(+/-) | ...)Show InChI InChI=1S/C16H21N3O2/c1-16(2,3)18-9-12(20)10-21-15-6-4-5-14-13(15)7-11(8-17)19-14/h4-7,12,18-20H,9-10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes by liquid scintillation counting |
J Med Chem 59: 5780-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00358
BindingDB Entry DOI: 10.7270/Q2GH9NF7 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM81499

(CAS_155346 | CYANOPINDOLOL | CYANOPINDOLOL(+/-) | ...)Show InChI InChI=1S/C16H21N3O2/c1-16(2,3)18-9-12(20)10-21-15-6-4-5-14-13(15)7-11(8-17)19-14/h4-7,12,18-20H,9-10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes by liquid scintillation counting |
J Med Chem 59: 5780-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00358
BindingDB Entry DOI: 10.7270/Q2GH9NF7 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50232757

(CHEMBL4092412)Show InChI InChI=1S/C19H25NO2/c1-19(2,3)20-13-16(21)14-22-18-12-8-7-11-17(18)15-9-5-4-6-10-15/h4-12,16,20-21H,13-14H2,1-3H3/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.0182 | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg
Curated by ChEMBL
| Assay Description
Partial agonist activity at human beta2-AR expressed in CHOK1 cells assessed as inhibition of cimaterol-induced CRE-SPAP production after 5 hrs |
ACS Med Chem Lett 8: 481-485 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00363
BindingDB Entry DOI: 10.7270/Q2WM1GNK |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50232766

(Berlafenone)Show InChI InChI=1S/C19H25NO2/c1-19(2,3)20-13-16(21)14-22-18-12-8-7-11-17(18)15-9-5-4-6-10-15/h4-12,16,20-21H,13-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg
Curated by ChEMBL
| Assay Description
Displacement of [3H](-)CGP12177 from human beta2-AR expressed in CHOK1 cells after 2 hrs by TopCount microscintillation counting method |
ACS Med Chem Lett 8: 481-485 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00363
BindingDB Entry DOI: 10.7270/Q2WM1GNK |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50232757

(CHEMBL4092412)Show InChI InChI=1S/C19H25NO2/c1-19(2,3)20-13-16(21)14-22-18-12-8-7-11-17(18)15-9-5-4-6-10-15/h4-12,16,20-21H,13-14H2,1-3H3/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.0355 | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg
Curated by ChEMBL
| Assay Description
Displacement of [3H](-)CGP12177 from human beta2-AR expressed in CHOK1 cells after 2 hrs by TopCount microscintillation counting method |
ACS Med Chem Lett 8: 481-485 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00363
BindingDB Entry DOI: 10.7270/Q2WM1GNK |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50568740
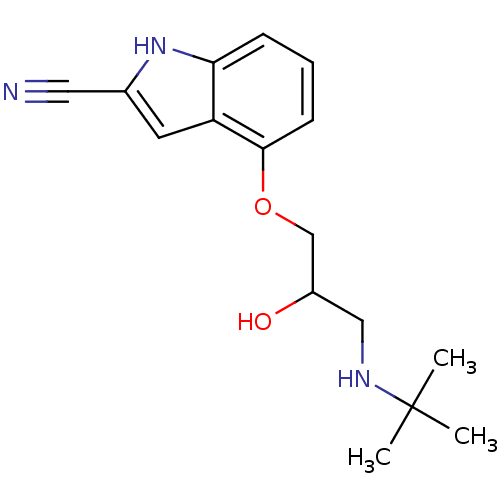
(CHEMBL4860528) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50601551
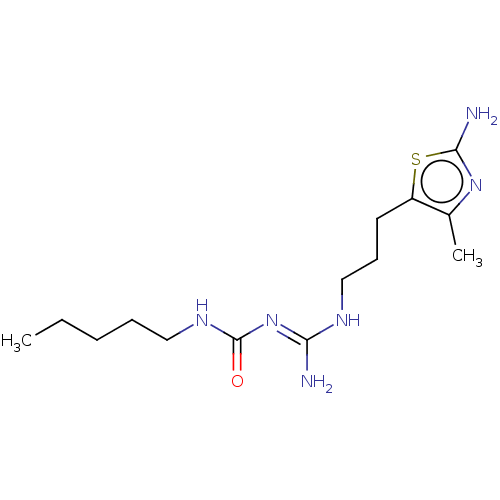
(CHEMBL5207281)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CCCCCNC(=O)\N=C(/N)NCCCc1nnc(N)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50601574
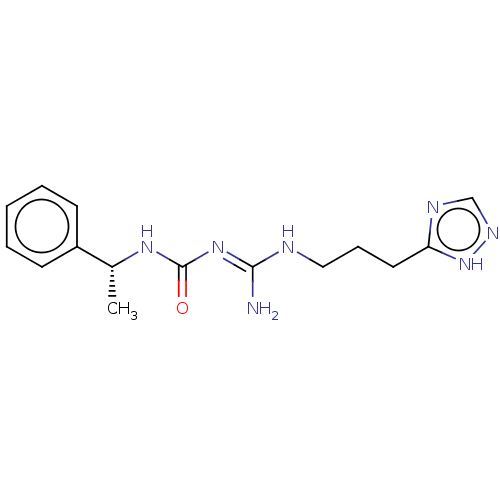
(CHEMBL5201074)Show SMILES Cl.Cl.C[C@@H](NC(=O)\N=C(/N)NCCCc1ncn[nH]1)c1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50601567
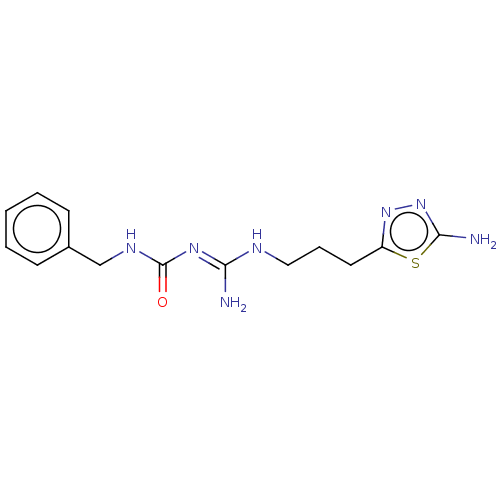
(CHEMBL5206565)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N\C(NCCCc1nnc(N)s1)=N/C(=O)NCc1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes by liquid scintillation counting |
J Med Chem 59: 5780-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00358
BindingDB Entry DOI: 10.7270/Q2GH9NF7 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes by liquid scintillation counting |
J Med Chem 59: 5780-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00358
BindingDB Entry DOI: 10.7270/Q2GH9NF7 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25765
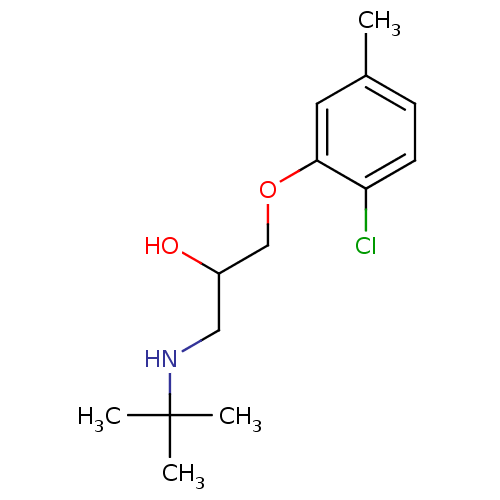
(Bupranolol | KL 255 | Ophtorenin | tert-butyl[3-(2...)Show InChI InChI=1S/C14H22ClNO2/c1-10-5-6-12(15)13(7-10)18-9-11(17)8-16-14(2,3)4/h5-7,11,16-17H,8-9H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
| Assay Description
The whole cell-binding studies were undertaken in CHO cell lines stably expressing each beta-adrenoceptor subtype. Nonspecific binding was determined... |
Br J Pharmacol 144: 317-22 (2005)
Article DOI: 10.1038/sj.bjp.0706048
BindingDB Entry DOI: 10.7270/Q28C9TKV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25759
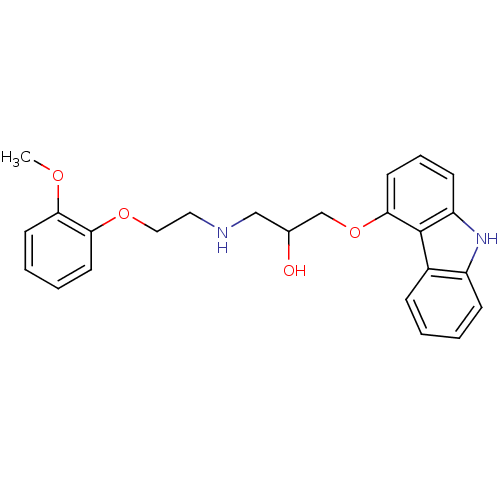
(CHEMBL723 | Carvedilol | Coreg | Dilatrend | Eucar...)Show SMILES COc1ccccc1OCCNCC(O)COc1cccc2[nH]c3ccccc3c12 Show InChI InChI=1S/C24H26N2O4/c1-28-21-10-4-5-11-22(21)29-14-13-25-15-17(27)16-30-23-12-6-9-20-24(23)18-7-2-3-8-19(18)26-20/h2-12,17,25-27H,13-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.195 | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from beta2 adrenergic receptor (unknown origin) stably expressed in HEK293 cell membranes measured after 90 mins by scintilla... |
J Med Chem 62: 7806-7839 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00595
BindingDB Entry DOI: 10.7270/Q2PG1W4K |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027879

