 Found 280 hits of ic50 for UniProtKB: O43174
Found 280 hits of ic50 for UniProtKB: O43174 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50353578
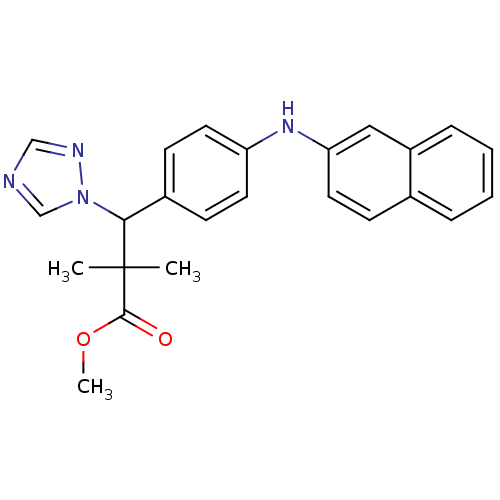
(CHEMBL1831092)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccc3ccccc3c2)cc1)n1cncn1 Show InChI InChI=1S/C24H24N4O2/c1-24(2,23(29)30-3)22(28-16-25-15-26-28)18-9-11-20(12-10-18)27-21-13-8-17-6-4-5-7-19(17)14-21/h4-16,22,27H,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 in human liver microsomes |
J Med Chem 54: 6803-11 (2011)
Article DOI: 10.1021/jm200695m
BindingDB Entry DOI: 10.7270/Q2WM1DSJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50353582

(CHEMBL1831083)Show SMILES CCOC(=O)C(C)(C)C(c1ccc(Nc2ccc3ccccc3c2)cc1)n1ccnc1 Show InChI InChI=1S/C26H27N3O2/c1-4-31-25(30)26(2,3)24(29-16-15-27-18-29)20-10-12-22(13-11-20)28-23-14-9-19-7-5-6-8-21(19)17-23/h5-18,24,28H,4H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP26A1 assessed using [11,12-3H]ATRA as substrate by scintillation counting |
J Med Chem 54: 6803-11 (2011)
Article DOI: 10.1021/jm200695m
BindingDB Entry DOI: 10.7270/Q2WM1DSJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50353578

(CHEMBL1831092)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccc3ccccc3c2)cc1)n1cncn1 Show InChI InChI=1S/C24H24N4O2/c1-24(2,23(29)30-3)22(28-16-25-15-26-28)18-9-11-20(12-10-18)27-21-13-8-17-6-4-5-7-19(17)14-21/h4-16,22,27H,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP26A1 assessed using [11,12-3H]ATRA as substrate by scintillation counting |
J Med Chem 54: 6803-11 (2011)
Article DOI: 10.1021/jm200695m
BindingDB Entry DOI: 10.7270/Q2WM1DSJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50183224
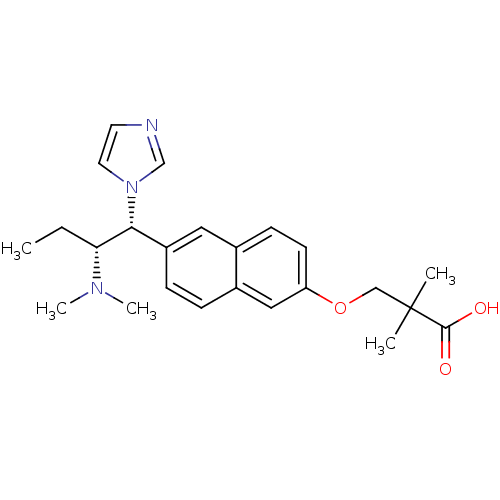
(3-[6-(2-dimethylamino-1-imidazol-1-yl-butyl)-napht...)Show SMILES CC[C@H]([C@@H](c1ccc2cc(OCC(C)(C)C(O)=O)ccc2c1)n1ccnc1)N(C)C Show InChI InChI=1S/C24H31N3O3/c1-6-21(26(4)5)22(27-12-11-25-16-27)19-8-7-18-14-20(10-9-17(18)13-19)30-15-24(2,3)23(28)29/h7-14,16,21-22H,6,15H2,1-5H3,(H,28,29)/t21-,22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50183243

(1-((6-((1R,2R)-2-(diethylamino)-1-(1H-imidazol-1-y...)Show SMILES CCN(CC)[C@H](C)[C@@H](c1ccc2cc(OCC3(CCCC3)C(O)=O)ccc2c1)n1ccnc1 Show InChI InChI=1S/C27H35N3O3/c1-4-29(5-2)20(3)25(30-15-14-28-19-30)23-9-8-22-17-24(11-10-21(22)16-23)33-18-27(26(31)32)12-6-7-13-27/h8-11,14-17,19-20,25H,4-7,12-13,18H2,1-3H3,(H,31,32)/t20-,25+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50183229
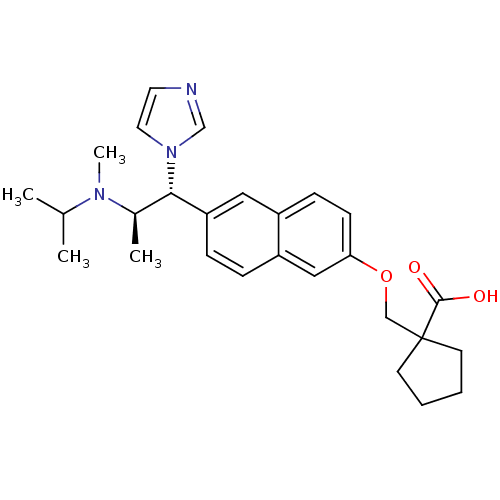
(1-((6-((1R,2R)-1-(1H-imidazol-1-yl)-2-(isopropyl(m...)Show SMILES CC(C)N(C)[C@H](C)[C@@H](c1ccc2cc(OCC3(CCCC3)C(O)=O)ccc2c1)n1ccnc1 Show InChI InChI=1S/C27H35N3O3/c1-19(2)29(4)20(3)25(30-14-13-28-18-30)23-8-7-22-16-24(10-9-21(22)15-23)33-17-27(26(31)32)11-5-6-12-27/h7-10,13-16,18-20,25H,5-6,11-12,17H2,1-4H3,(H,31,32)/t20-,25+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50091698
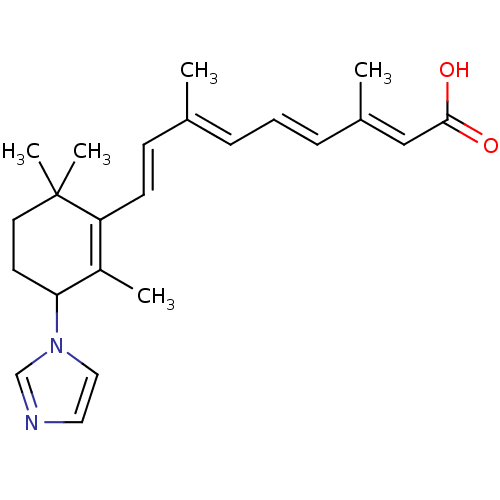
((2E,4E,6E,8E)-9-(3-Imidazol-1-yl-2,6,6-trimethyl-c...)Show SMILES C\C(\C=C\C1=C(C)C(CCC1(C)C)n1ccnc1)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C23H30N2O2/c1-17(7-6-8-18(2)15-22(26)27)9-10-20-19(3)21(11-12-23(20,4)5)25-14-13-24-16-25/h6-10,13-16,21H,11-12H2,1-5H3,(H,26,27)/b8-6+,10-9+,17-7+,18-15+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of ATRA-induced CYP26 in human T47D cell microsome assessed as ATRA metabolism using [11.12-3H]-ATRA |
J Med Chem 47: 6716-29 (2004)
Article DOI: 10.1021/jm0401457
BindingDB Entry DOI: 10.7270/Q2K64HJV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342128

