 Found 994 hits of ki data for polymerid = 5101
Found 994 hits of ki data for polymerid = 5101 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393244
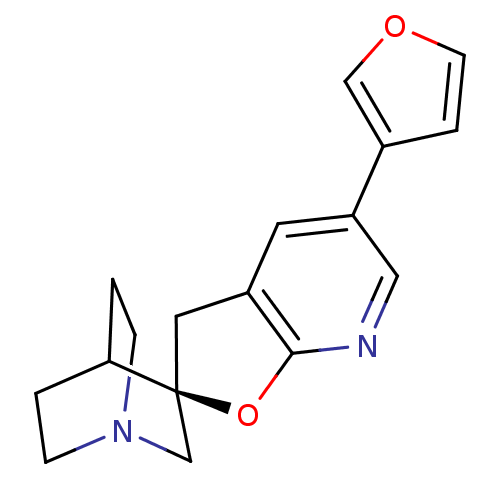
(CHEMBL2151438)Show SMILES C1c2cc(cnc2O[C@]11CN2CCC1CC2)-c1ccoc1 |r,wU:8.8,(21.23,-.78,;22.37,.25,;23.89,.09,;24.79,1.32,;24.17,2.73,;22.65,2.89,;21.75,1.65,;20.22,1.49,;19.9,-.01,;19.9,-1.55,;18.56,-2.31,;17.23,-1.55,;17.23,-.01,;18.56,.77,;19.32,-.56,;17.82,-.96,;26.33,1.34,;27.22,2.6,;28.69,2.15,;28.72,.6,;27.26,.11,)| Show InChI InChI=1S/C17H18N2O2/c1-4-19-5-2-15(1)17(11-19)8-13-7-14(9-18-16(13)21-17)12-3-6-20-10-12/h3,6-7,9-10,15H,1-2,4-5,8,11H2/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human alpha7 nAchR |
J Med Chem 54: 7943-61 (2011)
Article DOI: 10.1021/jm2007672
BindingDB Entry DOI: 10.7270/Q29P32QP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50364875
(CHEMBL1950156)Show SMILES CN1C[C@H]2CN(C[C@@H]12)c1ccc2-c3ccc(cc3C(=O)c2c1)N1C[C@@H]2CN(C)[C@@H]2C1 |r| Show InChI InChI=1S/C25H28N4O/c1-26-9-15-11-28(13-23(15)26)17-3-5-19-20-6-4-18(8-22(20)25(30)21(19)7-17)29-12-16-10-27(2)24(16)14-29/h3-8,15-16,23-24H,9-14H2,1-2H3/t15-,16-,23+,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to alpha7 nAChR (unknown origin) |
J Med Chem 56: 7574-89 (2013)
Article DOI: 10.1021/jm401184f
BindingDB Entry DOI: 10.7270/Q2Z3215R |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393252
(CHEMBL2151570)Show SMILES C1CN2CCC1[C@H](C2)Oc1ccc(nn1)-c1ccc2[nH]ccc2c1 |r,wU:6.9,(10.31,-30.96,;10.31,-32.5,;11.64,-33.26,;10.9,-31.9,;12.4,-31.51,;11.64,-30.18,;12.97,-30.96,;12.97,-32.5,;14.31,-30.2,;15.64,-30.97,;16.97,-30.21,;18.3,-30.98,;18.3,-32.52,;16.96,-33.29,;15.63,-32.51,;19.62,-33.3,;19.61,-34.84,;20.94,-35.61,;22.28,-34.84,;23.75,-35.31,;24.65,-34.07,;23.75,-32.82,;22.28,-33.3,;20.95,-32.53,)| Show InChI InChI=1S/C19H20N4O/c1-2-16-15(5-8-20-16)11-14(1)17-3-4-19(22-21-17)24-18-12-23-9-6-13(18)7-10-23/h1-5,8,11,13,18,20H,6-7,9-10,12H2/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]A-585539 from alpha7 nAChR human cortex |
J Med Chem 54: 7943-61 (2011)
Article DOI: 10.1021/jm2007672
BindingDB Entry DOI: 10.7270/Q29P32QP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393252

(CHEMBL2151570)Show SMILES C1CN2CCC1[C@H](C2)Oc1ccc(nn1)-c1ccc2[nH]ccc2c1 |r,wU:6.9,(10.31,-30.96,;10.31,-32.5,;11.64,-33.26,;10.9,-31.9,;12.4,-31.51,;11.64,-30.18,;12.97,-30.96,;12.97,-32.5,;14.31,-30.2,;15.64,-30.97,;16.97,-30.21,;18.3,-30.98,;18.3,-32.52,;16.96,-33.29,;15.63,-32.51,;19.62,-33.3,;19.61,-34.84,;20.94,-35.61,;22.28,-34.84,;23.75,-35.31,;24.65,-34.07,;23.75,-32.82,;22.28,-33.3,;20.95,-32.53,)| Show InChI InChI=1S/C19H20N4O/c1-2-16-15(5-8-20-16)11-14(1)17-3-4-19(22-21-17)24-18-12-23-9-6-13(18)7-10-23/h1-5,8,11,13,18,20H,6-7,9-10,12H2/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mahidol University
