 Found 906 hits of ic50 for UniProtKB: O76083
Found 906 hits of ic50 for UniProtKB: O76083 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50034641
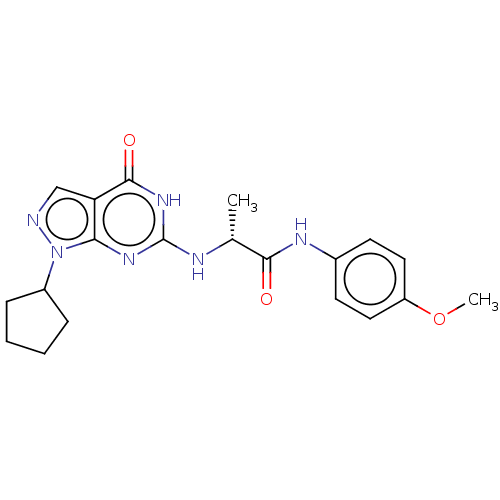
(CHEMBL3360415)Show SMILES COc1ccc(NC(=O)[C@@H](C)Nc2nc3n(ncc3c(=O)[nH]2)C2CCCC2)cc1 |r| Show InChI InChI=1S/C20H24N6O3/c1-12(18(27)23-13-7-9-15(29-2)10-8-13)22-20-24-17-16(19(28)25-20)11-21-26(17)14-5-3-4-6-14/h7-12,14H,3-6H2,1-2H3,(H,23,27)(H2,22,24,25,28)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A2 catalytic domain (unknown origin) using [3H]-cGMP/[3H]-cAMP as substrate after 15 mins by liquid scintillation counting analysis |
J Med Chem 57: 10304-13 (2014)
Article DOI: 10.1021/jm500836h
BindingDB Entry DOI: 10.7270/Q28P624W |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50034641

(CHEMBL3360415)Show SMILES COc1ccc(NC(=O)[C@@H](C)Nc2nc3n(ncc3c(=O)[nH]2)C2CCCC2)cc1 |r| Show InChI InChI=1S/C20H24N6O3/c1-12(18(27)23-13-7-9-15(29-2)10-8-13)22-20-24-17-16(19(28)25-20)11-21-26(17)14-5-3-4-6-14/h7-12,14H,3-6H2,1-2H3,(H,23,27)(H2,22,24,25,28)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127254
BindingDB Entry DOI: 10.7270/Q2S1862H |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50034641

(CHEMBL3360415)Show SMILES COc1ccc(NC(=O)[C@@H](C)Nc2nc3n(ncc3c(=O)[nH]2)C2CCCC2)cc1 |r| Show InChI InChI=1S/C20H24N6O3/c1-12(18(27)23-13-7-9-15(29-2)10-8-13)22-20-24-17-16(19(28)25-20)11-21-26(17)14-5-3-4-6-14/h7-12,14H,3-6H2,1-2H3,(H,23,27)(H2,22,24,25,28)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114170
BindingDB Entry DOI: 10.7270/Q2RX9H3Q |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM317140

(US9617269, Compound WYQ-90-D)Show SMILES COc1ccc(NC(=O)C(C)NC2=NC3C(C=NN3C3CCCC3)C(=O)N2)cc1 |c:16,t:12| Show InChI InChI=1S/C20H26N6O3/c1-12(18(27)23-13-7-9-15(29-2)10-8-13)22-20-24-17-16(19(28)25-20)11-21-26(17)14-5-3-4-6-14/h7-12,14,16-17H,3-6H2,1-2H3,(H,23,27)(H2,22,24,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University; University of North Carolina at Chapel Hill
US Patent
| Assay Description
Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera... |
US Patent US9617269 (2017)
BindingDB Entry DOI: 10.7270/Q2RX9F4B |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300099
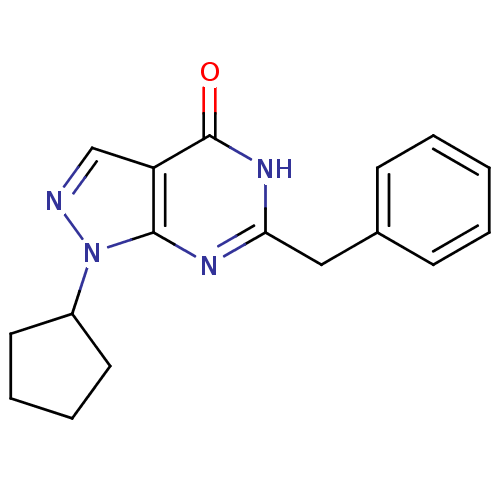
(6-benzyl-1-cyclopentyl-1,5-dihydro-4H-pyrazolo[3,4...)Show InChI InChI=1S/C17H18N4O/c22-17-14-11-18-21(13-8-4-5-9-13)16(14)19-15(20-17)10-12-6-2-1-3-7-12/h1-3,6-7,11,13H,4-5,8-10H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397844
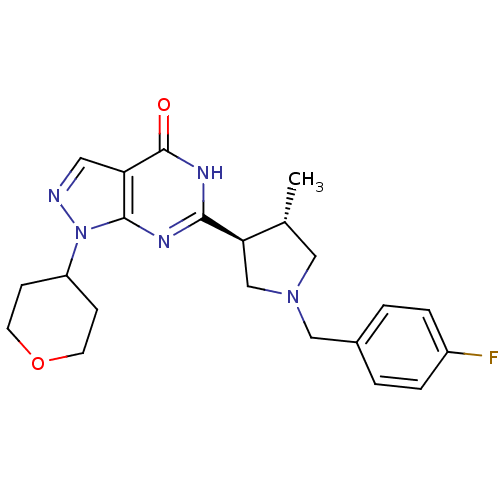
(CHEMBL2179099)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C22H26FN5O2/c1-14-11-27(12-15-2-4-16(23)5-3-15)13-19(14)20-25-21-18(22(29)26-20)10-24-28(21)17-6-8-30-9-7-17/h2-5,10,14,17,19H,6-9,11-13H2,1H3,(H,25,26,29)/t14-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397850
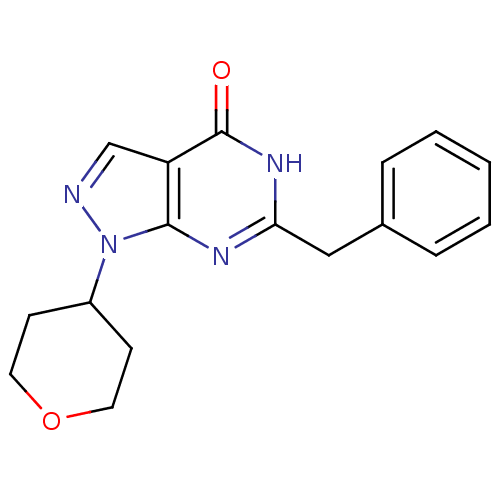
(CHEMBL2179093)Show InChI InChI=1S/C17H18N4O2/c22-17-14-11-18-21(13-6-8-23-9-7-13)16(14)19-15(20-17)10-12-4-2-1-3-5-12/h1-5,11,13H,6-10H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM317136