(3-Imidazol-1-yl-2-methyl-3-[4-(naphthalen-2-ylamin...)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccc3ccccc3c2)cc1)n1ccnc1 Show InChI InChI=1S/C25H25N3O2/c1-25(2,24(29)30-3)23(28-15-14-26-17-28)19-9-11-21(12-10-19)27-22-13-8-18-6-4-5-7-20(18)16-22/h4-17,23,27H,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 (unknown origin) |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342128

(3-Imidazol-1-yl-2-methyl-3-[4-(naphthalen-2-ylamin...)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccc3ccccc3c2)cc1)n1ccnc1 Show InChI InChI=1S/C25H25N3O2/c1-25(2,24(29)30-3)23(28-15-14-26-17-28)19-9-11-21(12-10-19)27-22-13-8-18-6-4-5-7-20(18)16-22/h4-17,23,27H,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in human MCF7 cell microsomes using [3H]ATRA after 1 hr by scintillation counting |
J Med Chem 54: 2778-91 (2011)
Article DOI: 10.1021/jm101583w
BindingDB Entry DOI: 10.7270/Q2TT4R8J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342128

(3-Imidazol-1-yl-2-methyl-3-[4-(naphthalen-2-ylamin...)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccc3ccccc3c2)cc1)n1ccnc1 Show InChI InChI=1S/C25H25N3O2/c1-25(2,24(29)30-3)23(28-15-14-26-17-28)19-9-11-21(12-10-19)27-22-13-8-18-6-4-5-7-20(18)16-22/h4-17,23,27H,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP26A1 assessed using [11,12-3H]ATRA as substrate by scintillation counting |
J Med Chem 54: 6803-11 (2011)
Article DOI: 10.1021/jm200695m
BindingDB Entry DOI: 10.7270/Q2WM1DSJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342128

(3-Imidazol-1-yl-2-methyl-3-[4-(naphthalen-2-ylamin...)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccc3ccccc3c2)cc1)n1ccnc1 Show InChI InChI=1S/C25H25N3O2/c1-25(2,24(29)30-3)23(28-15-14-26-17-28)19-9-11-21(12-10-19)27-22-13-8-18-6-4-5-7-20(18)16-22/h4-17,23,27H,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 in human liver microsomes |
J Med Chem 54: 6803-11 (2011)
Article DOI: 10.1021/jm200695m
BindingDB Entry DOI: 10.7270/Q2WM1DSJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50183237
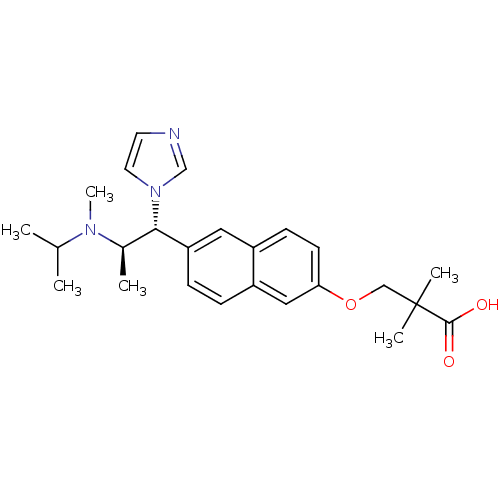
(3-(6-((1R,2R)-1-(1H-imidazol-1-yl)-2-(isopropyl(me...)Show SMILES CC(C)N(C)[C@H](C)[C@@H](c1ccc2cc(OCC(C)(C)C(O)=O)ccc2c1)n1ccnc1 Show InChI InChI=1S/C25H33N3O3/c1-17(2)27(6)18(3)23(28-12-11-26-16-28)21-8-7-20-14-22(10-9-19(20)13-21)31-15-25(4,5)24(29)30/h7-14,16-18,23H,15H2,1-6H3,(H,29,30)/t18-,23+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342130