Curated by ChEMBL
| Assay Description
Displacement of [3H]A-585539 from alpha7 nAChR in human cerebral cortex membranes after 75 mins by scintillation counting |
ACS Med Chem Lett 7: 890-895 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00146
BindingDB Entry DOI: 10.7270/Q2ZW1QDQ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393251
(CHEMBL2151569)Show InChI InChI=1S/C19H21N5/c1-23-9-15-11-24(12-16(15)10-23)19-5-4-18(21-22-19)13-2-3-17-14(8-13)6-7-20-17/h2-8,15-16,20H,9-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to alpha7 nAChR |
J Med Chem 54: 7943-61 (2011)
Article DOI: 10.1021/jm2007672
BindingDB Entry DOI: 10.7270/Q29P32QP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393251

(CHEMBL2151569)Show InChI InChI=1S/C19H21N5/c1-23-9-15-11-24(12-16(15)10-23)19-5-4-18(21-22-19)13-2-3-17-14(8-13)6-7-20-17/h2-8,15-16,20H,9-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to alpha7 nAChR (unknown origin) |
J Med Chem 56: 7574-89 (2013)
Article DOI: 10.1021/jm401184f
BindingDB Entry DOI: 10.7270/Q2Z3215R |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399780
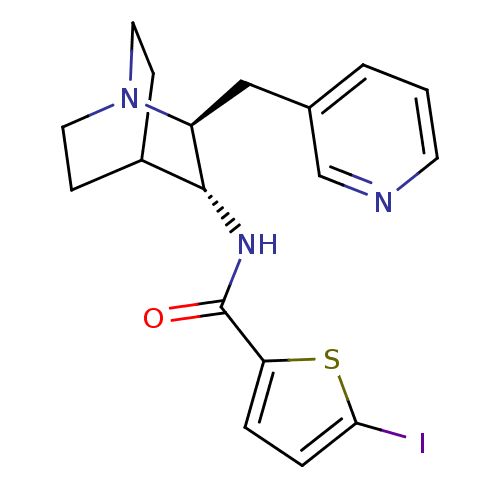
(CHEMBL2179874)Show SMILES Ic1ccc(s1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:9.9,wD:16.19,(34.89,-18.91,;33.35,-18.9,;32.45,-17.65,;30.99,-18.12,;30.98,-19.66,;32.44,-20.14,;29.83,-20.69,;29.83,-22.23,;28.5,-19.92,;27.16,-20.68,;25.83,-19.9,;24.51,-20.68,;24.51,-22.22,;25.83,-22.98,;25.05,-21.65,;26.43,-21.15,;27.16,-22.22,;28.5,-22.99,;28.49,-24.53,;27.16,-25.3,;27.15,-26.84,;28.49,-27.61,;29.83,-26.83,;29.82,-25.3,)| Show InChI InChI=1S/C18H20IN3OS/c19-16-4-3-15(24-16)18(23)21-17-13-5-8-22(9-6-13)14(17)10-12-2-1-7-20-11-12/h1-4,7,11,13-14,17H,5-6,8-10H2,(H,21,23)/t14-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399779
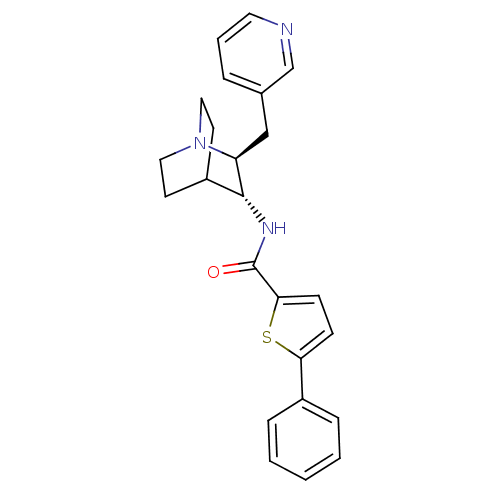
(CHEMBL2179875)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1)c1ccc(s1)-c1ccccc1 |r,wU:3.2,wD:10.12,(40.04,-22.15,;40.04,-20.61,;38.71,-19.84,;37.37,-20.6,;36.04,-19.83,;34.72,-20.6,;34.72,-22.14,;36.04,-22.91,;35.26,-21.57,;36.64,-21.07,;37.37,-22.14,;38.71,-22.92,;38.7,-24.46,;37.37,-25.22,;37.36,-26.76,;38.7,-27.53,;40.04,-26.76,;40.03,-25.22,;41.19,-19.58,;41.2,-18.04,;42.66,-17.58,;43.56,-18.82,;42.65,-20.06,;45.1,-18.83,;45.86,-20.17,;47.4,-20.18,;48.18,-18.85,;47.4,-17.51,;45.87,-17.51,)| Show InChI InChI=1S/C24H25N3OS/c28-24(22-9-8-21(29-22)18-6-2-1-3-7-18)26-23-19-10-13-27(14-11-19)20(23)15-17-5-4-12-25-16-17/h1-9,12,16,19-20,23H,10-11,13-15H2,(H,26,28)/t20-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399777
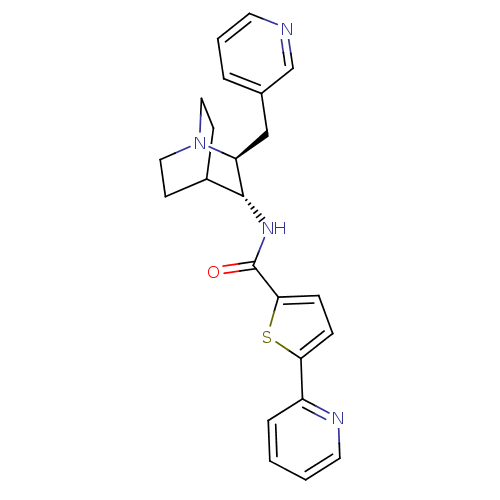