(CGP-12177 | CHEBI:73288)Show InChI InChI=1S/C14H21N3O3/c1-14(2,3)15-7-9(18)8-20-11-6-4-5-10-12(11)17-13(19)16-10/h4-6,9,15,18H,7-8H2,1-3H3,(H2,16,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes by liquid scintillation counting |
J Med Chem 59: 5780-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00358
BindingDB Entry DOI: 10.7270/Q2GH9NF7 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027879

(CGP-12177 | CHEBI:73288)Show InChI InChI=1S/C14H21N3O3/c1-14(2,3)15-7-9(18)8-20-11-6-4-5-10-12(11)17-13(19)16-10/h4-6,9,15,18H,7-8H2,1-3H3,(H2,16,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes by liquid scintillation counting |
J Med Chem 59: 5780-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00358
BindingDB Entry DOI: 10.7270/Q2GH9NF7 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25767
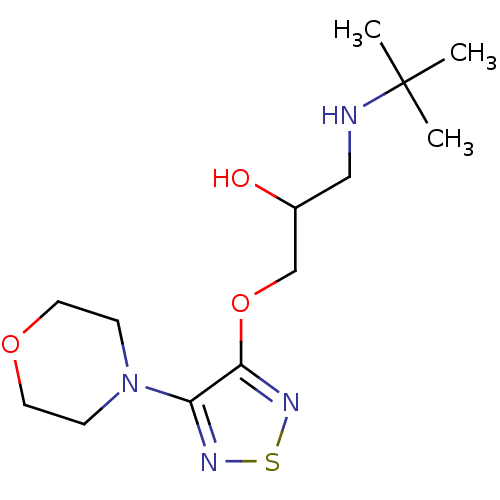
(Timolol | racemic-Timolol | tert-butyl(2-hydroxy-3...)Show InChI InChI=1S/C13H24N4O3S/c1-13(2,3)14-8-10(18)9-20-12-11(15-21-16-12)17-4-6-19-7-5-17/h10,14,18H,4-9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
| Assay Description
The whole cell-binding studies were undertaken in CHO cell lines stably expressing each beta-adrenoceptor subtype. Nonspecific binding was determined... |
Br J Pharmacol 144: 317-22 (2005)
Article DOI: 10.1038/sj.bjp.0706048
BindingDB Entry DOI: 10.7270/Q28C9TKV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human beta-2 adrenergic receptor expressed in salbutamol-stimulated CHO-K1 cells assessed as CRE-SPAP level by fluorescence co... |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM27960