(3-[4-(6-Hydroxy-naphthalen-2-ylamino)-phenyl]-3-im...)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccc3cc(O)ccc3c2)cc1)n1ccnc1 Show InChI InChI=1S/C25H25N3O3/c1-25(2,24(30)31-3)23(28-13-12-26-16-28)17-4-8-20(9-5-17)27-21-10-6-19-15-22(29)11-7-18(19)14-21/h4-16,23,27,29H,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in human MCF7 cell microsomes using [3H]ATRA after 1 hr by scintillation counting |
J Med Chem 54: 2778-91 (2011)
Article DOI: 10.1021/jm101583w
BindingDB Entry DOI: 10.7270/Q2TT4R8J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50162787
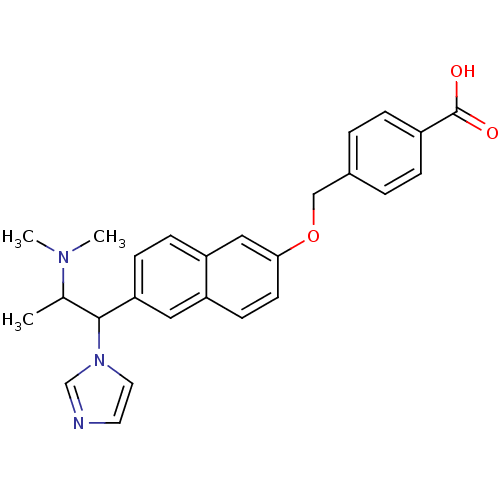
(4-[6-(2-Dimethylamino-1-imidazol-1-yl-propyl)-naph...)Show SMILES CC(C(c1ccc2cc(OCc3ccc(cc3)C(O)=O)ccc2c1)n1ccnc1)N(C)C Show InChI InChI=1S/C26H27N3O3/c1-18(28(2)3)25(29-13-12-27-17-29)23-9-8-22-15-24(11-10-21(22)14-23)32-16-19-4-6-20(7-5-19)26(30)31/h4-15,17-18,25H,16H2,1-3H3,(H,30,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Potency towards cytochrome P 450 26 enzyme activity |
Bioorg Med Chem Lett 15: 1669-73 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.044
BindingDB Entry DOI: 10.7270/Q2WW7H5R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50162787

(4-[6-(2-Dimethylamino-1-imidazol-1-yl-propyl)-naph...)Show SMILES CC(C(c1ccc2cc(OCc3ccc(cc3)C(O)=O)ccc2c1)n1ccnc1)N(C)C Show InChI InChI=1S/C26H27N3O3/c1-18(28(2)3)25(29-13-12-27-17-29)23-9-8-22-15-24(11-10-21(22)14-23)32-16-19-4-6-20(7-5-19)26(30)31/h4-15,17-18,25H,16H2,1-3H3,(H,30,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Potency towards cytochrome P 450 26 enzyme activity |
Bioorg Med Chem Lett 15: 1669-73 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.044
BindingDB Entry DOI: 10.7270/Q2WW7H5R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50162776
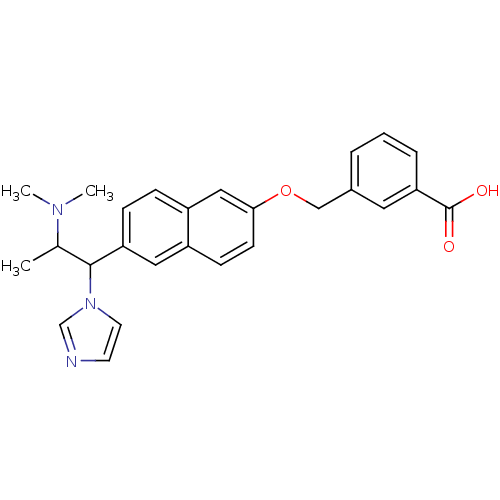
(3-[6-(2-Dimethylamino-1-imidazol-1-yl-propyl)-naph...)Show SMILES CC(C(c1ccc2cc(OCc3cccc(c3)C(O)=O)ccc2c1)n1ccnc1)N(C)C Show InChI InChI=1S/C26H27N3O3/c1-18(28(2)3)25(29-12-11-27-17-29)22-8-7-21-15-24(10-9-20(21)14-22)32-16-19-5-4-6-23(13-19)26(30)31/h4-15,17-18,25H,16H2,1-3H3,(H,30,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Potency towards cytochrome P 450 26 enzyme activity |
Bioorg Med Chem Lett 15: 1669-73 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.044
BindingDB Entry DOI: 10.7270/Q2WW7H5R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50162776

(3-[6-(2-Dimethylamino-1-imidazol-1-yl-propyl)-naph...)Show SMILES CC(C(c1ccc2cc(OCc3cccc(c3)C(O)=O)ccc2c1)n1ccnc1)N(C)C Show InChI InChI=1S/C26H27N3O3/c1-18(28(2)3)25(29-12-11-27-17-29)22-8-7-21-15-24(10-9-20(21)14-22)32-16-19-5-4-6-23(13-19)26(30)31/h4-15,17-18,25H,16H2,1-3H3,(H,30,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Potency towards cytochrome P 450 26 enzyme activity |
Bioorg Med Chem Lett 15: 1669-73 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.044
BindingDB Entry DOI: 10.7270/Q2WW7H5R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50377963
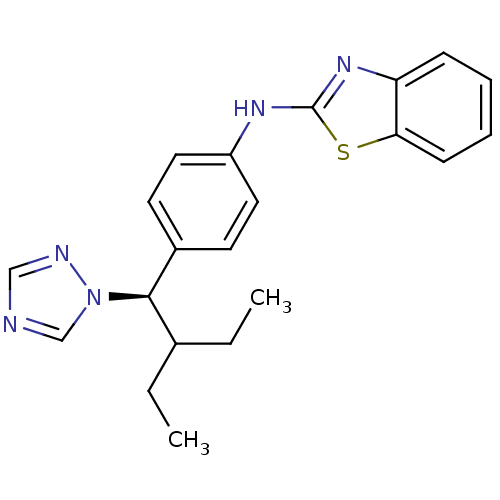
(CHEMBL224305 | R-115866)Show SMILES CCC(CC)[C@H](c1ccc(Nc2nc3ccccc3s2)cc1)n1cncn1 |r| Show InChI InChI=1S/C21H23N5S/c1-3-15(4-2)20(26-14-22-13-23-26)16-9-11-17(12-10-16)24-21-25-18-7-5-6-8-19(18)27-21/h5-15,20H,3-4H2,1-2H3,(H,24,25)/t20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 (unknown origin) |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50183231

(1-((6-((1R,2R)-2-(ethyl(methyl)amino)-1-(1H-imidaz...)Show SMILES CCN(C)[C@H](C)[C@@H](c1ccc2cc(OCC3(CCCC3)C(O)=O)ccc2c1)n1ccnc1 Show InChI InChI=1S/C26H33N3O3/c1-4-28(3)19(2)24(29-14-13-27-18-29)22-8-7-21-16-23(10-9-20(21)15-22)32-17-26(25(30)31)11-5-6-12-26/h7-10,13-16,18-19,24H,4-6,11-12,17H2,1-3H3,(H,30,31)/t19-,24+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM393281