(CHEMBL2179877)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1)c1ccc(s1)-c1ccccn1 |r,wU:3.2,wD:10.12,(17.82,-36.07,;17.82,-34.53,;16.49,-33.76,;15.15,-34.53,;13.82,-33.75,;12.49,-34.53,;12.49,-36.07,;13.82,-36.83,;13.04,-35.49,;14.42,-34.99,;15.15,-36.07,;16.48,-36.84,;16.48,-38.38,;15.15,-39.14,;15.14,-40.68,;16.48,-41.46,;17.82,-40.68,;17.81,-39.14,;18.97,-33.5,;18.98,-31.97,;20.44,-31.5,;21.34,-32.75,;20.43,-33.98,;22.88,-32.76,;23.65,-31.43,;25.18,-31.43,;25.95,-32.77,;25.18,-34.1,;23.64,-34.1,)| Show InChI InChI=1S/C23H24N4OS/c28-23(21-7-6-20(29-21)18-5-1-2-11-25-18)26-22-17-8-12-27(13-9-17)19(22)14-16-4-3-10-24-15-16/h1-7,10-11,15,17,19,22H,8-9,12-14H2,(H,26,28)/t19-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393247
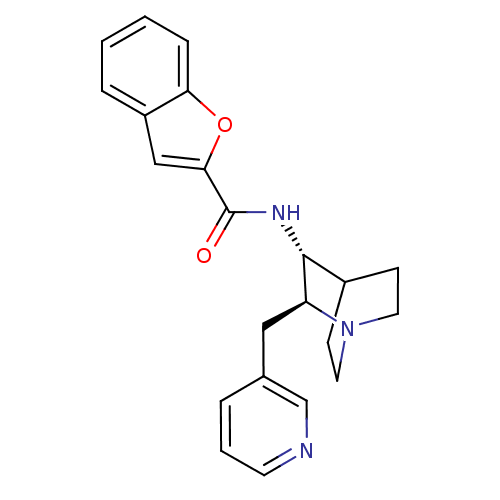
(CHEMBL1258006)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1)c1cc2ccccc2o1 |r,wU:3.2,wD:10.12,TLB:11:10:6.5:8.9,THB:2:3:6.5:8.9,(5.71,-17.16,;5.73,-15.62,;4.4,-14.83,;3.06,-15.59,;1.59,-14.95,;.24,-15.55,;-.04,-16.95,;1.33,-16.32,;1.07,-14.41,;1.52,-13.3,;2.87,-16.97,;3.63,-18.31,;2.84,-19.64,;1.3,-19.62,;.52,-20.94,;1.28,-22.29,;2.83,-22.3,;3.61,-20.97,;7.07,-14.87,;7.24,-13.35,;8.76,-13.04,;9.54,-11.72,;11.07,-11.74,;11.83,-13.08,;11.04,-14.41,;9.51,-14.39,;8.47,-15.52,)| Show InChI InChI=1S/C22H23N3O2/c26-22(20-13-17-5-1-2-6-19(17)27-20)24-21-16-7-10-25(11-8-16)18(21)12-15-4-3-9-23-14-15/h1-6,9,13-14,16,18,21H,7-8,10-12H2,(H,24,26)/t18-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human alpha7 nAChR expressed in HEK293 cells co-expressing RIC3 cDNA |
J Med Chem 54: 7943-61 (2011)
Article DOI: 10.1021/jm2007672
BindingDB Entry DOI: 10.7270/Q29P32QP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50309873

(4-(6-Chlorooxazolo[4,5-b]pyridin-2-yl)-1,4-diazabi...)Show SMILES Clc1cnc2nc(oc2c1)N1CCN2CCC1CC2 |TLB:6:10:15.14:17.18,(-1.41,-19.11,;-1.97,-20.56,;-.99,-21.77,;-1.55,-23.21,;-3.08,-23.45,;-3.94,-24.73,;-5.43,-24.32,;-5.49,-22.78,;-4.05,-22.24,;-3.49,-20.8,;-6.76,-25.09,;-6.39,-26.56,;-7.4,-25.84,;-8.78,-25.91,;-8.85,-24.2,;-8.23,-23.06,;-8.17,-24.47,;-9.47,-25.17,;-9.77,-26.61,)| Show InChI InChI=1S/C13H15ClN4O/c14-9-7-11-12(15-8-9)16-13(19-11)18-6-5-17-3-1-10(18)2-4-17/h7-8,10H,1-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to alpha7 nAChR |
J Med Chem 54: 7943-61 (2011)
Article DOI: 10.1021/jm2007672
BindingDB Entry DOI: 10.7270/Q29P32QP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393247

(CHEMBL1258006)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1)c1cc2ccccc2o1 |r,wU:3.2,wD:10.12,TLB:11:10:6.5:8.9,THB:2:3:6.5:8.9,(5.71,-17.16,;5.73,-15.62,;4.4,-14.83,;3.06,-15.59,;1.59,-14.95,;.24,-15.55,;-.04,-16.95,;1.33,-16.32,;1.07,-14.41,;1.52,-13.3,;2.87,-16.97,;3.63,-18.31,;2.84,-19.64,;1.3,-19.62,;.52,-20.94,;1.28,-22.29,;2.83,-22.3,;3.61,-20.97,;7.07,-14.87,;7.24,-13.35,;8.76,-13.04,;9.54,-11.72,;11.07,-11.74,;11.83,-13.08,;11.04,-14.41,;9.51,-14.39,;8.47,-15.52,)| Show InChI InChI=1S/C22H23N3O2/c26-22(20-13-17-5-1-2-6-19(17)27-20)24-21-16-7-10-25(11-8-16)18(21)12-15-4-3-9-23-14-15/h1-6,9,13-14,16,18,21H,7-8,10-12H2,(H,24,26)/t18-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50366779