(US9617269, Compound WYQ-87-D | US9617269, Compound...)Show SMILES COc1ccc(NC(=O)C(C)Nc2nc3n(ncc3c(=O)[nH]2)C(C)C)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University; University of North Carolina at Chapel Hill
US Patent
| Assay Description
Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera... |
US Patent US9617269 (2017)
BindingDB Entry DOI: 10.7270/Q2RX9F4B |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50034648
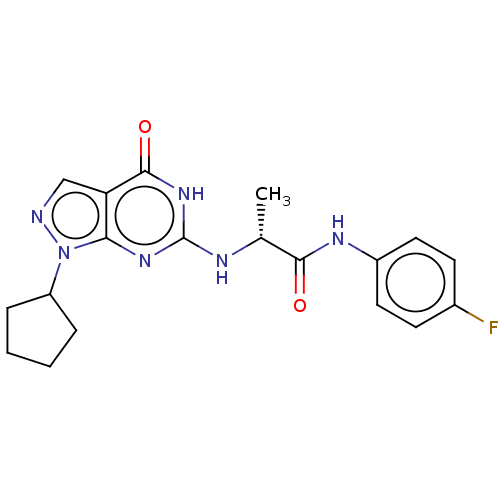
(CHEMBL3360421)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)[nH]1)C1CCCC1)C(=O)Nc1ccc(F)cc1 |r| Show InChI InChI=1S/C19H21FN6O2/c1-11(17(27)23-13-8-6-12(20)7-9-13)22-19-24-16-15(18(28)25-19)10-21-26(16)14-4-2-3-5-14/h6-11,14H,2-5H2,1H3,(H,23,27)(H2,22,24,25,28)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A2 catalytic domain (unknown origin) using [3H]-cGMP/[3H]-cAMP as substrate after 15 mins by liquid scintillation counting analysis |
J Med Chem 57: 10304-13 (2014)
Article DOI: 10.1021/jm500836h
BindingDB Entry DOI: 10.7270/Q28P624W |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50034645
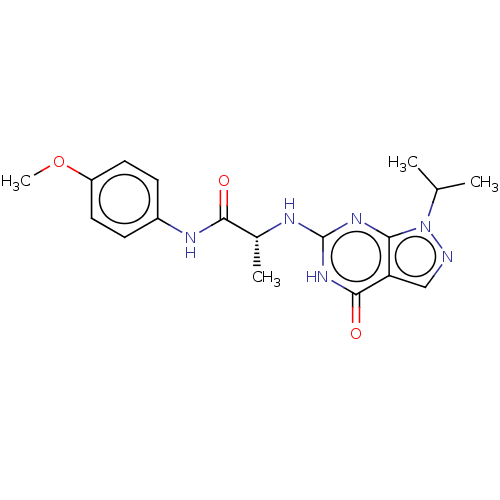
(CHEMBL3360419)Show SMILES COc1ccc(NC(=O)[C@@H](C)Nc2nc3n(ncc3c(=O)[nH]2)C(C)C)cc1 |r| Show InChI InChI=1S/C18H22N6O3/c1-10(2)24-15-14(9-19-24)17(26)23-18(22-15)20-11(3)16(25)21-12-5-7-13(27-4)8-6-12/h5-11H,1-4H3,(H,21,25)(H2,20,22,23,26)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A2 catalytic domain (unknown origin) using [3H]-cGMP/[3H]-cAMP as substrate after 15 mins by liquid scintillation counting analysis |
J Med Chem 57: 10304-13 (2014)
Article DOI: 10.1021/jm500836h
BindingDB Entry DOI: 10.7270/Q28P624W |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397845
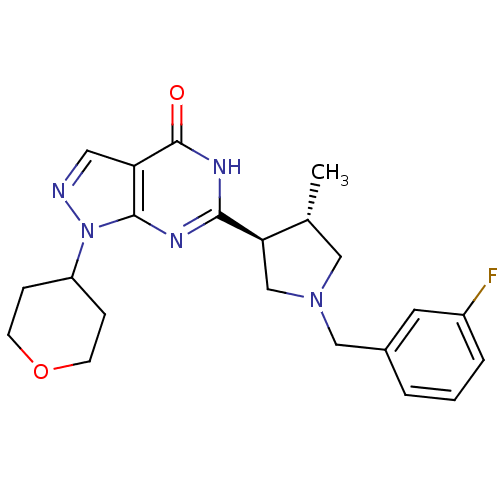
(CHEMBL2177125)Show SMILES C[C@@H]1CN(Cc2cccc(F)c2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C22H26FN5O2/c1-14-11-27(12-15-3-2-4-16(23)9-15)13-19(14)20-25-21-18(22(29)26-20)10-24-28(21)17-5-7-30-8-6-17/h2-4,9-10,14,17,19H,5-8,11-13H2,1H3,(H,25,26,29)/t14-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM131008
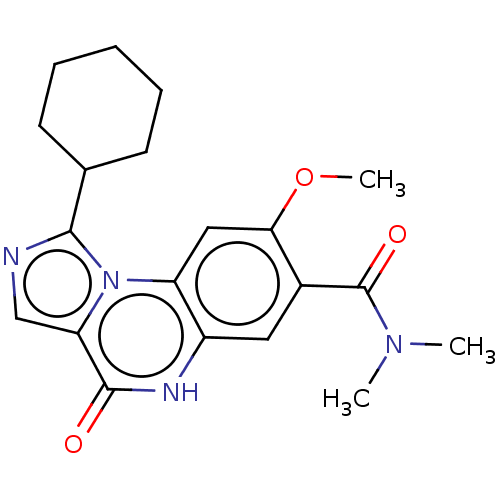
(US8829000, 70)Show SMILES COc1cc2c(cc1C(=O)N(C)C)[nH]c(=O)c1cnc(C3CCCCC3)n21 Show InChI InChI=1S/C20H24N4O3/c1-23(2)20(26)13-9-14-15(10-17(13)27-3)24-16(19(25)22-14)11-21-18(24)12-7-5-4-6-8-12/h9-12H,4-8H2,1-3H3,(H,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | 30 |
ASKA Pharmaceutical Co., Ltd.
US Patent
| Assay Description
To 150 uL of buffer B (70 mmol/L Tris-HCl, pH7.5, 16.7 mmol/L MgCl2, 33.3 nmol/L [3H]-cGMP) solution containing [3H]-cGMP (specific activity=244.2 GB... |
US Patent US8829000 (2014)
BindingDB Entry DOI: 10.7270/Q2K072ZX |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397848
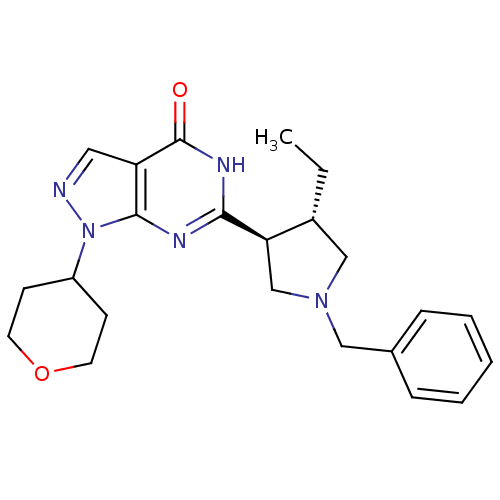
(CHEMBL2179096)Show SMILES CC[C@@H]1CN(Cc2ccccc2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C23H29N5O2/c1-2-17-14-27(13-16-6-4-3-5-7-16)15-20(17)21-25-22-19(23(29)26-21)12-24-28(22)18-8-10-30-11-9-18/h3-7,12,17-18,20H,2,8-11,13-15H2,1H3,(H,25,26,29)/t17-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300113
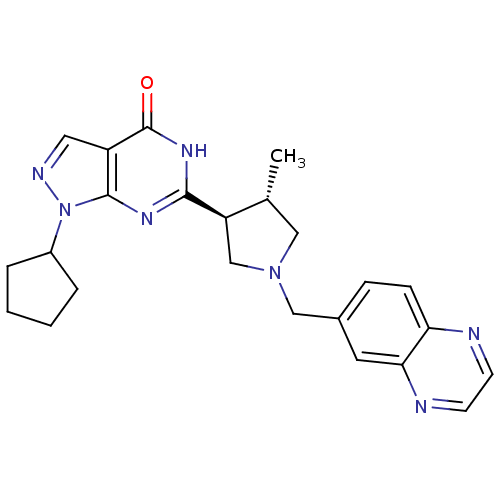
(1-cyclopentyl-6-[(3S,4S)-4-methyl-1-(quinoxalin-6-...)Show SMILES C[C@@H]1CN(Cc2ccc3nccnc3c2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C24H27N7O/c1-15-12-30(13-16-6-7-20-21(10-16)26-9-8-25-20)14-19(15)22-28-23-18(24(32)29-22)11-27-31(23)17-4-2-3-5-17/h6-11,15,17,19H,2-5,12-14H2,1H3,(H,28,29,32)/t15-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50543967