(US9963439, R116010)Show SMILES CC(C(c1ccc(Nc2nc3ccccc3s2)cc1)n1ccnc1)N(C)C Show InChI InChI=1S/C21H23N5S/c1-15(25(2)3)20(26-13-12-22-14-26)16-8-10-17(11-9-16)23-21-24-18-6-4-5-7-19(18)27-21/h4-15,20H,1-3H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle
| Assay Description
Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... |
J Med Chem 52: 1864-72 (2009)
BindingDB Entry DOI: 10.7270/Q2FN18J2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50377963

(CHEMBL224305 | R-115866)Show SMILES CCC(CC)[C@H](c1ccc(Nc2nc3ccccc3s2)cc1)n1cncn1 |r| Show InChI InChI=1S/C21H23N5S/c1-3-15(4-2)20(26-14-22-13-23-26)16-9-11-17(12-10-16)24-21-25-18-7-5-6-8-19(18)27-21/h5-15,20H,3-4H2,1-2H3,(H,24,25)/t20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in human MCF7 cells |
Bioorg Med Chem 16: 8301-13 (2008)
Article DOI: 10.1016/j.bmc.2007.06.048
BindingDB Entry DOI: 10.7270/Q2M61M5B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50183225
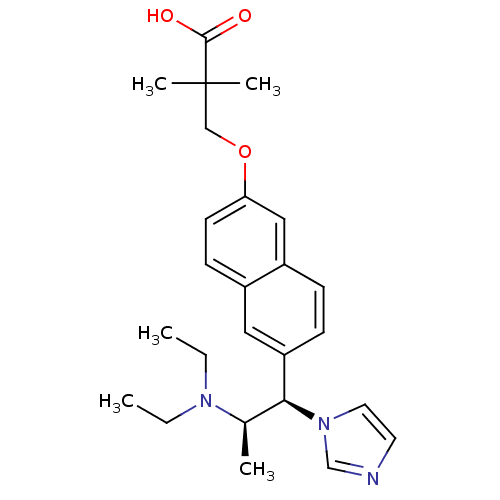
(3-(6-((1R,2R)-2-(diethylamino)-1-(1H-imidazol-1-yl...)Show SMILES CCN(CC)[C@H](C)[C@@H](c1ccc2cc(OCC(C)(C)C(O)=O)ccc2c1)n1ccnc1 Show InChI InChI=1S/C25H33N3O3/c1-6-27(7-2)18(3)23(28-13-12-26-17-28)21-9-8-20-15-22(11-10-19(20)14-21)31-16-25(4,5)24(29)30/h8-15,17-18,23H,6-7,16H2,1-5H3,(H,29,30)/t18-,23+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50253810
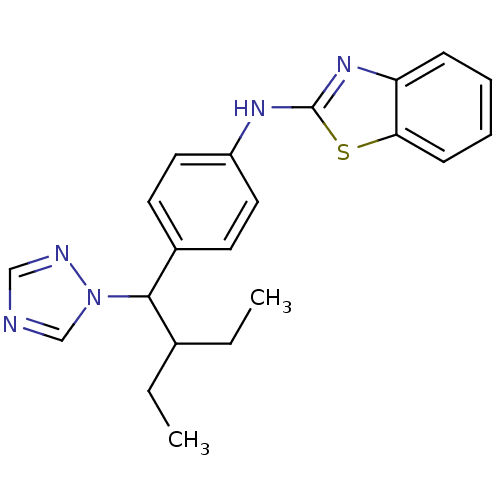
(CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...)Show InChI InChI=1S/C21H23N5S/c1-3-15(4-2)20(26-14-22-13-23-26)16-9-11-17(12-10-16)24-21-25-18-7-5-6-8-19(18)27-21/h5-15,20H,3-4H2,1-2H3,(H,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle
| Assay Description
Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... |
J Med Chem 52: 1864-72 (2009)
BindingDB Entry DOI: 10.7270/Q2FN18J2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50253810

(CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...)Show InChI InChI=1S/C21H23N5S/c1-3-15(4-2)20(26-14-22-13-23-26)16-9-11-17(12-10-16)24-21-25-18-7-5-6-8-19(18)27-21/h5-15,20H,3-4H2,1-2H3,(H,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Inhibition of microsomal fraction of human CYP26A1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... |
J Med Chem 59: 2579-95 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01780
BindingDB Entry DOI: 10.7270/Q2J38VG8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50158410
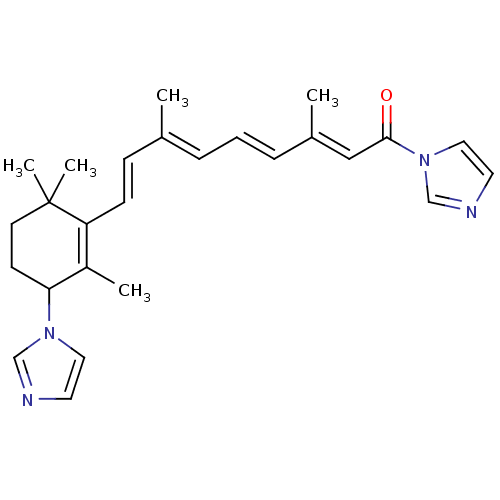
(4-((+/-)-(1H-imidazol-1-yl)-N-(imidazolyl)-(E)-ret...)Show SMILES C\C(\C=C\C1=C(C)C(CCC1(C)C)n1ccnc1)=C/C=C/C(/C)=C/C(=O)n1ccnc1 |c:4| Show InChI InChI=1S/C26H32N4O/c1-20(7-6-8-21(2)17-25(31)30-16-14-28-19-30)9-10-23-22(3)24(11-12-26(23,4)5)29-15-13-27-18-29/h6-10,13-19,24H,11-12H2,1-5H3/b8-6+,10-9+,20-7+,21-17+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of ATRA-induced CYP26 in human T47D cell microsome assessed as ATRA metabolism using [11.12-3H]-ATRA |
J Med Chem 47: 6716-29 (2004)
Article DOI: 10.1021/jm0401457
BindingDB Entry DOI: 10.7270/Q2K64HJV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50391726
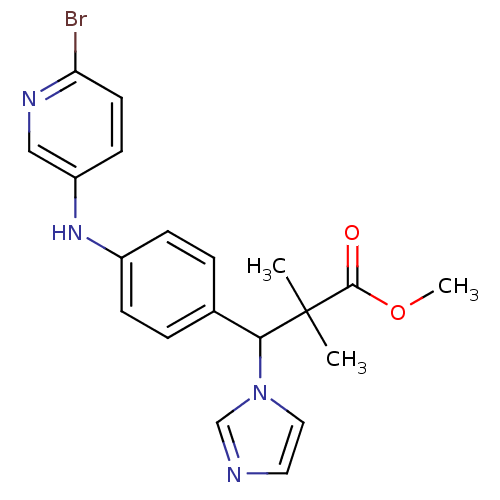
(CHEMBL2146987)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccc(Br)nc2)cc1)n1ccnc1 Show InChI InChI=1S/C20H21BrN4O2/c1-20(2,19(26)27-3)18(25-11-10-22-13-25)14-4-6-15(7-5-14)24-16-8-9-17(21)23-12-16/h4-13,18,24H,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1-mediated retionic acid metabolism in human MCF7 cell microsomes using [3H]ATRA as substrate after 1 hr by scintillation countin... |
Bioorg Med Chem 20: 6080-8 (2012)
Article DOI: 10.1016/j.bmc.2012.08.044
BindingDB Entry DOI: 10.7270/Q2D21ZQD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50162791
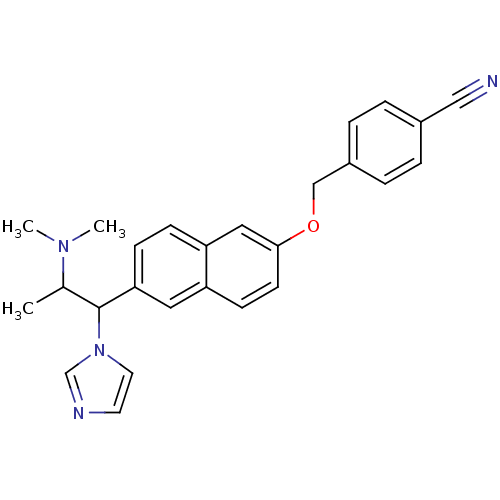
(4-[6-(2-Dimethylamino-1-imidazol-1-yl-propyl)-naph...)Show SMILES CC(C(c1ccc2cc(OCc3ccc(cc3)C#N)ccc2c1)n1ccnc1)N(C)C Show InChI InChI=1S/C26H26N4O/c1-19(29(2)3)26(30-13-12-28-18-30)24-9-8-23-15-25(11-10-22(23)14-24)31-17-21-6-4-20(16-27)5-7-21/h4-15,18-19,26H,17H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Potency towards cytochrome P 450 26 enzyme activity |
Bioorg Med Chem Lett 15: 1669-73 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.044
BindingDB Entry DOI: 10.7270/Q2WW7H5R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50162791

(4-[6-(2-Dimethylamino-1-imidazol-1-yl-propyl)-naph...)Show SMILES CC(C(c1ccc2cc(OCc3ccc(cc3)C#N)ccc2c1)n1ccnc1)N(C)C Show InChI InChI=1S/C26H26N4O/c1-19(29(2)3)26(30-13-12-28-18-30)24-9-8-23-15-25(11-10-22(23)14-24)31-17-21-6-4-20(16-27)5-7-21/h4-15,18-19,26H,17H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Potency towards cytochrome P 450 26 enzyme activity |
Bioorg Med Chem Lett 15: 1669-73 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.044
BindingDB Entry DOI: 10.7270/Q2WW7H5R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342143
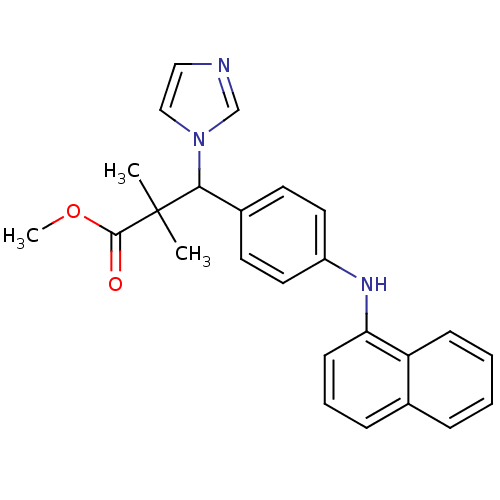
(3-Imidazol-1-yl-2,2-dimethyl-3-[4-(naphthalen-1-yl...)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2cccc3ccccc23)cc1)n1ccnc1 Show InChI InChI=1S/C25H25N3O2/c1-25(2,24(29)30-3)23(28-16-15-26-17-28)19-11-13-20(14-12-19)27-22-10-6-8-18-7-4-5-9-21(18)22/h4-17,23,27H,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in human MCF7 cell microsomes using [3H]ATRA after 1 hr by scintillation counting |
J Med Chem 54: 2778-91 (2011)
Article DOI: 10.1021/jm101583w
BindingDB Entry DOI: 10.7270/Q2TT4R8J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50091698

((2E,4E,6E,8E)-9-(3-Imidazol-1-yl-2,6,6-trimethyl-c...)Show SMILES C\C(\C=C\C1=C(C)C(CCC1(C)C)n1ccnc1)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C23H30N2O2/c1-17(7-6-8-18(2)15-22(26)27)9-10-20-19(3)21(11-12-23(20,4)5)25-14-13-24-16-25/h6-10,13-16,21H,11-12H2,1-5H3,(H,26,27)/b8-6+,10-9+,17-7+,18-15+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of ATRA-induced CYP26 in human T47D cells assessed as ATRA metabolism using [11.12-3H]-ATRA |
J Med Chem 47: 6716-29 (2004)
Article DOI: 10.1021/jm0401457
BindingDB Entry DOI: 10.7270/Q2K64HJV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50391728

(CHEMBL2147090)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccc3OCOc3c2)cc1)n1cncn1 Show InChI InChI=1S/C21H22N4O4/c1-21(2,20(26)27-3)19(25-12-22-11-23-25)14-4-6-15(7-5-14)24-16-8-9-17-18(10-16)29-13-28-17/h4-12,19,24H,13H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1-mediated retionic acid metabolism in human MCF7 cell microsomes using [3H]ATRA as substrate after 1 hr by scintillation countin... |
Bioorg Med Chem 20: 6080-8 (2012)
Article DOI: 10.1016/j.bmc.2012.08.044
BindingDB Entry DOI: 10.7270/Q2D21ZQD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50162788