(METHYLLYCACONITINE)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)cc(C)c3O)CC[C@H](OC)[C@@]34[C@@H]5C[C@H]6[C@H](OC)[C@@H]5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)[C@H]23)[C@H]14 |r,wU:4.4,48.54,44.48,42.46,36.39,39.43,wD:28.52,35.36,31.32,29.31,47.50,25.27,32.34,TLB:23:4:28:44.42,4:47:36.29.35:48,1:2:28:44.42,45:44:36.29.35:48,44:47:24.23.25:3.48.2,42:36:32:29.30,THB:37:36:32:29.30,40:39:32:29.30,5:4:28:44.42,(5.29,-1.99,;6.48,-1.02,;7.91,-1.57,;7.91,-4.71,;8.6,-3.19,;9.67,-4.27,;9.27,-5.75,;10.35,-6.84,;11.83,-6.45,;9.94,-8.32,;8.46,-8.7,;8.06,-10.18,;9.14,-11.27,;10.63,-10.87,;11.02,-9.39,;12.51,-8.99,;13.7,-9.95,;13.62,-11.48,;14.98,-9.12,;14.58,-7.64,;15.55,-6.45,;13.05,-7.56,;11.52,-7.55,;7.27,-2.43,;7.27,-.88,;8.6,-.11,;8.59,1.42,;7.27,2.19,;9.93,-.88,;11.13,.09,;10.97,1.62,;12.38,2.24,;13.41,1.1,;17.14,1.1,;17.91,-.25,;12.64,-.24,;13.31,-1.62,;14.39,-2.7,;15.18,-1.64,;15.11,2.18,;16.19,3.26,;15.8,4.74,;12.66,-3.02,;13.74,-4.1,;11.15,-3.38,;10.82,-4.87,;12.29,-5.26,;9.94,-2.42,;9.88,1.15,)| Show InChI InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,14,21-22,24-25,27-30,33,40-41,43-44H,7,12-13,15-18H2,1-6H3/t21-,22-,24+,25+,27-,28+,29-,30+,33-,34+,35-,36+,37+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllycaconitine from human alpha-7 in tsA201 cells coexpressed with 5HT3A receptor |
J Med Chem 50: 4616-29 (2007)
Article DOI: 10.1021/jm070574f
BindingDB Entry DOI: 10.7270/Q2J67HRC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399781
(CHEMBL2179873)Show SMILES Brc1ccc(s1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:9.9,wD:16.19,(21.88,-19.63,;20.34,-19.62,;19.44,-18.37,;17.98,-18.84,;17.97,-20.37,;19.43,-20.86,;16.82,-21.41,;16.81,-22.95,;15.48,-20.63,;14.15,-21.4,;12.82,-20.62,;11.49,-21.4,;11.49,-22.94,;12.82,-23.7,;12.03,-22.36,;13.41,-21.86,;14.15,-22.94,;15.48,-23.71,;15.48,-25.25,;14.14,-26.01,;14.14,-27.55,;15.47,-28.33,;16.81,-27.55,;16.81,-26.01,)| Show InChI InChI=1S/C18H20BrN3OS/c19-16-4-3-15(24-16)18(23)21-17-13-5-8-22(9-6-13)14(17)10-12-2-1-7-20-11-12/h1-4,7,11,13-14,17H,5-6,8-10H2,(H,21,23)/t14-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50173945
(CHEMBL195190 | [2,2']Bithiophenyl-5-carboxylic aci...)Show SMILES O=C(NC1CN2CCC1CC2)c1ccc(s1)-c1cccs1 |THB:2:3:9.10:7.6,(5.22,-2.93,;4.66,-4.37,;3.13,-4.59,;2.18,-3.38,;1.89,-1.97,;.55,-1.36,;.61,.27,;1.06,-.84,;.8,-2.74,;-.74,-3.4,;-.94,-2.02,;5.62,-5.57,;5.2,-7.07,;6.49,-7.93,;7.69,-6.97,;7.16,-5.52,;9.18,-7.39,;9.64,-8.87,;11.18,-8.87,;11.66,-7.41,;10.43,-6.49,)| Show InChI InChI=1S/C16H18N2OS2/c19-16(17-12-10-18-7-5-11(12)6-8-18)15-4-3-14(21-15)13-2-1-9-20-13/h1-4,9,11-12H,5-8,10H2,(H,17,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human nicotinic acetylcholine receptor alpha 7 expressed in GH4C1 cell using [3H]methyllycaconitine |