(CHEMBL4638644)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)[nH]1)C1CCCC1)C(=O)Nc1ccc2[nH]ncc2c1 |r| Show InChI InChI=1S/C20H22N8O2/c1-11(18(29)24-13-6-7-16-12(8-13)9-21-27-16)23-20-25-17-15(19(30)26-20)10-22-28(17)14-4-2-3-5-14/h6-11,14H,2-5H2,1H3,(H,21,27)(H,24,29)(H2,23,25,26,30)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127254
BindingDB Entry DOI: 10.7270/Q2S1862H |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300113

(1-cyclopentyl-6-[(3S,4S)-4-methyl-1-(quinoxalin-6-...)Show SMILES C[C@@H]1CN(Cc2ccc3nccnc3c2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C24H27N7O/c1-15-12-30(13-16-6-7-20-21(10-16)26-9-8-25-20)14-19(15)22-28-23-18(24(32)29-22)11-27-31(23)17-4-2-3-5-17/h6-11,15,17,19H,2-5,12-14H2,1H3,(H,28,29,32)/t15-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300112
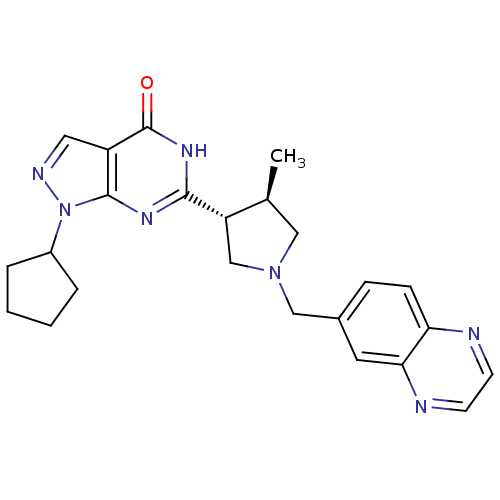
(CHEMBL565667 | trans-1-cyclopentyl-6-(4-methyl-1-(...)Show SMILES C[C@H]1CN(Cc2ccc3nccnc3c2)C[C@@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C24H27N7O/c1-15-12-30(13-16-6-7-20-21(10-16)26-9-8-25-20)14-19(15)22-28-23-18(24(32)29-22)11-27-31(23)17-4-2-3-5-17/h6-11,15,17,19H,2-5,12-14H2,1H3,(H,28,29,32)/t15-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300112