((2R,3S)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy...)Show InChI InChI=1S/C17H27NO2/c1-11(2)18-13(4)16(19)10-20-17-9-8-12(3)14-6-5-7-15(14)17/h8-9,11,13,16,18-19H,5-7,10H2,1-4H3/t13-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.245 | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg
Curated by ChEMBL
| Assay Description
Displacement of [3H](-)CGP12177 from human beta2-AR expressed in CHOK1 cells after 2 hrs by TopCount microscintillation counting method |
ACS Med Chem Lett 8: 481-485 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00363
BindingDB Entry DOI: 10.7270/Q2WM1GNK |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421329
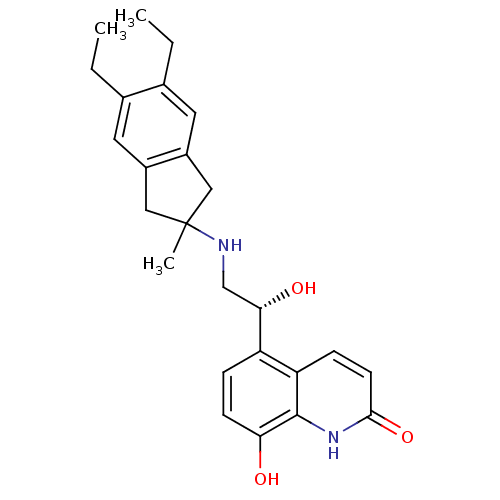
(CHEMBL2088201)Show SMILES CCc1cc2CC(C)(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C25H30N2O3/c1-4-15-10-17-12-25(3,13-18(17)11-16(15)5-2)26-14-22(29)19-6-8-21(28)24-20(19)7-9-23(30)27-24/h6-11,22,26,28-29H,4-5,12-14H2,1-3H3,(H,27,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Kinetic binding affinity to human beta2-adrenoceptor |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50423821
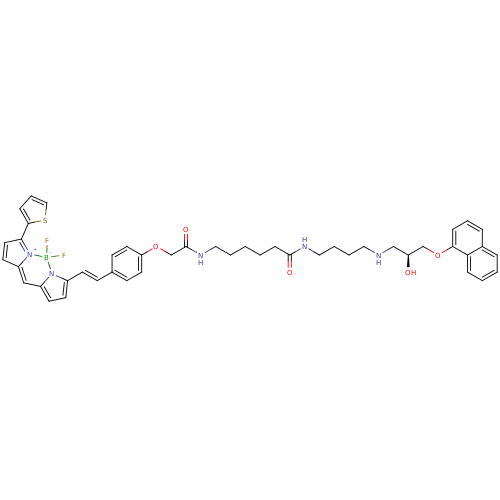
(CHEMBL1830486)Show SMILES O[C@@H](CNCCCCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\c2ccc3C=C4C=CC(c5cccs5)=[N+]4[B-](F)(F)n23)cc1)COc1cccc2ccccc12 |r,c:33,41,t:31| Show InChI InChI=1S/C46H50BF2N5O5S/c48-47(49)53-36(20-21-37(53)30-38-22-25-42(54(38)47)44-14-9-29-60-44)19-16-34-17-23-40(24-18-34)58-33-46(57)52-27-5-1-2-15-45(56)51-28-7-6-26-50-31-39(55)32-59-43-13-8-11-35-10-3-4-12-41(35)43/h3-4,8-14,16-25,29-30,39,50,55H,1-2,5-7,15,26-28,31-33H2,(H,51,56)(H,52,57)/b19-16+/t39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.275 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human beta-2 adrenergic receptor expressed in salbutamol-stimulated CHO-K1 cells assessed as CRE-SPAP level by fluorescence co... |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50423827
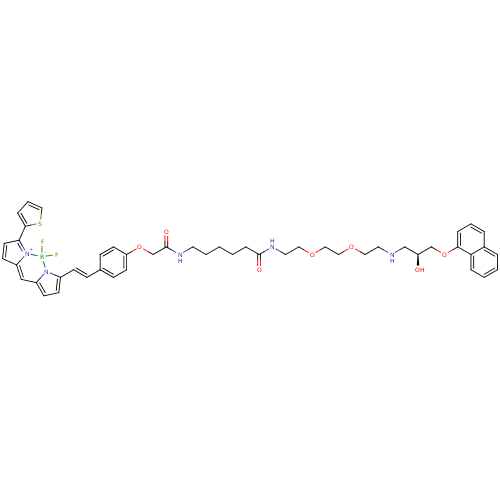
(CHEMBL1830491)Show SMILES O[C@@H](CNCCOCCOCCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\c2ccc3C=C4C=CC(c5cccs5)=[N+]4[B-](F)(F)n23)cc1)COc1cccc2ccccc12 |r,c:37,45,t:35| Show InChI InChI=1S/C48H54BF2N5O7S/c50-49(51)55-38(18-19-39(55)32-40-20-23-44(56(40)49)46-12-7-31-64-46)17-14-36-15-21-42(22-16-36)62-35-48(59)53-24-5-1-2-13-47(58)54-26-28-61-30-29-60-27-25-52-33-41(57)34-63-45-11-6-9-37-8-3-4-10-43(37)45/h3-4,6-12,14-23,31-32,41,52,57H,1-2,5,13,24-30,33-35H2,(H,53,59)(H,54,58)/b17-14+/t41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.295 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human beta-2 adrenergic receptor expressed in salbutamol-stimulated CHO-K1 cells assessed as CRE-SPAP level by fluorescence co... |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25764
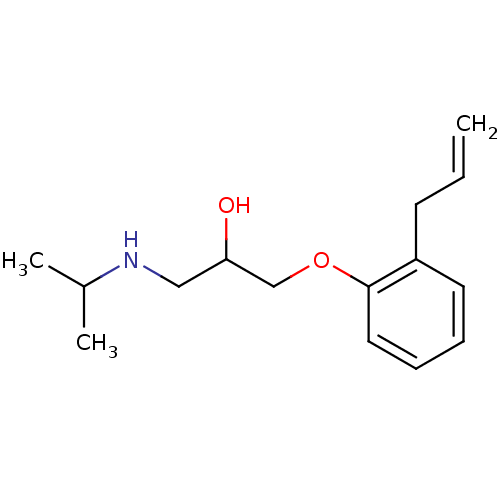
(ALPRENOLOL,(+) | ALPRENOLOL,(-) | Alfeprol | Alphe...)Show InChI InChI=1S/C15H23NO2/c1-4-7-13-8-5-6-9-15(13)18-11-14(17)10-16-12(2)3/h4-6,8-9,12,14,16-17H,1,7,10-11H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.295 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human beta-2 adrenergic receptor expressed in salbutamol-stimulated CHO-K1 cells assessed as CRE-SPAP level by fluorescence co... |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771
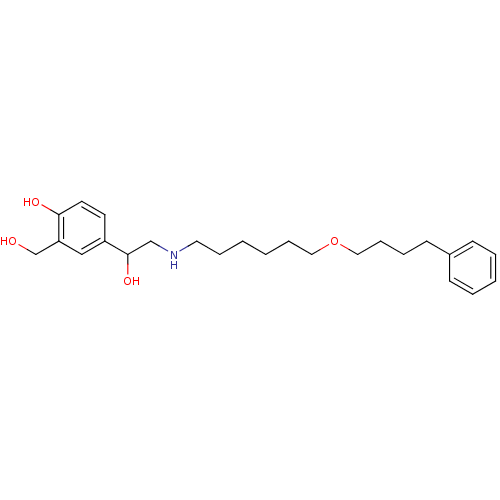
(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes by liquid scintillation counting |
J Med Chem 59: 5780-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00358
BindingDB Entry DOI: 10.7270/Q2GH9NF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50532364
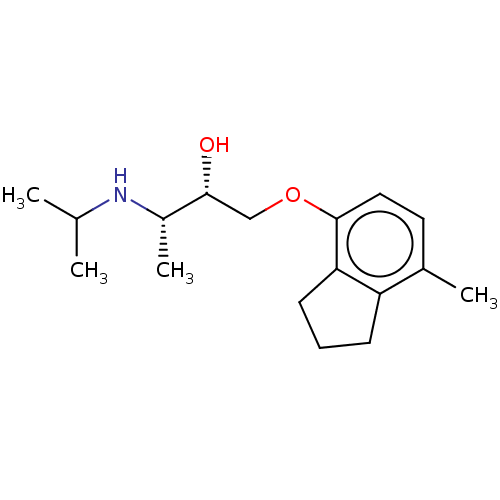
(CHEMBL1233766)Show SMILES CC(C)N[C@@H](C)[C@H](O)COc1ccc(C)c2CCCc12 |r| Show InChI InChI=1S/C17H27NO2/c1-11(2)18-13(4)16(19)10-20-17-9-8-12(3)14-6-5-7-15(14)17/h8-9,11,13,16,18-19H,5-7,10H2,1-4H3/t13-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes by liquid scintillation counting |
J Med Chem 59: 5780-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00358
BindingDB Entry DOI: 10.7270/Q2GH9NF7 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771