Bioorg Med Chem Lett 15: 4727-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.070
BindingDB Entry DOI: 10.7270/Q28K79W0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50398820

(CHEMBL2177538)Show InChI InChI=1S/C13H16N2/c1-2-12(9-14-3-1)13-5-10-4-11(6-13)8-15-7-10/h1-3,5,9-11,15H,4,6-8H2/t10-,11+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha7 nAChR expressed in human HEK/RIC3 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399776
(CHEMBL2179878)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1)c1ccc(s1)-c1cccs1 |r,wU:3.2,wD:10.12,(34.31,-36.29,;34.32,-34.75,;32.99,-33.98,;31.65,-34.74,;30.32,-33.97,;28.99,-34.74,;28.99,-36.28,;30.32,-37.05,;29.54,-35.71,;30.91,-35.21,;31.65,-36.28,;32.98,-37.06,;32.98,-38.6,;31.64,-39.36,;31.64,-40.9,;32.97,-41.67,;34.31,-40.9,;34.31,-39.36,;35.47,-33.72,;35.48,-32.19,;36.94,-31.72,;37.84,-32.96,;36.93,-34.2,;39.38,-32.97,;40.29,-31.73,;41.75,-32.21,;41.74,-33.75,;40.27,-34.22,)| Show InChI InChI=1S/C22H23N3OS2/c26-22(20-6-5-19(28-20)18-4-2-12-27-18)24-21-16-7-10-25(11-8-16)17(21)13-15-3-1-9-23-14-15/h1-6,9,12,14,16-17,21H,7-8,10-11,13H2,(H,24,26)/t17-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50206243

(CHEMBL3918431)Show SMILES C1N=C(Nc2cc3ccccc3cn2)O[C@]11CN2CCC1CC2 |r,wU:15.16,t:1,TLB:14:15:18.19:22.21,THB:0:15:18.19:22.21,(17.63,-8.02,;19,-7.33,;18.76,-5.81,;19.85,-4.72,;21.34,-5.11,;22.41,-4.02,;23.9,-4.41,;24.98,-3.32,;26.47,-3.71,;26.88,-5.2,;25.79,-6.3,;24.3,-5.9,;23.22,-7,;21.73,-6.6,;17.24,-5.56,;16.54,-6.93,;15.8,-8.21,;14.52,-7.62,;14.52,-5.71,;15.36,-4.63,;15.36,-6.25,;13.79,-6.93,;13,-8.11,)| Show InChI InChI=1S/C18H20N4O/c1-2-4-14-10-19-16(9-13(14)3-1)21-17-20-11-18(23-17)12-22-7-5-15(18)6-8-22/h1-4,9-10,15H,5-8,11-12H2,(H,19,20,21)/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [125I]alpha-bungarotoxin from human alpha7 nAChR expressed in HEK293 cell membranes incubated for 2 hrs and measured by gamma countin... |
ACS Med Chem Lett 8: 366-371 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00032
BindingDB Entry DOI: 10.7270/Q2S46V8B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50398821
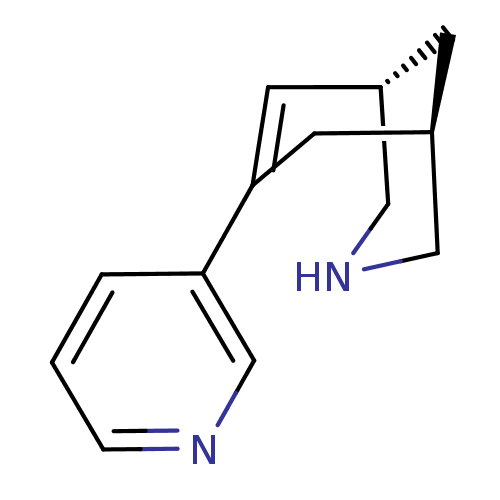
(CHEMBL2177537)Show InChI InChI=1S/C13H16N2/c1-2-12(9-14-3-1)13-5-10-4-11(6-13)8-15-7-10/h1-3,5,9-11,15H,4,6-8H2/t10-,11+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha7 nAChR expressed in human HEK/RIC3 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50173953
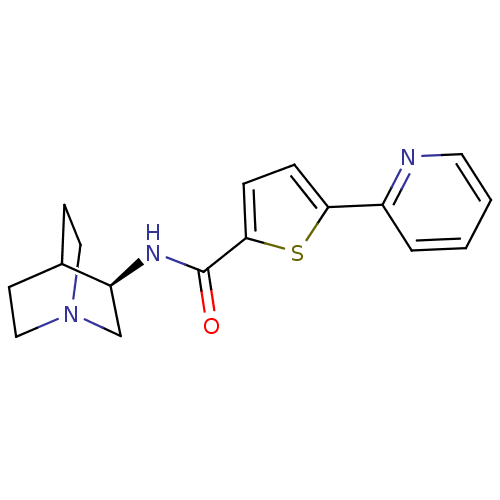