(CHEMBL565667 | trans-1-cyclopentyl-6-(4-methyl-1-(...)Show SMILES C[C@H]1CN(Cc2ccc3nccnc3c2)C[C@@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C24H27N7O/c1-15-12-30(13-16-6-7-20-21(10-16)26-9-8-25-20)14-19(15)22-28-23-18(24(32)29-22)11-27-31(23)17-4-2-3-5-17/h6-11,15,17,19H,2-5,12-14H2,1H3,(H,28,29,32)/t15-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM111911
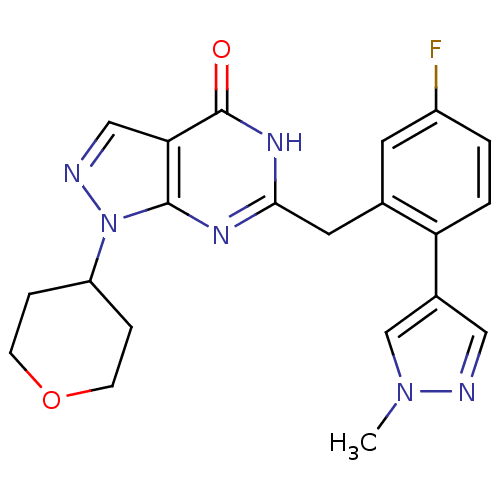
(US8623901, 239)Show SMILES Cn1cc(cn1)-c1ccc(F)cc1Cc1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C21H21FN6O2/c1-27-12-14(10-23-27)17-3-2-15(22)8-13(17)9-19-25-20-18(21(29)26-19)11-24-28(20)16-4-6-30-7-5-16/h2-3,8,10-12,16H,4-7,9H2,1H3,(H,25,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 0 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
The PDE9A2 enzymatic activity assay was run as scintillation proximity assay (SPA), in general according to the protocol of the manufacturer (GE Heal... |
US Patent US8623901 (2014)
BindingDB Entry DOI: 10.7270/Q2Z036TP |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50543966

(CHEMBL4642683)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)[nH]1)C1CCCC1)C(=O)Nc1ccc2[nH]cnc2c1 |r| Show InChI InChI=1S/C20H22N8O2/c1-11(18(29)25-12-6-7-15-16(8-12)22-10-21-15)24-20-26-17-14(19(30)27-20)9-23-28(17)13-4-2-3-5-13/h6-11,13H,2-5H2,1H3,(H,21,22)(H,25,29)(H2,24,26,27,30)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127254
BindingDB Entry DOI: 10.7270/Q2S1862H |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM131013
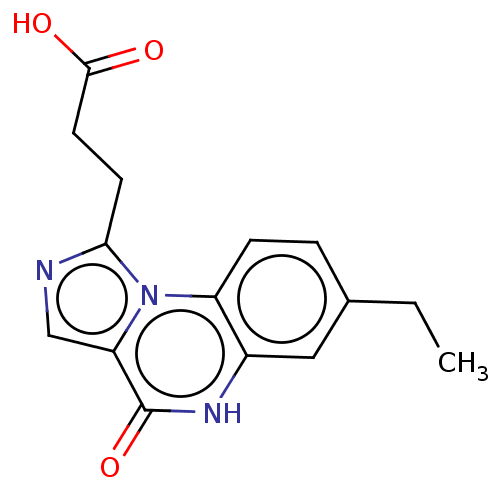
(US8829000, 185)Show InChI InChI=1S/C15H15N3O3/c1-2-9-3-4-11-10(7-9)17-15(21)12-8-16-13(18(11)12)5-6-14(19)20/h3-4,7-8H,2,5-6H2,1H3,(H,17,21)(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 30 |
ASKA Pharmaceutical Co., Ltd.
US Patent
| Assay Description
To 150 uL of buffer B (70 mmol/L Tris-HCl, pH7.5, 16.7 mmol/L MgCl2, 33.3 nmol/L [3H]-cGMP) solution containing [3H]-cGMP (specific activity=244.2 GB... |
US Patent US8829000 (2014)
BindingDB Entry DOI: 10.7270/Q2K072ZX |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM131014
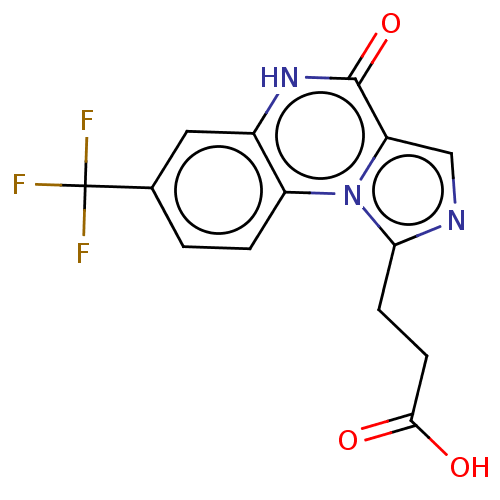
(US8829000, 187)Show InChI InChI=1S/C14H10F3N3O3/c15-14(16,17)7-1-2-9-8(5-7)19-13(23)10-6-18-11(20(9)10)3-4-12(21)22/h1-2,5-6H,3-4H2,(H,19,23)(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 30 |
ASKA Pharmaceutical Co., Ltd.
US Patent
| Assay Description
To 150 uL of buffer B (70 mmol/L Tris-HCl, pH7.5, 16.7 mmol/L MgCl2, 33.3 nmol/L [3H]-cGMP) solution containing [3H]-cGMP (specific activity=244.2 GB... |
US Patent US8829000 (2014)
BindingDB Entry DOI: 10.7270/Q2K072ZX |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300099

(6-benzyl-1-cyclopentyl-1,5-dihydro-4H-pyrazolo[3,4...)Show InChI InChI=1S/C17H18N4O/c22-17-14-11-18-21(13-8-4-5-9-13)16(14)19-15(20-17)10-12-6-2-1-3-7-12/h1-3,6-7,11,13H,4-5,8-10H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50543965

(CHEMBL4640285)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)[nH]1)C1CCCC1)C(=O)Nc1ccc2nccn2c1 |r| Show InChI InChI=1S/C20H22N8O2/c1-12(18(29)24-13-6-7-16-21-8-9-27(16)11-13)23-20-25-17-15(19(30)26-20)10-22-28(17)14-4-2-3-5-14/h6-12,14H,2-5H2,1H3,(H,24,29)(H2,23,25,26,30)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127254
BindingDB Entry DOI: 10.7270/Q2S1862H |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50300099