(3-[6-(2-Dimethylamino-1-imidazol-1-yl-propyl)-naph...)Show SMILES COC(=O)c1cccc(COc2ccc3cc(ccc3c2)C(C(C)N(C)C)n2ccnc2)c1 Show InChI InChI=1S/C27H29N3O3/c1-19(29(2)3)26(30-13-12-28-18-30)23-9-8-22-16-25(11-10-21(22)15-23)33-17-20-6-5-7-24(14-20)27(31)32-4/h5-16,18-19,26H,17H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Potency towards cytochrome P 450 26 enzyme activity |
Bioorg Med Chem Lett 15: 1669-73 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.044
BindingDB Entry DOI: 10.7270/Q2WW7H5R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50162788

(3-[6-(2-Dimethylamino-1-imidazol-1-yl-propyl)-naph...)Show SMILES COC(=O)c1cccc(COc2ccc3cc(ccc3c2)C(C(C)N(C)C)n2ccnc2)c1 Show InChI InChI=1S/C27H29N3O3/c1-19(29(2)3)26(30-13-12-28-18-30)23-9-8-22-16-25(11-10-21(22)15-23)33-17-20-6-5-7-24(14-20)27(31)32-4/h5-16,18-19,26H,17H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Potency towards cytochrome P 450 26 enzyme activity |
Bioorg Med Chem Lett 15: 1669-73 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.044
BindingDB Entry DOI: 10.7270/Q2WW7H5R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50183228
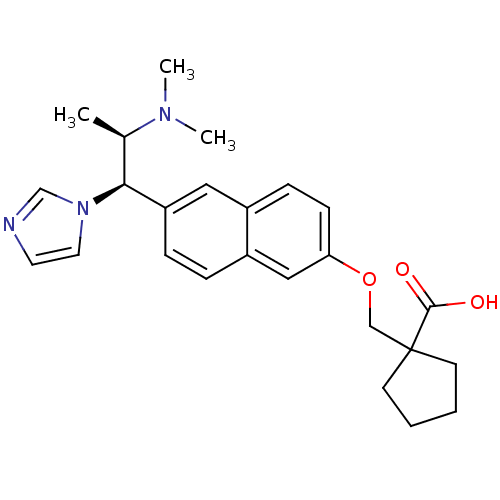
(1-((6-((1R,2R)-2-(dimethylamino)-1-(1H-imidazol-1-...)Show SMILES C[C@H]([C@@H](c1ccc2cc(OCC3(CCCC3)C(O)=O)ccc2c1)n1ccnc1)N(C)C Show InChI InChI=1S/C25H31N3O3/c1-18(27(2)3)23(28-13-12-26-17-28)21-7-6-20-15-22(9-8-19(20)14-21)31-16-25(24(29)30)10-4-5-11-25/h6-9,12-15,17-18,23H,4-5,10-11,16H2,1-3H3,(H,29,30)/t18-,23+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50162782
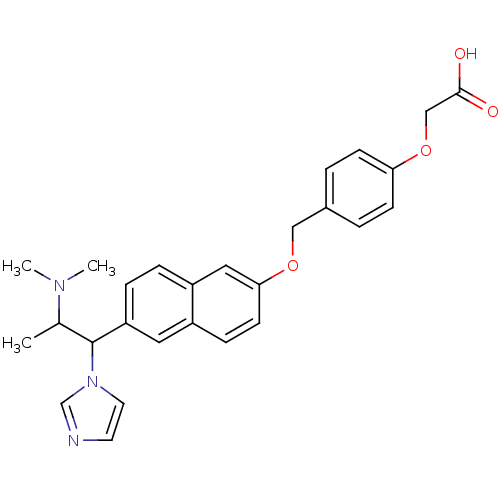
(CHEMBL179550 | {4-[6-(2-Dimethylamino-1-imidazol-1...)Show SMILES CC(C(c1ccc2cc(OCc3ccc(OCC(O)=O)cc3)ccc2c1)n1ccnc1)N(C)C Show InChI InChI=1S/C27H29N3O4/c1-19(29(2)3)27(30-13-12-28-18-30)23-7-6-22-15-25(11-8-21(22)14-23)33-16-20-4-9-24(10-5-20)34-17-26(31)32/h4-15,18-19,27H,16-17H2,1-3H3,(H,31,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Potency towards cytochrome P 450 26 enzyme activity |
Bioorg Med Chem Lett 15: 1669-73 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.044
BindingDB Entry DOI: 10.7270/Q2WW7H5R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342141
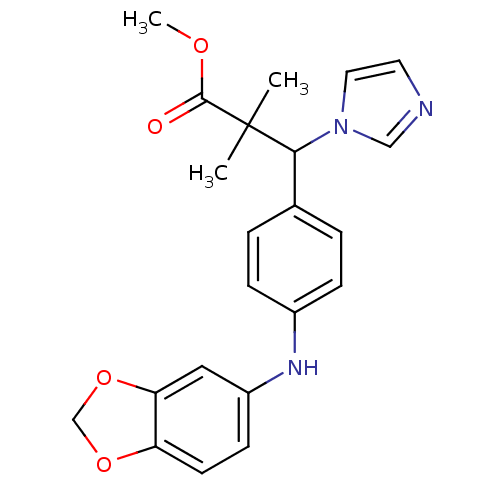
(3-[4-(Benzo[1,3]dioxol-5-ylamino)-phenyl]-3-imidaz...)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccc3OCOc3c2)cc1)n1ccnc1 Show InChI InChI=1S/C22H23N3O4/c1-22(2,21(26)27-3)20(25-11-10-23-13-25)15-4-6-16(7-5-15)24-17-8-9-18-19(12-17)29-14-28-18/h4-13,20,24H,14H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1-mediated retionic acid metabolism in human MCF7 cell microsomes using [3H]ATRA as substrate after 1 hr by scintillation countin... |
Bioorg Med Chem 20: 6080-8 (2012)
Article DOI: 10.1016/j.bmc.2012.08.044
BindingDB Entry DOI: 10.7270/Q2D21ZQD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342129

(3-Imidazol-1-yl-3-[4-(6-methoxy-naphthalen-2-ylami...)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccc3cc(OC)ccc3c2)cc1)n1ccnc1 Show InChI InChI=1S/C26H27N3O3/c1-26(2,25(30)32-4)24(29-14-13-27-17-29)18-5-9-21(10-6-18)28-22-11-7-20-16-23(31-3)12-8-19(20)15-22/h5-17,24,28H,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in human MCF7 cell microsomes using [3H]ATRA after 1 hr by scintillation counting |
J Med Chem 54: 2778-91 (2011)
Article DOI: 10.1021/jm101583w
BindingDB Entry DOI: 10.7270/Q2TT4R8J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342141