(CHEMBL364069 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc(s1)-c1ccccn1 |wD:3.2,THB:2:3:9.10:7.6,(3.4,-.23,;2.83,-1.67,;1.29,-1.88,;.35,-.66,;.05,.74,;-1.29,1.35,;-1.23,2.99,;-.77,1.87,;-1.02,-.03,;-2.57,-.69,;-2.78,.69,;3.79,-2.88,;3.37,-4.36,;4.66,-5.22,;5.86,-4.27,;5.32,-2.82,;7.35,-4.69,;8.45,-3.6,;9.93,-4.01,;10.32,-5.52,;9.22,-6.6,;7.73,-6.18,)| Show InChI InChI=1S/C17H19N3OS/c21-17(19-14-11-20-9-6-12(14)7-10-20)16-5-4-15(22-16)13-3-1-2-8-18-13/h1-5,8,12,14H,6-7,9-11H2,(H,19,21)/t14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human nicotinic acetylcholine receptor alpha 7 expressed in GH4C1 cell using [3H]methyllycaconitine |
Bioorg Med Chem Lett 15: 4727-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.070
BindingDB Entry DOI: 10.7270/Q28K79W0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50398849
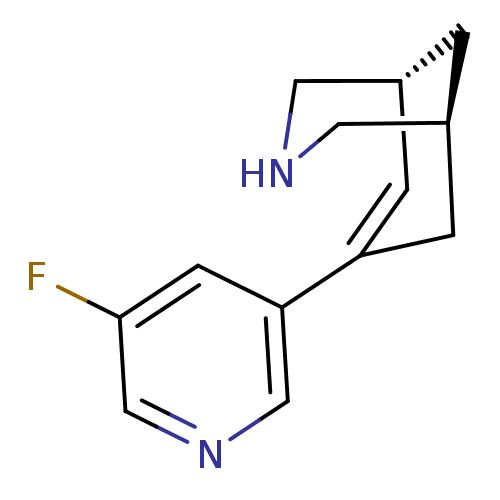
(CHEMBL2177553)Show SMILES Fc1cncc(c1)C1=C[C@@H]2CNC[C@@H](C2)C1 |r,t:8| Show InChI InChI=1S/C13H15FN2/c14-13-4-12(7-16-8-13)11-2-9-1-10(3-11)6-15-5-9/h2,4,7-10,15H,1,3,5-6H2/t9-,10+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha7 nAChR expressed in human HEK/RIC3 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50397944
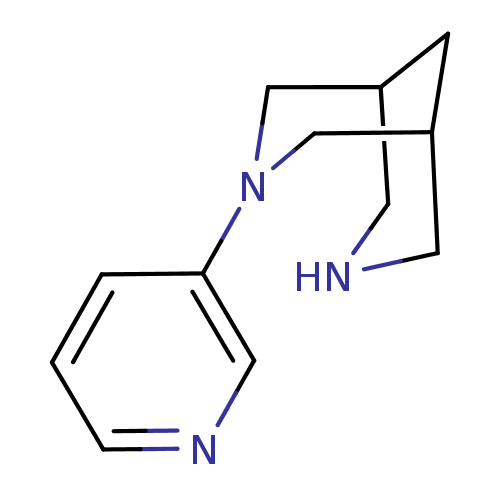
(CHEMBL2177512)Show InChI InChI=1S/C12H17N3/c1-2-12(7-13-3-1)15-8-10-4-11(9-15)6-14-5-10/h1-3,7,10-11,14H,4-6,8-9H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha7 nAChR expressed in human HEK/RIC3 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50190788
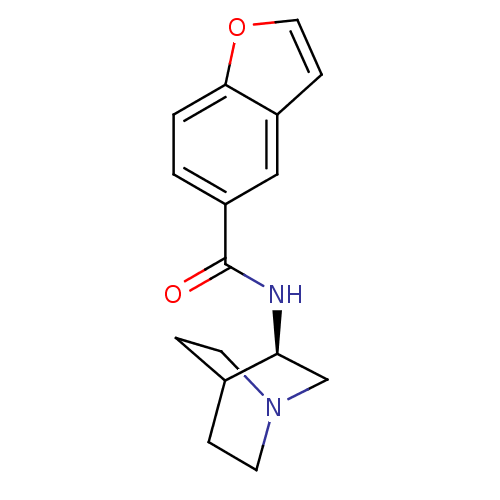
(CHEMBL378471 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2occc2c1 |wU:3.2,(-3.91,-15.15,;-3.91,-16.69,;-2.57,-17.45,;-1.24,-16.67,;-1.25,-15.14,;.09,-14.37,;1.43,-15.13,;1.43,-16.67,;.1,-17.45,;-.78,-16.3,;.04,-15.5,;-5.24,-17.46,;-5.23,-19.02,;-6.57,-19.79,;-7.91,-19.02,;-9.37,-19.49,;-10.28,-18.24,;-9.37,-17,;-7.9,-17.48,;-6.58,-16.7,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to alpha7 nAChR |
J Med Chem 54: 7943-61 (2011)
Article DOI: 10.1021/jm2007672
BindingDB Entry DOI: 10.7270/Q29P32QP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399767
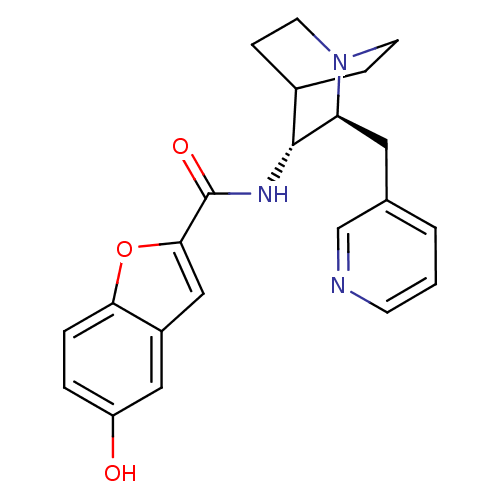
(CHEMBL2180253)Show SMILES Oc1ccc2oc(cc2c1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:13.14,wD:20.24,(39.86,-14.21,;39.23,-15.62,;40.13,-16.87,;39.49,-18.26,;37.97,-18.41,;37.07,-19.65,;35.61,-19.17,;35.61,-17.63,;37.08,-17.16,;37.71,-15.77,;34.45,-20.2,;34.45,-21.74,;33.12,-19.43,;31.78,-20.19,;30.45,-19.41,;29.12,-20.19,;29.12,-21.74,;30.45,-22.5,;29.66,-21.16,;31.04,-20.66,;31.78,-21.74,;33.12,-22.51,;33.11,-24.05,;31.77,-24.82,;31.77,-26.36,;33.11,-27.13,;34.45,-26.36,;34.45,-24.82,)| Show InChI InChI=1S/C22H23N3O3/c26-17-3-4-19-16(11-17)12-20(28-19)22(27)24-21-15-5-8-25(9-6-15)18(21)10-14-2-1-7-23-13-14/h1-4,7,11-13,15,18,21,26H,5-6,8-10H2,(H,24,27)/t18-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50173956