(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes by liquid scintillation counting |
J Med Chem 59: 5780-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00358
BindingDB Entry DOI: 10.7270/Q2GH9NF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50532364

(CHEMBL1233766)Show SMILES CC(C)N[C@@H](C)[C@H](O)COc1ccc(C)c2CCCc12 |r| Show InChI InChI=1S/C17H27NO2/c1-11(2)18-13(4)16(19)10-20-17-9-8-12(3)14-6-5-7-15(14)17/h8-9,11,13,16,18-19H,5-7,10H2,1-4H3/t13-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes by liquid scintillation counting |
J Med Chem 59: 5780-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00358
BindingDB Entry DOI: 10.7270/Q2GH9NF7 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25759

(CHEMBL723 | Carvedilol | Coreg | Dilatrend | Eucar...)Show SMILES COc1ccccc1OCCNCC(O)COc1cccc2[nH]c3ccccc3c12 Show InChI InChI=1S/C24H26N2O4/c1-28-21-10-4-5-11-22(21)29-14-13-25-15-17(27)16-30-23-12-6-9-20-24(23)18-7-2-3-8-19(18)26-20/h2-12,17,25-27H,13-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
| Assay Description
The whole cell-binding studies were undertaken in CHO cell lines stably expressing each beta-adrenoceptor subtype. Nonspecific binding was determined... |
Br J Pharmacol 144: 317-22 (2005)
Article DOI: 10.1038/sj.bjp.0706048
BindingDB Entry DOI: 10.7270/Q28C9TKV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25747
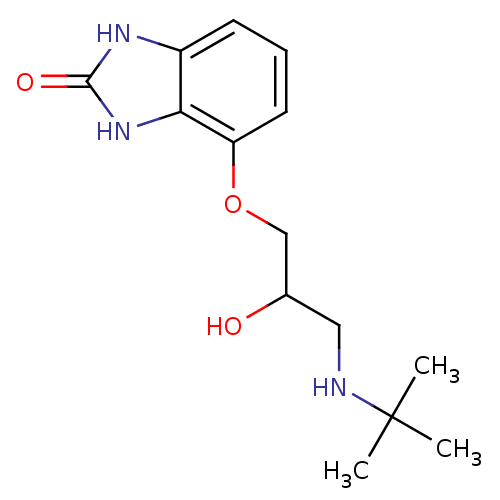
(4-[3-(tert-butylamino)-2-hydroxypropoxy]-2,3-dihyd...)Show InChI InChI=1S/C14H21N3O3/c1-14(2,3)15-7-9(18)8-20-11-6-4-5-10-12(11)17-13(19)16-10/h4-6,9,15,18H,7-8H2,1-3H3,(H2,16,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
| Assay Description
The whole cell-binding studies were undertaken in CHO cell lines stably expressing each beta-adrenoceptor subtype. Nonspecific binding was determined... |
Br J Pharmacol 144: 317-22 (2005)
Article DOI: 10.1038/sj.bjp.0706048
BindingDB Entry DOI: 10.7270/Q28C9TKV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50423824
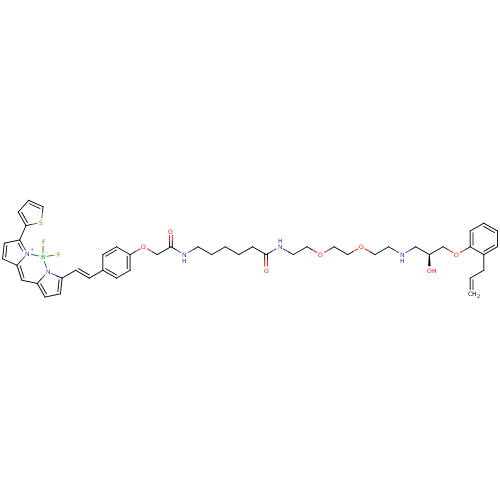
(CHEMBL1830618)Show SMILES O[C@@H](CNCCOCCOCCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\c2ccc3C=C4C=CC(c5cccs5)=[N+]4[B-](F)(F)n23)cc1)COc1ccccc1CC=C |r,c:37,45,t:35| Show InChI InChI=1S/C47H56BF2N5O7S/c1-2-9-37-10-5-6-11-44(37)62-34-41(56)33-51-25-27-59-29-30-60-28-26-53-46(57)13-4-3-7-24-52-47(58)35-61-42-21-15-36(16-22-42)14-17-38-18-19-39-32-40-20-23-43(45-12-8-31-63-45)55(40)48(49,50)54(38)39/h2,5-6,8,10-12,14-23,31-32,41,51,56H,1,3-4,7,9,13,24-30,33-35H2,(H,52,58)(H,53,57)/b17-14+/t41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.427 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human beta-2 adrenergic receptor expressed in salbutamol-stimulated CHO-K1 cells assessed as CRE-SPAP level by fluorescence co... |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50423829