(6-benzyl-1-cyclopentyl-1,5-dihydro-4H-pyrazolo[3,4...)Show InChI InChI=1S/C17H18N4O/c22-17-14-11-18-21(13-8-4-5-9-13)16(14)19-15(20-17)10-12-6-2-1-3-7-12/h1-3,6-7,11,13H,4-5,8-10H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells by SPA |
J Med Chem 52: 7946-9 (2009)
Article DOI: 10.1021/jm9015334
BindingDB Entry DOI: 10.7270/Q2RV0NRS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50462275
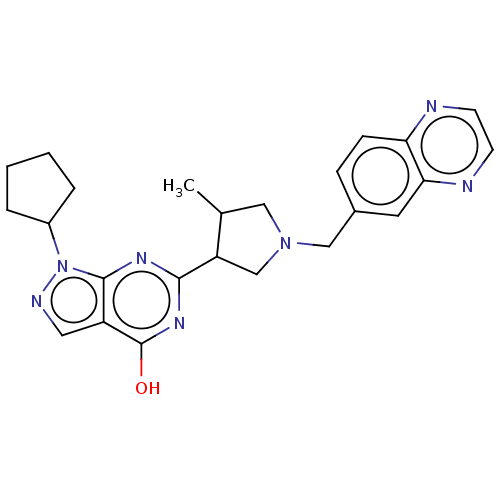
(CHEMBL4249954)Show SMILES CC1CN(Cc2ccc3nccnc3c2)CC1c1nc(O)c2cnn(C3CCCC3)c2n1 Show InChI InChI=1S/C24H27N7O/c1-15-12-30(13-16-6-7-20-21(10-16)26-9-8-25-20)14-19(15)22-28-23-18(24(32)29-22)11-27-31(23)17-4-2-3-5-17/h6-11,15,17,19H,2-5,12-14H2,1H3,(H,28,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan Academy of Medical Science & Sichuan Provincial People's Hospital
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged human PDE9A2 expressed in insect cells using cGMP as substrate after 1 hr by IMAP TR-FRET assay |
Eur J Med Chem 150: 742-756 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.046
BindingDB Entry DOI: 10.7270/Q2JM2D83 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50602405

(CHEMBL5205373)Show SMILES CCCC1CN(CC(=O)N2CCc3ccccc23)Cc2c1[nH]c(=O)c(C#N)c2-c1ccc(C)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01267
BindingDB Entry DOI: 10.7270/Q20P143W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM477990
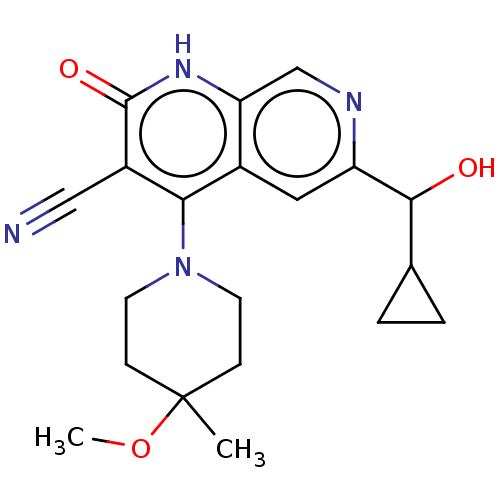
(6-(cyclopropyl(hydroxy)methyl)-4-(4-methoxy-4-meth...)Show SMILES COC1(C)CCN(CC1)c1c(C#N)c(=O)[nH]c2cnc(cc12)C(O)C1CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Test substance: Compounds of the invention, prepared by the corresponding examples of the invention1. Experimental materials and instrumentsPDE9A2 En... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M3300C |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM477990

(6-(cyclopropyl(hydroxy)methyl)-4-(4-methoxy-4-meth...)Show SMILES COC1(C)CCN(CC1)c1c(C#N)c(=O)[nH]c2cnc(cc12)C(O)C1CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing TransThera Biosciences Co. Ltd.
US Patent
| Assay Description
1. Experimental materials and instrumentsPDE9A2 Enzyme (BPS, Cat. No. 60090)384-well plate (Perkin Elmer, Cat. No. 6007279)2. Experimental procedureP... |
US Patent US10889591 (2021)
BindingDB Entry DOI: 10.7270/Q2QR5175 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM317141

(US9617269, Compound WYQ-90-L)Show SMILES COc1ccc(NC(=O)C(C)Nc2nc3n(ncc3c(=O)[nH]2)C2CCCC2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University; University of North Carolina at Chapel Hill
US Patent
| Assay Description
Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera... |
US Patent US9617269 (2017)
BindingDB Entry DOI: 10.7270/Q2RX9F4B |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM131010
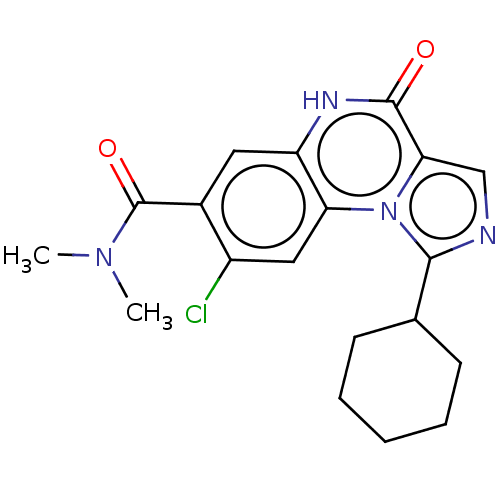
(US8829000, 85)Show SMILES CN(C)C(=O)c1cc2[nH]c(=O)c3cnc(C4CCCCC4)n3c2cc1Cl Show InChI InChI=1S/C19H21ClN4O2/c1-23(2)19(26)12-8-14-15(9-13(12)20)24-16(18(25)22-14)10-21-17(24)11-6-4-3-5-7-11/h8-11H,3-7H2,1-2H3,(H,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 30 |
ASKA Pharmaceutical Co., Ltd.
US Patent
| Assay Description
To 150 uL of buffer B (70 mmol/L Tris-HCl, pH7.5, 16.7 mmol/L MgCl2, 33.3 nmol/L [3H]-cGMP) solution containing [3H]-cGMP (specific activity=244.2 GB... |
US Patent US8829000 (2014)
BindingDB Entry DOI: 10.7270/Q2K072ZX |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM131012
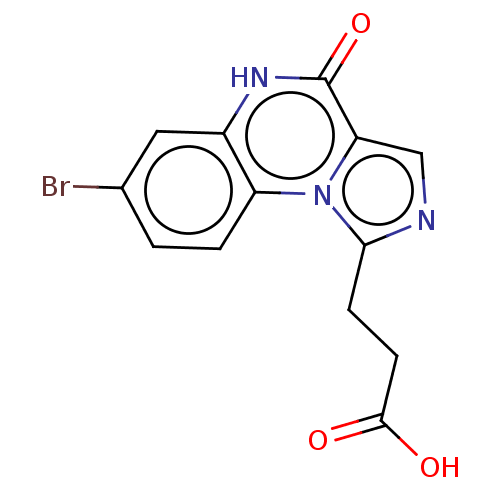
(US8829000, 180)Show InChI InChI=1S/C13H10BrN3O3/c14-7-1-2-9-8(5-7)16-13(20)10-6-15-11(17(9)10)3-4-12(18)19/h1-2,5-6H,3-4H2,(H,16,20)(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 30 |
ASKA Pharmaceutical Co., Ltd.
US Patent
| Assay Description
To 150 uL of buffer B (70 mmol/L Tris-HCl, pH7.5, 16.7 mmol/L MgCl2, 33.3 nmol/L [3H]-cGMP) solution containing [3H]-cGMP (specific activity=244.2 GB... |
US Patent US8829000 (2014)
BindingDB Entry DOI: 10.7270/Q2K072ZX |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50034642
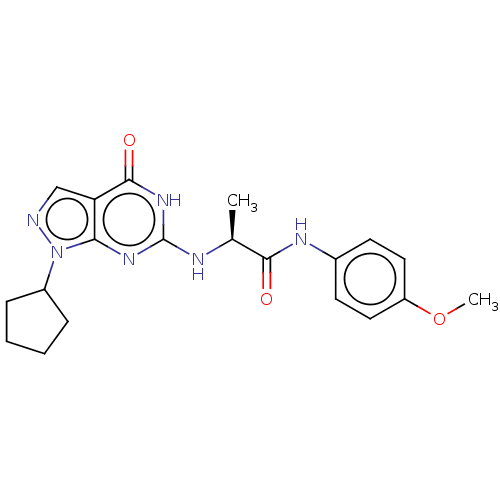
(CHEMBL3360416)Show SMILES COc1ccc(NC(=O)[C@H](C)Nc2nc3n(ncc3c(=O)[nH]2)C2CCCC2)cc1 |r| Show InChI InChI=1S/C20H24N6O3/c1-12(18(27)23-13-7-9-15(29-2)10-8-13)22-20-24-17-16(19(28)25-20)11-21-26(17)14-5-3-4-6-14/h7-12,14H,3-6H2,1-2H3,(H,23,27)(H2,22,24,25,28)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A2 catalytic domain (unknown origin) using [3H]-cGMP/[3H]-cAMP as substrate after 15 mins by liquid scintillation counting analysis |
J Med Chem 57: 10304-13 (2014)
Article DOI: 10.1021/jm500836h
BindingDB Entry DOI: 10.7270/Q28P624W |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM111910