(3-[4-(Benzo[1,3]dioxol-5-ylamino)-phenyl]-3-imidaz...)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccc3OCOc3c2)cc1)n1ccnc1 Show InChI InChI=1S/C22H23N3O4/c1-22(2,21(26)27-3)20(25-11-10-23-13-25)15-4-6-16(7-5-15)24-17-8-9-18-19(12-17)29-14-28-18/h4-13,20,24H,14H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in human MCF7 cell microsomes using [3H]ATRA after 1 hr by scintillation counting |
J Med Chem 54: 2778-91 (2011)
Article DOI: 10.1021/jm101583w
BindingDB Entry DOI: 10.7270/Q2TT4R8J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50183241

(3-(6-((1R,2R)-2-(ethyl(methyl)amino)-1-(1H-imidazo...)Show SMILES CCN(C)[C@H](C)[C@@H](c1ccc2cc(OCC(C)(C)C(O)=O)ccc2c1)n1ccnc1 Show InChI InChI=1S/C24H31N3O3/c1-6-26(5)17(2)22(27-12-11-25-16-27)20-8-7-19-14-21(10-9-18(19)13-20)30-15-24(3,4)23(28)29/h7-14,16-17,22H,6,15H2,1-5H3,(H,28,29)/t17-,22+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50391722

(CHEMBL2146981)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccc(cc2)C(F)(F)F)cc1)n1ccnc1 Show InChI InChI=1S/C22H22F3N3O2/c1-21(2,20(29)30-3)19(28-13-12-26-14-28)15-4-8-17(9-5-15)27-18-10-6-16(7-11-18)22(23,24)25/h4-14,19,27H,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1-mediated retionic acid metabolism in human MCF7 cell microsomes using [3H]ATRA as substrate after 1 hr by scintillation countin... |
Bioorg Med Chem 20: 6080-8 (2012)
Article DOI: 10.1016/j.bmc.2012.08.044
BindingDB Entry DOI: 10.7270/Q2D21ZQD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342133
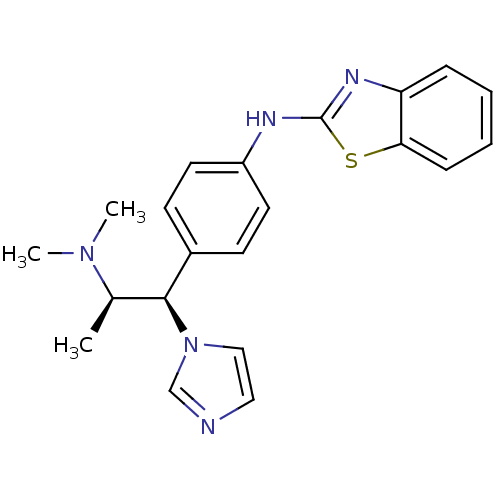
(Benzothiazol-2-yl-[4-((1S,2S)-2-dimethylamino-1-im...)Show SMILES C[C@H]([C@@H](c1ccc(Nc2nc3ccccc3s2)cc1)n1ccnc1)N(C)C |r| Show InChI InChI=1S/C21H23N5S/c1-15(25(2)3)20(26-13-12-22-14-26)16-8-10-17(11-9-16)23-21-24-18-6-4-5-7-19(18)27-21/h4-15,20H,1-3H3,(H,23,24)/t15-,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in human MCF7 cell microsomes using [3H]ATRA after 1 hr by scintillation counting |
J Med Chem 54: 2778-91 (2011)
Article DOI: 10.1021/jm101583w
BindingDB Entry DOI: 10.7270/Q2TT4R8J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342140
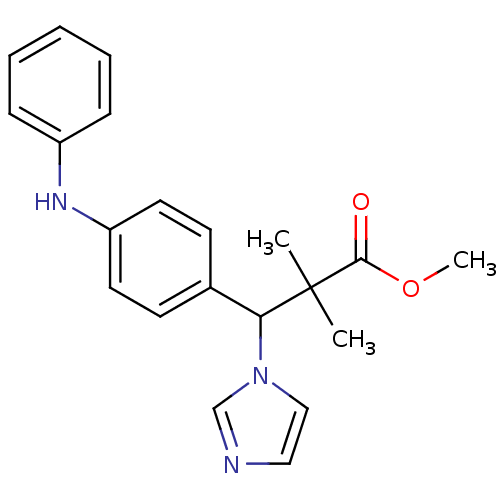
(3-Imidazol-1-yl-2,2-dimethyl-3-(4-phenylaminopheny...)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccccc2)cc1)n1ccnc1 Show InChI InChI=1S/C21H23N3O2/c1-21(2,20(25)26-3)19(24-14-13-22-15-24)16-9-11-18(12-10-16)23-17-7-5-4-6-8-17/h4-15,19,23H,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in human MCF7 cell microsomes using [3H]ATRA after 1 hr by scintillation counting |
J Med Chem 54: 2778-91 (2011)
Article DOI: 10.1021/jm101583w
BindingDB Entry DOI: 10.7270/Q2TT4R8J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342133

(Benzothiazol-2-yl-[4-((1S,2S)-2-dimethylamino-1-im...)Show SMILES C[C@H]([C@@H](c1ccc(Nc2nc3ccccc3s2)cc1)n1ccnc1)N(C)C |r| Show InChI InChI=1S/C21H23N5S/c1-15(25(2)3)20(26-13-12-22-14-26)16-8-10-17(11-9-16)23-21-24-18-6-4-5-7-19(18)27-21/h4-15,20H,1-3H3,(H,23,24)/t15-,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP26A1 assessed using [11,12-3H]ATRA as substrate by scintillation counting |
J Med Chem 54: 6803-11 (2011)
Article DOI: 10.1021/jm200695m
BindingDB Entry DOI: 10.7270/Q2WM1DSJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342133