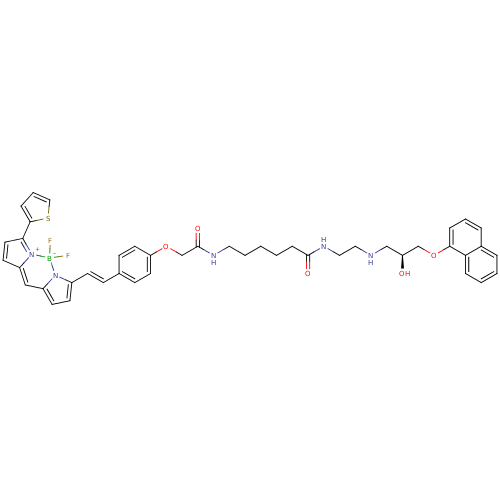
(CHEMBL1830485)Show SMILES O[C@@H](CNCCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\c2ccc3C=C4C=CC(c5cccs5)=[N+]4[B-](F)(F)n23)cc1)COc1cccc2ccccc12 |r,c:31,39,t:29| Show InChI InChI=1S/C44H46BF2N5O5S/c46-45(47)51-34(18-19-35(51)28-36-20-23-40(52(36)45)42-12-7-27-58-42)17-14-32-15-21-38(22-16-32)56-31-44(55)49-24-5-1-2-13-43(54)50-26-25-48-29-37(53)30-57-41-11-6-9-33-8-3-4-10-39(33)41/h3-4,6-12,14-23,27-28,37,48,53H,1-2,5,13,24-26,29-31H2,(H,49,55)(H,50,54)/b17-14+/t37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.427 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human beta-2 adrenergic receptor expressed in salbutamol-stimulated CHO-K1 cells assessed as CRE-SPAP level by fluorescence co... |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50423826
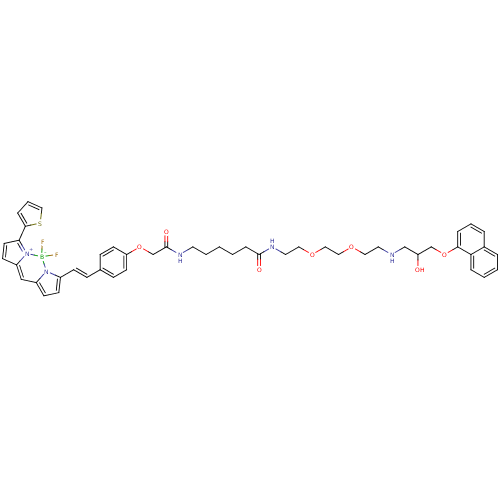
(CHEMBL1830492)Show SMILES OC(CNCCOCCOCCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\c2ccc3C=C4C=CC(c5cccs5)=[N+]4[B-](F)(F)n23)cc1)COc1cccc2ccccc12 |c:37,45,t:35| Show InChI InChI=1S/C48H54BF2N5O7S/c50-49(51)55-38(18-19-39(55)32-40-20-23-44(56(40)49)46-12-7-31-64-46)17-14-36-15-21-42(22-16-36)62-35-48(59)53-24-5-1-2-13-47(58)54-26-28-61-30-29-60-27-25-52-33-41(57)34-63-45-11-6-9-37-8-3-4-10-43(37)45/h3-4,6-12,14-23,31-32,41,52,57H,1-2,5,13,24-30,33-35H2,(H,53,59)(H,54,58)/b17-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.437 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human beta-2 adrenergic receptor expressed in salbutamol-stimulated CHO-K1 cells assessed as CRE-SPAP level by fluorescence co... |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50493311
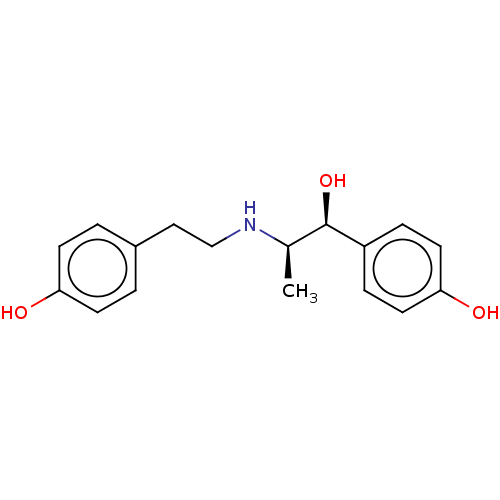
(rel-RITODRINE)Show SMILES C[C@@H](NCCc1ccc(O)cc1)[C@@H](O)c1ccc(O)cc1 Show InChI InChI=1S/C17H21NO3/c1-12(17(21)14-4-8-16(20)9-5-14)18-11-10-13-2-6-15(19)7-3-13/h2-9,12,17-21H,10-11H2,1H3/t12-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Binding affinity to beta-2 adrenergic receptor (unknown origin) at 1 to 10000 nM |
Bioorg Med Chem Lett 23: 5376-81 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.052
BindingDB Entry DOI: 10.7270/Q2F76GHV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25764