(US8623901, 234)Show SMILES Cc1nnc([nH]1)-c1ccccc1Cc1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C20H21N7O2/c1-12-22-18(26-25-12)15-5-3-2-4-13(15)10-17-23-19-16(20(28)24-17)11-21-27(19)14-6-8-29-9-7-14/h2-5,11,14H,6-10H2,1H3,(H,22,25,26)(H,23,24,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 0 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
The PDE9A2 enzymatic activity assay was run as scintillation proximity assay (SPA), in general according to the protocol of the manufacturer (GE Heal... |
US Patent US8623901 (2014)
BindingDB Entry DOI: 10.7270/Q2Z036TP |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM131009
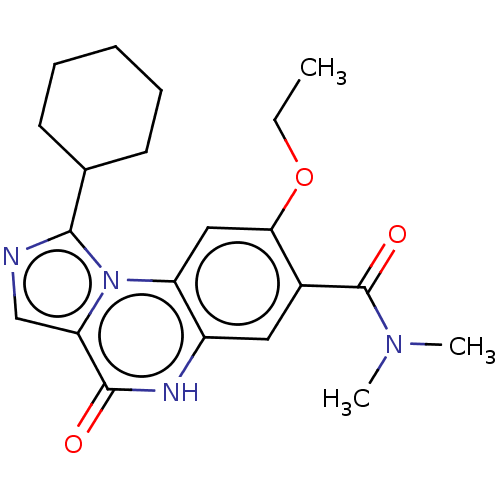
(US8829000, 82)Show SMILES CCOc1cc2c(cc1C(=O)N(C)C)[nH]c(=O)c1cnc(C3CCCCC3)n21 Show InChI InChI=1S/C21H26N4O3/c1-4-28-18-11-16-15(10-14(18)21(27)24(2)3)23-20(26)17-12-22-19(25(16)17)13-8-6-5-7-9-13/h10-13H,4-9H2,1-3H3,(H,23,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | 30 |
ASKA Pharmaceutical Co., Ltd.
US Patent
| Assay Description
To 150 uL of buffer B (70 mmol/L Tris-HCl, pH7.5, 16.7 mmol/L MgCl2, 33.3 nmol/L [3H]-cGMP) solution containing [3H]-cGMP (specific activity=244.2 GB... |
US Patent US8829000 (2014)
BindingDB Entry DOI: 10.7270/Q2K072ZX |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397843
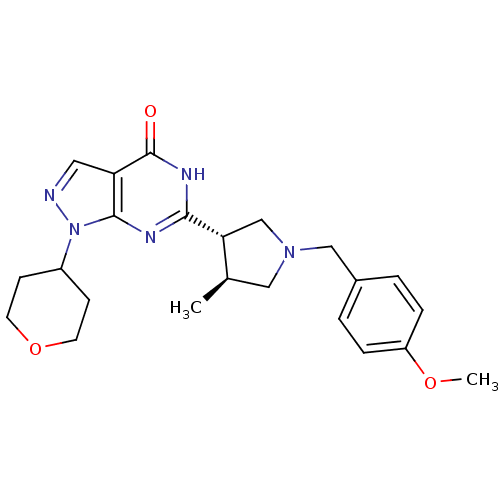
(CHEMBL2179100)Show SMILES COc1ccc(CN2C[C@@H](C)[C@@H](C2)c2nc3n(ncc3c(=O)[nH]2)C2CCOCC2)cc1 |r| Show InChI InChI=1S/C23H29N5O3/c1-15-12-27(13-16-3-5-18(30-2)6-4-16)14-20(15)21-25-22-19(23(29)26-21)11-24-28(22)17-7-9-31-10-8-17/h3-6,11,15,17,20H,7-10,12-14H2,1-2H3,(H,25,26,29)/t15-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397841
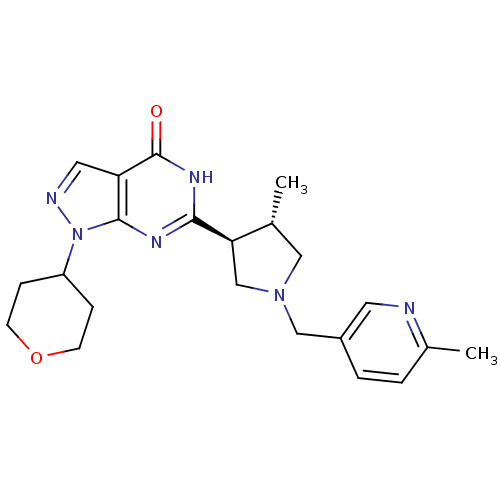
(CHEMBL2179102)Show SMILES C[C@@H]1CN(Cc2ccc(C)nc2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C22H28N6O2/c1-14-11-27(12-16-4-3-15(2)23-9-16)13-19(14)20-25-21-18(22(29)26-20)10-24-28(21)17-5-7-30-8-6-17/h3-4,9-10,14,17,19H,5-8,11-13H2,1-2H3,(H,25,26,29)/t14-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM477987