(CHEMBL370535 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc(s1)-c1ccccc1 |wD:3.2,THB:2:3:9.10:7.6,(.02,-.24,;-.55,-1.68,;-2.07,-1.9,;-3.03,-.69,;-3.31,.72,;-4.67,1.32,;-4.6,2.96,;-4.14,1.85,;-4.4,-.05,;-5.94,-.71,;-6.14,.67,;.41,-2.88,;-.02,-4.38,;1.27,-5.24,;2.49,-4.28,;1.95,-2.83,;3.96,-4.7,;3.96,-6.23,;5.29,-7,;6.62,-6.24,;6.64,-4.7,;5.29,-3.93,)| Show InChI InChI=1S/C18H20N2OS/c21-18(19-15-12-20-10-8-13(15)9-11-20)17-7-6-16(22-17)14-4-2-1-3-5-14/h1-7,13,15H,8-12H2,(H,19,21)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human nicotinic acetylcholine receptor alpha 7 expressed in GH4C1 cell using [3H]methyllycaconitine |
Bioorg Med Chem Lett 15: 4727-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.070
BindingDB Entry DOI: 10.7270/Q28K79W0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50173944
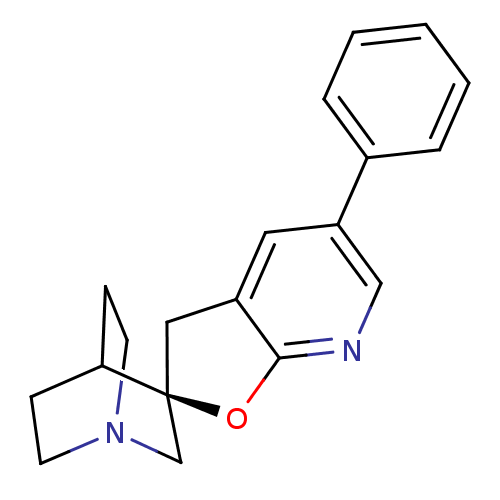
(5'-phenyl-(2'R)-spiro[4-azabicyclo[2.2.2]octane-2,...)Show SMILES C1c2cc(cnc2O[C@]11CN2CCC1CC2)-c1ccccc1 |wD:8.8,TLB:7:8:14.15:12.11,THB:0:8:14.15:12.11,(2.37,-1.18,;2.91,-2.63,;4.33,-3.19,;4.54,-4.71,;3.34,-5.67,;1.92,-5.1,;1.69,-3.58,;.41,-2.72,;.83,-1.24,;.55,.16,;-.81,.77,;-2.28,.12,;-2.08,-1.26,;-.54,-.59,;-.28,1.3,;-.74,2.41,;5.99,-5.27,;7.18,-4.31,;8.61,-4.87,;8.85,-6.41,;7.63,-7.37,;6.2,-6.8,)| Show InChI InChI=1S/C19H20N2O/c1-2-4-14(5-3-1)16-10-15-11-19(22-18(15)20-12-16)13-21-8-6-17(19)7-9-21/h1-5,10,12,17H,6-9,11,13H2/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human alpha7 nAChR |
Bioorg Med Chem Lett 27: 5002-5005 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.009
BindingDB Entry DOI: 10.7270/Q24Q7XJ3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM139032
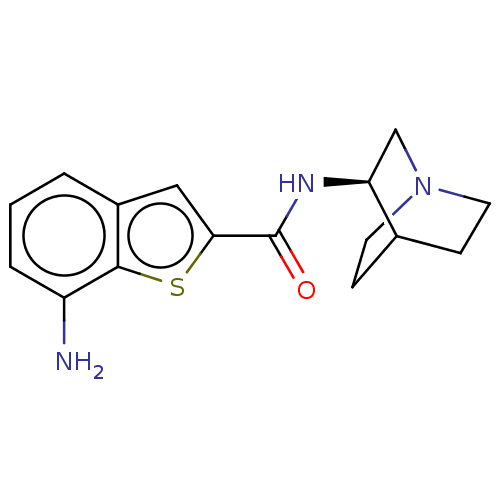
(US8884017, 68)Show SMILES Nc1cccc2cc(sc12)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:13.14,(5.71,-.87,;4.94,.47,;5.71,1.8,;4.94,3.13,;3.4,3.13,;2.63,1.8,;1.12,1.48,;.96,-.05,;2.37,-.68,;3.4,.47,;-.37,-.82,;-.37,-2.36,;-1.71,-.05,;-3.04,-.82,;-3.04,-2.36,;-4.37,-3.13,;-5.71,-2.36,;-5.71,-.82,;-4.37,-.05,;-3.6,-1.39,;-5.14,-1.8,)| Show InChI InChI=1S/C16H19N3OS/c17-12-3-1-2-11-8-14(21-15(11)12)16(20)18-13-9-19-6-4-10(13)5-7-19/h1-3,8,10,13H,4-7,9,17H2,(H,18,20)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2 | -11.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
The [3H]-methyllycaconitine binding assay is a modification of the method described by Davies et al. in Neuropharmacol. 1999, 38, 679-690.Rat brain t... |
US Patent US8884017 (2014)
BindingDB Entry DOI: 10.7270/Q2MK6BK3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50190785
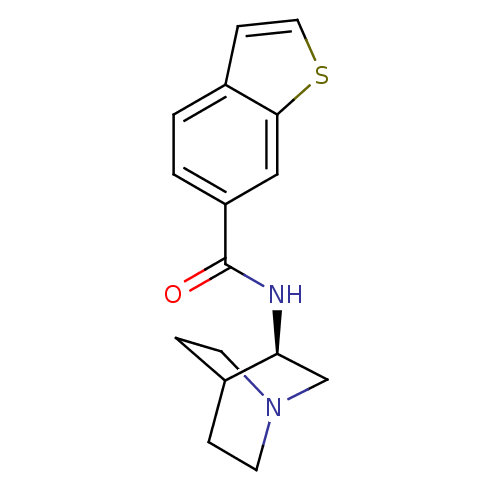
(CHEMBL378349 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccsc2c1 |wU:3.2,(28.02,-3.67,;28.02,-5.21,;29.36,-5.97,;30.69,-5.19,;30.68,-3.66,;32.02,-2.89,;33.36,-3.65,;33.36,-5.2,;32.03,-5.97,;31.15,-4.82,;31.97,-4.02,;26.69,-5.98,;26.7,-7.54,;25.35,-8.31,;24.02,-7.54,;22.56,-8.01,;21.65,-6.76,;22.56,-5.52,;24.03,-6,;25.35,-5.22,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to alpha7 nAChR |
J Med Chem 54: 7943-61 (2011)
Article DOI: 10.1021/jm2007672