(ALPRENOLOL,(+) | ALPRENOLOL,(-) | Alfeprol | Alphe...)Show InChI InChI=1S/C15H23NO2/c1-4-7-13-8-5-6-9-15(13)18-11-14(17)10-16-12(2)3/h4-6,8-9,12,14,16-17H,1,7,10-11H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGP 12177 from human beta-2 adrenergic receptor expressed in CHOK1 cells |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50019443
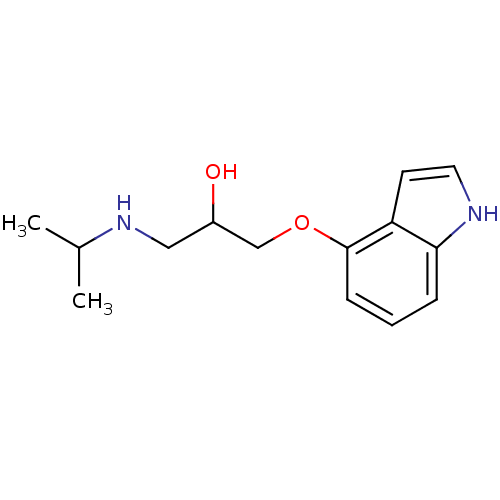
(1-(1H-Indol-4-yloxy)-3-isopropylamino-propan-2-ol ...)Show InChI InChI=1S/C14H20N2O2/c1-10(2)16-8-11(17)9-18-14-5-3-4-13-12(14)6-7-15-13/h3-7,10-11,15-17H,8-9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.537 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGP 12177 from human beta-2 adrenergic receptor expressed in CHOK1 cells |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25768
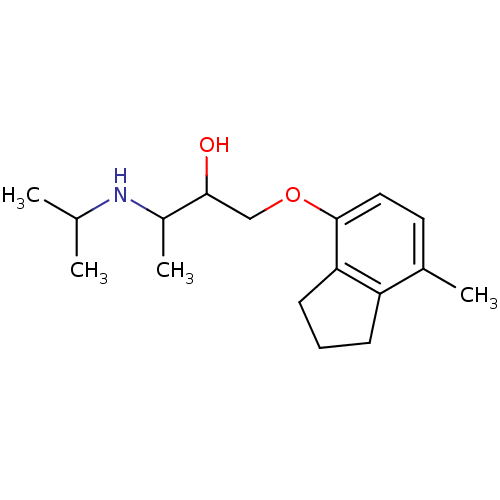
(1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(pro...)Show InChI InChI=1S/C17H27NO2/c1-11(2)18-13(4)16(19)10-20-17-9-8-12(3)14-6-5-7-15(14)17/h8-9,11,13,16,18-19H,5-7,10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
| Assay Description
The whole cell-binding studies were undertaken in CHO cell lines stably expressing each beta-adrenoceptor subtype. Nonspecific binding was determined... |
Br J Pharmacol 144: 317-22 (2005)
Article DOI: 10.1038/sj.bjp.0706048
BindingDB Entry DOI: 10.7270/Q28C9TKV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGP 12177 from human beta-2 adrenergic receptor expressed in CHOK1 cells |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50423818
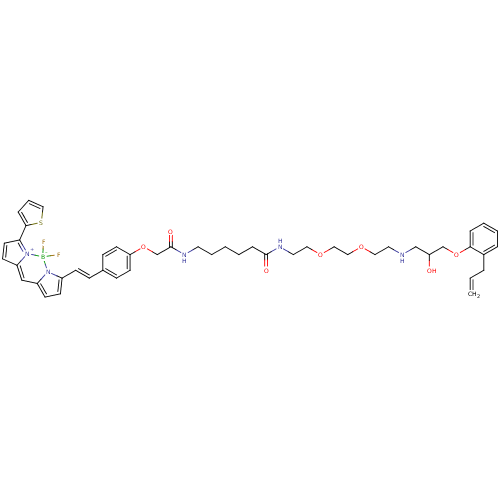
(CHEMBL1830619)Show SMILES OC(CNCCOCCOCCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\c2ccc3C=C4C=CC(c5cccs5)=[N+]4[B-](F)(F)n23)cc1)COc1ccccc1CC=C |c:37,45,t:35| Show InChI InChI=1S/C47H56BF2N5O7S/c1-2-9-37-10-5-6-11-44(37)62-34-41(56)33-51-25-27-59-29-30-60-28-26-53-46(57)13-4-3-7-24-52-47(58)35-61-42-21-15-36(16-22-42)14-17-38-18-19-39-32-40-20-23-43(45-12-8-31-63-45)55(40)48(49,50)54(38)39/h2,5-6,8,10-12,14-23,31-32,41,51,56H,1,3-4,7,9,13,24-30,33-35H2,(H,52,58)(H,53,57)/b17-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.617 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human beta-2 adrenergic receptor expressed in salbutamol-stimulated CHO-K1 cells assessed as CRE-SPAP level by fluorescence co... |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50423827
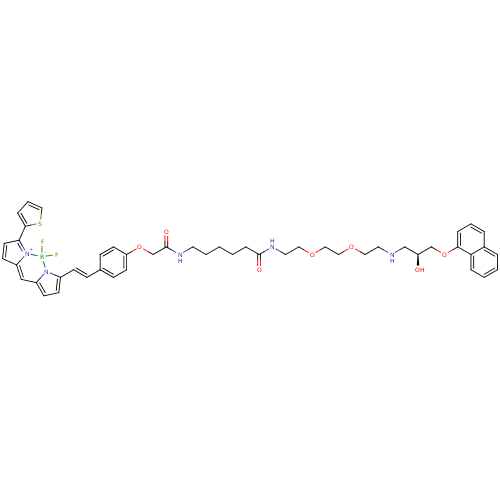
(CHEMBL1830491)Show SMILES O[C@@H](CNCCOCCOCCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\c2ccc3C=C4C=CC(c5cccs5)=[N+]4[B-](F)(F)n23)cc1)COc1cccc2ccccc12 |r,c:37,45,t:35| Show InChI InChI=1S/C48H54BF2N5O7S/c50-49(51)55-38(18-19-39(55)32-40-20-23-44(56(40)49)46-12-7-31-64-46)17-14-36-15-21-42(22-16-36)62-35-48(59)53-24-5-1-2-13-47(58)54-26-28-61-30-29-60-27-25-52-33-41(57)34-63-45-11-6-9-37-8-3-4-10-43(37)45/h3-4,6-12,14-23,31-32,41,52,57H,1-2,5,13,24-30,33-35H2,(H,53,59)(H,54,58)/b17-14+/t41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.617 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGP 12177 from human beta-2 adrenergic receptor expressed in CHOK1 cells |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50019443