(US10889591, Compound 108 | US11434248, Compound 10...)Show SMILES COC1(C)CCN(CC1)c1c(C#N)c(=O)[nH]c2cnc(cc12)C(C)O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Test substance: Compounds of the invention, prepared by the corresponding examples of the invention1. Experimental materials and instrumentsPDE9A2 En... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M3300C |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM477987

(US10889591, Compound 108 | US11434248, Compound 10...)Show SMILES COC1(C)CCN(CC1)c1c(C#N)c(=O)[nH]c2cnc(cc12)C(C)O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing TransThera Biosciences Co. Ltd.
US Patent
| Assay Description
1. Experimental materials and instrumentsPDE9A2 Enzyme (BPS, Cat. No. 60090)384-well plate (Perkin Elmer, Cat. No. 6007279)2. Experimental procedureP... |
US Patent US10889591 (2021)
BindingDB Entry DOI: 10.7270/Q2QR5175 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM317074

(US9617269, Compound WYQ-25)Show SMILES Clc1ccc(CNc2nc3n(ncc3c(=O)[nH]2)C2CCCC2)cc1 Show InChI InChI=1S/C17H18ClN5O/c18-12-7-5-11(6-8-12)9-19-17-21-15-14(16(24)22-17)10-20-23(15)13-3-1-2-4-13/h5-8,10,13H,1-4,9H2,(H2,19,21,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University; University of North Carolina at Chapel Hill
US Patent
| Assay Description
Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera... |
US Patent US9617269 (2017)
BindingDB Entry DOI: 10.7270/Q2RX9F4B |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM111932
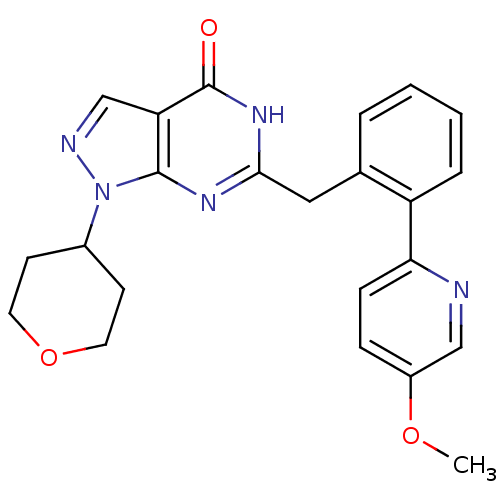
(US8623901, 261)Show SMILES COc1ccc(nc1)-c1ccccc1Cc1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C23H23N5O3/c1-30-17-6-7-20(24-13-17)18-5-3-2-4-15(18)12-21-26-22-19(23(29)27-21)14-25-28(22)16-8-10-31-11-9-16/h2-7,13-14,16H,8-12H2,1H3,(H,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 0 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
The PDE9A2 enzymatic activity assay was run as scintillation proximity assay (SPA), in general according to the protocol of the manufacturer (GE Heal... |
US Patent US8623901 (2014)
BindingDB Entry DOI: 10.7270/Q2Z036TP |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397846
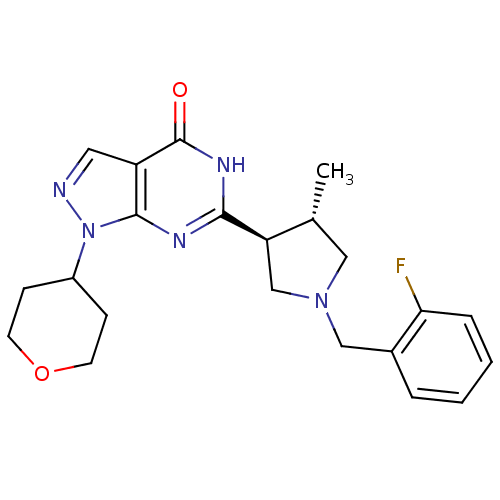
(CHEMBL2179098)Show SMILES C[C@@H]1CN(Cc2ccccc2F)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C22H26FN5O2/c1-14-11-27(12-15-4-2-3-5-19(15)23)13-18(14)20-25-21-17(22(29)26-20)10-24-28(21)16-6-8-30-9-7-16/h2-5,10,14,16,18H,6-9,11-13H2,1H3,(H,25,26,29)/t14-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397839
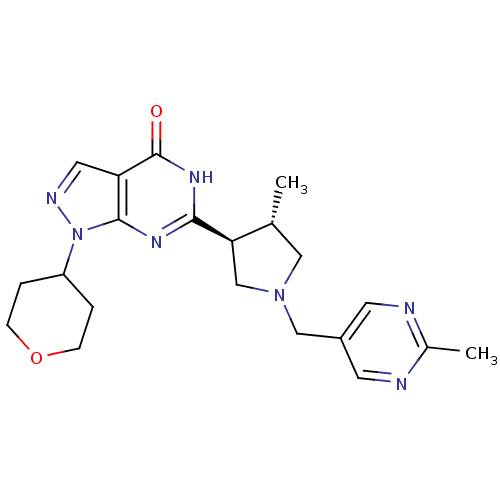
(CHEMBL2179104)Show SMILES C[C@@H]1CN(Cc2cnc(C)nc2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C21H27N7O2/c1-13-10-27(11-15-7-22-14(2)23-8-15)12-18(13)19-25-20-17(21(29)26-19)9-24-28(20)16-3-5-30-6-4-16/h7-9,13,16,18H,3-6,10-12H2,1-2H3,(H,25,26,29)/t13-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50397851
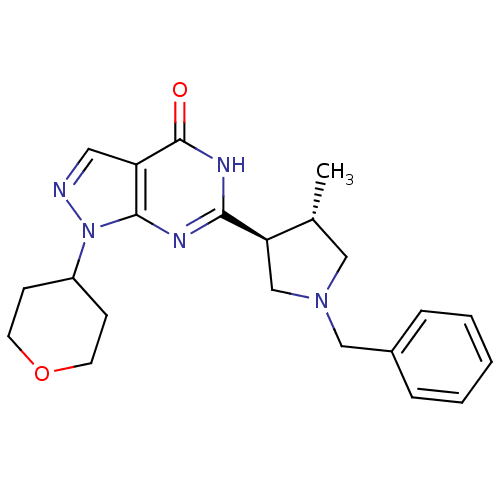
(CHEMBL2179094)Show SMILES C[C@@H]1CN(Cc2ccccc2)C[C@H]1c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C22H27N5O2/c1-15-12-26(13-16-5-3-2-4-6-16)14-19(15)20-24-21-18(22(28)25-20)11-23-27(21)17-7-9-29-10-8-17/h2-6,11,15,17,19H,7-10,12-14H2,1H3,(H,24,25,28)/t15-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE9A expressed in Sf9 cells using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9045-54 (2012)
Article DOI: 10.1021/jm3007799
BindingDB Entry DOI: 10.7270/Q24J0G79 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM570495