(Benzothiazol-2-yl-[4-((1S,2S)-2-dimethylamino-1-im...)Show SMILES C[C@H]([C@@H](c1ccc(Nc2nc3ccccc3s2)cc1)n1ccnc1)N(C)C |r| Show InChI InChI=1S/C21H23N5S/c1-15(25(2)3)20(26-13-12-22-14-26)16-8-10-17(11-9-16)23-21-24-18-6-4-5-7-19(18)27-21/h4-15,20H,1-3H3,(H,23,24)/t15-,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 in human liver microsomes |
J Med Chem 54: 6803-11 (2011)
Article DOI: 10.1021/jm200695m
BindingDB Entry DOI: 10.7270/Q2WM1DSJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342140

(3-Imidazol-1-yl-2,2-dimethyl-3-(4-phenylaminopheny...)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccccc2)cc1)n1ccnc1 Show InChI InChI=1S/C21H23N3O2/c1-21(2,20(25)26-3)19(24-14-13-22-15-24)16-9-11-18(12-10-16)23-17-7-5-4-6-8-17/h4-15,19,23H,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1-mediated retionic acid metabolism in human MCF7 cell microsomes using [3H]ATRA as substrate after 1 hr by scintillation countin... |
Bioorg Med Chem 20: 6080-8 (2012)
Article DOI: 10.1016/j.bmc.2012.08.044
BindingDB Entry DOI: 10.7270/Q2D21ZQD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50391719
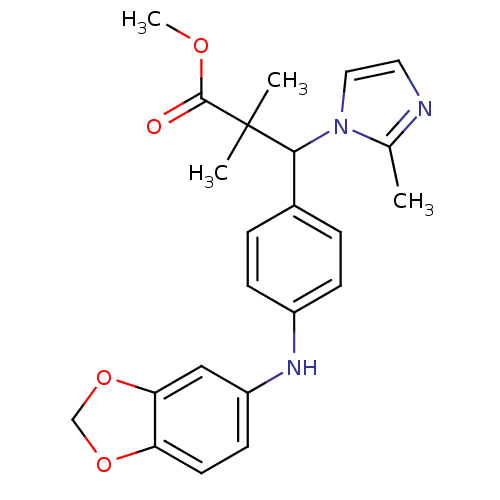
(CHEMBL2147092)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccc3OCOc3c2)cc1)n1ccnc1C Show InChI InChI=1S/C23H25N3O4/c1-15-24-11-12-26(15)21(23(2,3)22(27)28-4)16-5-7-17(8-6-16)25-18-9-10-19-20(13-18)30-14-29-19/h5-13,21,25H,14H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1-mediated retionic acid metabolism in human MCF7 cell microsomes using [3H]ATRA as substrate after 1 hr by scintillation countin... |
Bioorg Med Chem 20: 6080-8 (2012)
Article DOI: 10.1016/j.bmc.2012.08.044
BindingDB Entry DOI: 10.7270/Q2D21ZQD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50158410

(4-((+/-)-(1H-imidazol-1-yl)-N-(imidazolyl)-(E)-ret...)Show SMILES C\C(\C=C\C1=C(C)C(CCC1(C)C)n1ccnc1)=C/C=C/C(/C)=C/C(=O)n1ccnc1 |c:4| Show InChI InChI=1S/C26H32N4O/c1-20(7-6-8-21(2)17-25(31)30-16-14-28-19-30)9-10-23-22(3)24(11-12-26(23,4)5)29-15-13-27-18-29/h6-10,13-19,24H,11-12H2,1-5H3/b8-6+,10-9+,20-7+,21-17+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of ATRA-induced CYP26 in human T47D cells assessed as ATRA metabolism using [11.12-3H]-ATRA |
J Med Chem 47: 6716-29 (2004)
Article DOI: 10.1021/jm0401457
BindingDB Entry DOI: 10.7270/Q2K64HJV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50120455

(CHEMBL3617993)Show SMILES CC(C(c1ccc(Nc2nc3ccccc3s2)cc1)n1cncn1)N(C)C Show InChI InChI=1S/C20H22N6S/c1-14(25(2)3)19(26-13-21-12-22-26)15-8-10-16(11-9-15)23-20-24-17-6-4-5-7-18(17)27-20/h4-14,19H,1-3H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 (unknown origin) |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342131
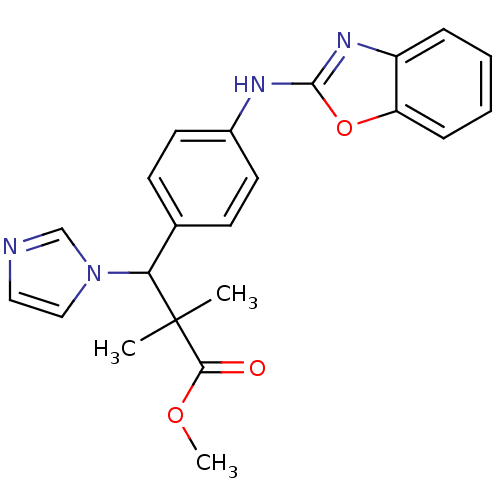
(3-[4-(Benzooxazol-2-ylamino)-phenyl]-3-imidazol-1-...)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2nc3ccccc3o2)cc1)n1ccnc1 Show InChI InChI=1S/C22H22N4O3/c1-22(2,20(27)28-3)19(26-13-12-23-14-26)15-8-10-16(11-9-15)24-21-25-17-6-4-5-7-18(17)29-21/h4-14,19H,1-3H3,(H,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in human MCF7 cell microsomes using [3H]ATRA after 1 hr by scintillation counting |
J Med Chem 54: 2778-91 (2011)
Article DOI: 10.1021/jm101583w
BindingDB Entry DOI: 10.7270/Q2TT4R8J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50091698

((2E,4E,6E,8E)-9-(3-Imidazol-1-yl-2,6,6-trimethyl-c...)Show SMILES C\C(\C=C\C1=C(C)C(CCC1(C)C)n1ccnc1)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C23H30N2O2/c1-17(7-6-8-18(2)15-22(26)27)9-10-20-19(3)21(11-12-23(20,4)5)25-14-13-24-16-25/h6-10,13-16,21H,11-12H2,1-5H3,(H,26,27)/b8-6+,10-9+,17-7+,18-15+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of ATRA-induced CYP26 in human MCF7 cells assessed ATRA as metabolism using [11.12-3H]-ATRA |
J Med Chem 47: 6716-29 (2004)
Article DOI: 10.1021/jm0401457
BindingDB Entry DOI: 10.7270/Q2K64HJV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data