(1-(1H-Indol-4-yloxy)-3-isopropylamino-propan-2-ol ...)Show InChI InChI=1S/C14H20N2O2/c1-10(2)16-8-11(17)9-18-14-5-3-4-13-12(14)6-7-15-13/h3-7,10-11,15-17H,8-9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.661 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human beta-2 adrenergic receptor expressed in salbutamol-stimulated CHO-K1 cells assessed as CRE-SPAP level by fluorescence co... |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25768

(1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(pro...)Show InChI InChI=1S/C17H27NO2/c1-11(2)18-13(4)16(19)10-20-17-9-8-12(3)14-6-5-7-15(14)17/h8-9,11,13,16,18-19H,5-7,10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.676 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGP12177 from human beta2 adrenoceptor expressed in CHOK1 cells after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3852-65 (2013)
Article DOI: 10.1021/jm400348g
BindingDB Entry DOI: 10.7270/Q2445NV6 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM81499

(CAS_155346 | CYANOPINDOLOL | CYANOPINDOLOL(+/-) | ...)Show InChI InChI=1S/C16H21N3O2/c1-16(2,3)18-9-12(20)10-21-15-6-4-5-14-13(15)7-11(8-17)19-14/h4-7,12,18-20H,9-10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes assessed as intrinsic Kd by liquid scintilla... |
J Med Chem 59: 5780-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00358
BindingDB Entry DOI: 10.7270/Q2GH9NF7 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM81499

(CAS_155346 | CYANOPINDOLOL | CYANOPINDOLOL(+/-) | ...)Show InChI InChI=1S/C16H21N3O2/c1-16(2,3)18-9-12(20)10-21-15-6-4-5-14-13(15)7-11(8-17)19-14/h4-7,12,18-20H,9-10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes assessed as intrinsic Kd by liquid scintilla... |
J Med Chem 59: 5780-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00358
BindingDB Entry DOI: 10.7270/Q2GH9NF7 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50423821

(CHEMBL1830486)Show SMILES O[C@@H](CNCCCCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\c2ccc3C=C4C=CC(c5cccs5)=[N+]4[B-](F)(F)n23)cc1)COc1cccc2ccccc12 |r,c:33,41,t:31| Show InChI InChI=1S/C46H50BF2N5O5S/c48-47(49)53-36(20-21-37(53)30-38-22-25-42(54(38)47)44-14-9-29-60-44)19-16-34-17-23-40(24-18-34)58-33-46(57)52-27-5-1-2-15-45(56)51-28-7-6-26-50-31-39(55)32-59-43-13-8-11-35-10-3-4-12-41(35)43/h3-4,8-14,16-25,29-30,39,50,55H,1-2,5-7,15,26-28,31-33H2,(H,51,56)(H,52,57)/b19-16+/t39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.724 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGP 12177 from human beta-2 adrenergic receptor expressed in CHOK1 cells |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50423821