(US11434248, Compound 160)Show SMILES OC(C1CC1)c1cc2c(N3CCC4(CCC4)CC3)c(C#N)c(=O)[nH]c2cn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Test substance: Compounds of the invention, prepared by the corresponding examples of the invention1. Experimental materials and instrumentsPDE9A2 En... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M3300C |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM111896
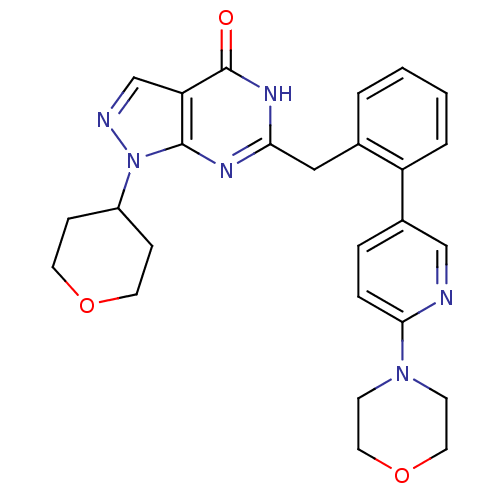
(US8623901, 223)Show SMILES O=c1[nH]c(Cc2ccccc2-c2ccc(nc2)N2CCOCC2)nc2n(ncc12)C1CCOCC1 Show InChI InChI=1S/C26H28N6O3/c33-26-22-17-28-32(20-7-11-34-12-8-20)25(22)29-23(30-26)15-18-3-1-2-4-21(18)19-5-6-24(27-16-19)31-9-13-35-14-10-31/h1-6,16-17,20H,7-15H2,(H,29,30,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 0 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
The PDE9A2 enzymatic activity assay was run as scintillation proximity assay (SPA), in general according to the protocol of the manufacturer (GE Heal... |
US Patent US8623901 (2014)
BindingDB Entry DOI: 10.7270/Q2Z036TP |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM123686

(US8741907, 1)Show InChI InChI=1S/C17H24N4O/c22-17-14-11-18-21(13-8-4-5-9-13)16(14)19-15(20-17)10-12-6-2-1-3-7-12/h11-13H,1-10H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
The test substances are dissolved in 100% DMSO and serially diluted to determine their in vitro effect on PDE 9A. 2 uL portions of the diluted substa... |
US Patent US8741907 (2014)
BindingDB Entry DOI: 10.7270/Q21V5CNH |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM477954

(6-(3-hydroxyazetidin-1-yl)-2-oxo-4-(7-azaspiro[3.5...)Show SMILES OC1CN(C1)c1cc2c(N3CCC4(CCC4)CC3)c(C#N)c(=O)[nH]c2cn1 Show InChI InChI=1S/C20H23N5O2/c21-9-15-18(24-6-4-20(5-7-24)2-1-3-20)14-8-17(25-11-13(26)12-25)22-10-16(14)23-19(15)27/h8,10,13,26H,1-7,11-12H2,(H,23,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Test substance: Compounds of the invention, prepared by the corresponding examples of the invention1. Experimental materials and instrumentsPDE9A2 En... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M3300C |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM477954

(6-(3-hydroxyazetidin-1-yl)-2-oxo-4-(7-azaspiro[3.5...)Show SMILES OC1CN(C1)c1cc2c(N3CCC4(CCC4)CC3)c(C#N)c(=O)[nH]c2cn1 Show InChI InChI=1S/C20H23N5O2/c21-9-15-18(24-6-4-20(5-7-24)2-1-3-20)14-8-17(25-11-13(26)12-25)22-10-16(14)23-19(15)27/h8,10,13,26H,1-7,11-12H2,(H,23,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing TransThera Biosciences Co. Ltd.
US Patent
| Assay Description
1. Experimental materials and instrumentsPDE9A2 Enzyme (BPS, Cat. No. 60090)384-well plate (Perkin Elmer, Cat. No. 6007279)2. Experimental procedureP... |
US Patent US10889591 (2021)
BindingDB Entry DOI: 10.7270/Q2QR5175 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM111892
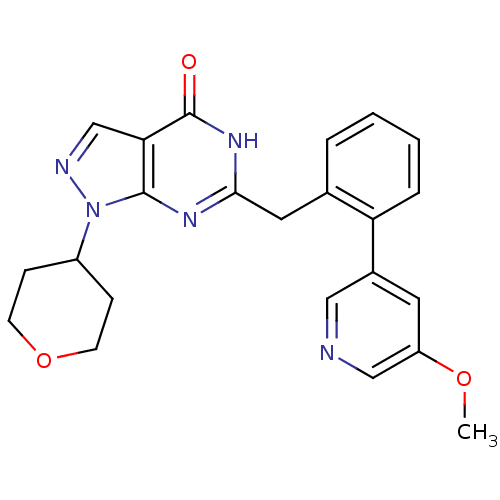
(US8623901, 220)Show SMILES COc1cncc(c1)-c1ccccc1Cc1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C23H23N5O3/c1-30-18-10-16(12-24-13-18)19-5-3-2-4-15(19)11-21-26-22-20(23(29)27-21)14-25-28(22)17-6-8-31-9-7-17/h2-5,10,12-14,17H,6-9,11H2,1H3,(H,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 0 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
The PDE9A2 enzymatic activity assay was run as scintillation proximity assay (SPA), in general according to the protocol of the manufacturer (GE Heal... |
US Patent US8623901 (2014)
BindingDB Entry DOI: 10.7270/Q2Z036TP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data