(CHEMBL1830486)Show SMILES O[C@@H](CNCCCCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\c2ccc3C=C4C=CC(c5cccs5)=[N+]4[B-](F)(F)n23)cc1)COc1cccc2ccccc12 |r,c:33,41,t:31| Show InChI InChI=1S/C46H50BF2N5O5S/c48-47(49)53-36(20-21-37(53)30-38-22-25-42(54(38)47)44-14-9-29-60-44)19-16-34-17-23-40(24-18-34)58-33-46(57)52-27-5-1-2-15-45(56)51-28-7-6-26-50-31-39(55)32-59-43-13-8-11-35-10-3-4-12-41(35)43/h3-4,8-14,16-25,29-30,39,50,55H,1-2,5-7,15,26-28,31-33H2,(H,51,56)(H,52,57)/b19-16+/t39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.741 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human beta-2 adrenergic receptor expressed in salbutamol-stimulated CHO-K1 cells assessed as CRE-SPAP level by fluorescence co... |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
| Assay Description
The whole cell-binding studies were undertaken in CHO cell lines stably expressing each beta-adrenoceptor subtype. Nonspecific binding was determined... |
Br J Pharmacol 144: 317-22 (2005)
Article DOI: 10.1038/sj.bjp.0706048
BindingDB Entry DOI: 10.7270/Q28C9TKV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50518977
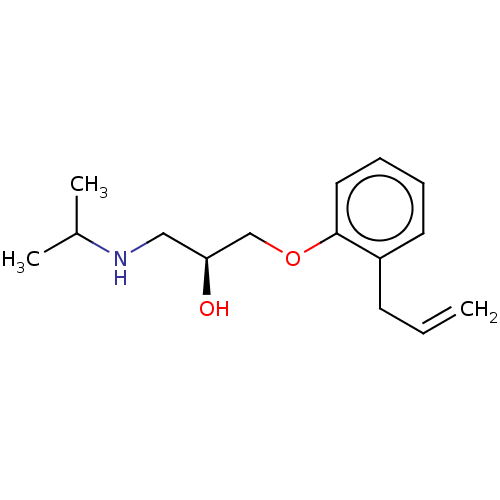
(CHEMBL1160734)Show InChI InChI=1S/C15H23NO2/c1-4-7-13-8-5-6-9-15(13)18-11-14(17)10-16-12(2)3/h4-6,8-9,12,14,16-17H,1,7,10-11H2,2-3H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.891 | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from beta2 adrenergic receptor (unknown origin) stably expressed in HEK293 cell membranes measured after 90 mins by scintilla... |
J Med Chem 62: 7806-7839 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00595
BindingDB Entry DOI: 10.7270/Q2PG1W4K |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25764

(ALPRENOLOL,(+) | ALPRENOLOL,(-) | Alfeprol | Alphe...)Show InChI InChI=1S/C15H23NO2/c1-4-7-13-8-5-6-9-15(13)18-11-14(17)10-16-12(2)3/h4-6,8-9,12,14,16-17H,1,7,10-11H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
| Assay Description
The whole cell-binding studies were undertaken in CHO cell lines stably expressing each beta-adrenoceptor subtype. Nonspecific binding was determined... |
Br J Pharmacol 144: 317-22 (2005)
Article DOI: 10.1038/sj.bjp.0706048
BindingDB Entry DOI: 10.7270/Q28C9TKV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50423824
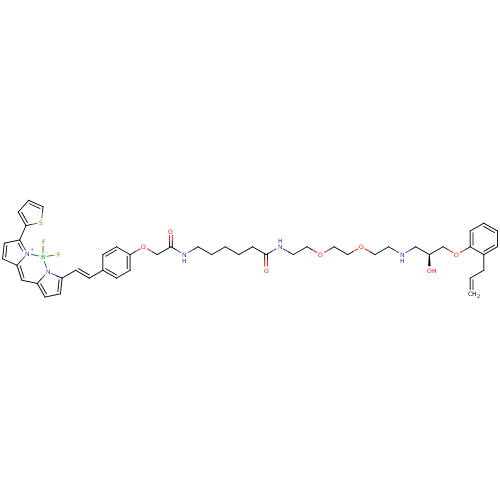
(CHEMBL1830618)Show SMILES O[C@@H](CNCCOCCOCCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\c2ccc3C=C4C=CC(c5cccs5)=[N+]4[B-](F)(F)n23)cc1)COc1ccccc1CC=C |r,c:37,45,t:35| Show InChI InChI=1S/C47H56BF2N5O7S/c1-2-9-37-10-5-6-11-44(37)62-34-41(56)33-51-25-27-59-29-30-60-28-26-53-46(57)13-4-3-7-24-52-47(58)35-61-42-21-15-36(16-22-42)14-17-38-18-19-39-32-40-20-23-43(45-12-8-31-63-45)55(40)48(49,50)54(38)39/h2,5-6,8,10-12,14-23,31-32,41,51,56H,1,3-4,7,9,13,24-30,33-35H2,(H,52,58)(H,53,57)/b17-14+/t41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.933 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGP 12177 from human beta-2 adrenergic receptor expressed in CHOK1 cells |
J Med Chem 54: 6874-87 (2011)
Article DOI: 10.1021/jm2008562
BindingDB Entry DOI: 10.7270/Q29S1SBQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595336
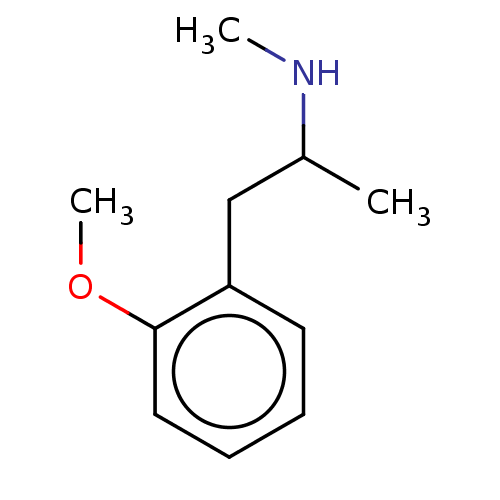
(METHOXYPHENAMINE | Methoxyphenamine) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114212
BindingDB Entry DOI: 10.7270/Q2N01BNJ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25769
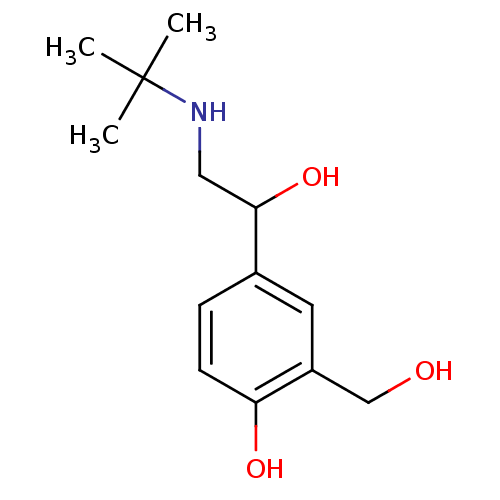
(4-[2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxym...)Show InChI InChI=1S/C13H21NO3/c1-13(2,3)14-7-12(17)9-4-5-11(16)10(6-9)8-15/h4-6,12,14-17H,7-8H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116864
BindingDB Entry DOI: 10.7270/Q241722K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data