BindingDB Entry DOI: 10.7270/Q29P32QP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399773

(CHEMBL2179881)Show SMILES COc1ccc2oc(cc2c1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:14.15,wD:21.25,(40.87,-44.06,;39.33,-44.22,;38.7,-45.63,;39.6,-46.88,;38.97,-48.27,;37.44,-48.42,;36.54,-49.66,;35.08,-49.18,;35.09,-47.64,;36.55,-47.17,;37.18,-45.78,;33.93,-50.21,;33.92,-51.75,;32.59,-49.44,;31.25,-50.2,;29.92,-49.42,;28.59,-50.2,;28.59,-51.75,;29.92,-52.51,;29.14,-51.17,;30.52,-50.67,;31.25,-51.75,;32.59,-52.52,;32.59,-54.06,;31.25,-54.83,;31.24,-56.37,;32.58,-57.15,;33.92,-56.37,;33.92,-54.83,)| Show InChI InChI=1S/C23H25N3O3/c1-28-18-4-5-20-17(12-18)13-21(29-20)23(27)25-22-16-6-9-26(10-7-16)19(22)11-15-3-2-8-24-14-15/h2-5,8,12-14,16,19,22H,6-7,9-11H2,1H3,(H,25,27)/t19-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]alpha-bungarotoxin from human alpha7 nAChR expressed in K28 cell membrane |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115820
BindingDB Entry DOI: 10.7270/Q2WW7N96 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399770

(CHEMBL2180250)Show SMILES Cc1c(oc2ccccc12)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:13.14,wD:20.24,(33.38,-1.89,;34.63,-2.8,;34.62,-4.34,;36.08,-4.82,;36.98,-3.58,;38.5,-3.43,;39.14,-2.04,;38.24,-.79,;36.72,-.94,;36.09,-2.33,;33.47,-5.37,;33.46,-6.91,;32.13,-4.6,;30.79,-5.36,;29.46,-4.59,;28.13,-5.36,;28.13,-6.91,;29.46,-7.67,;28.68,-6.33,;30.06,-5.83,;30.79,-6.91,;32.13,-7.68,;32.13,-9.22,;30.79,-9.99,;30.79,-11.53,;32.12,-12.3,;33.46,-11.53,;33.46,-9.99,)| Show InChI InChI=1S/C23H25N3O2/c1-15-18-6-2-3-7-20(18)28-22(15)23(27)25-21-17-8-11-26(12-9-17)19(21)13-16-5-4-10-24-14-16/h2-7,10,14,17,19,21H,8-9,11-13H2,1H3,(H,25,27)/t19-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50433470
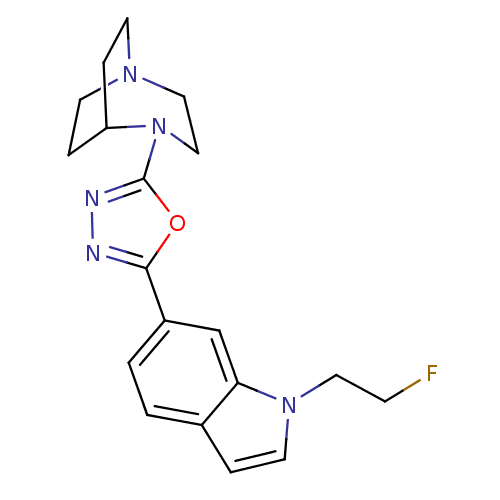
(CHEMBL2380928)Show SMILES FCCn1ccc2ccc(cc12)-c1nnc(o1)N1CCN2CCC1CC2 |(20.47,-8.19,;19.69,-9.52,;18.15,-9.5,;17.39,-8.17,;18.31,-6.92,;17.41,-5.67,;15.94,-6.14,;14.6,-5.37,;13.27,-6.14,;13.27,-7.67,;14.6,-8.44,;15.93,-7.68,;11.94,-8.44,;11.46,-9.91,;9.92,-9.91,;9.44,-8.44,;10.69,-7.53,;7.97,-7.96,;7.76,-6.43,;6.44,-5.64,;4.99,-6.19,;4.52,-7.65,;5.37,-8.93,;6.91,-9.07,;5.9,-8.06,;6.43,-7.06,)| Show InChI InChI=1S/C19H22FN5O/c20-6-10-24-9-3-14-1-2-15(13-17(14)24)18-21-22-19(26-18)25-12-11-23-7-4-16(25)5-8-23/h1-3,9,13,16H,4-8,10-12H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyllycaconitine from human alpha7 nAChR transfected in human SH-SY5Y cells after 240 mins by liquid scintillation counting an... |
Bioorg Med Chem 21: 2635-42 (2013)
Article DOI: 10.1016/j.bmc.2013.02.018
BindingDB Entry DOI: 10.7270/Q28K7BFK |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50417105
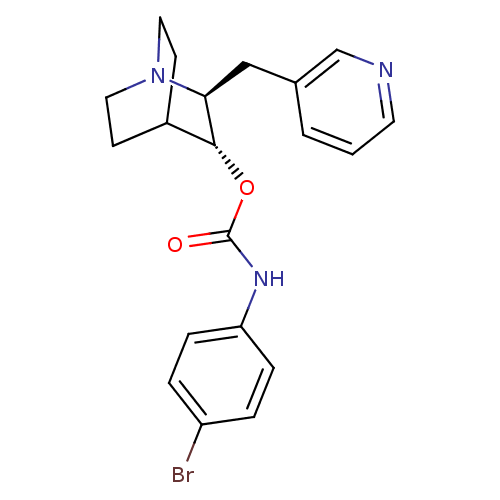
(CHEMBL1258240)Show SMILES Brc1ccc(NC(=O)O[C@@H]2C3CCN(CC3)[C@H]2Cc2cccnc2)cc1 |r,wU:9.8,wD:16.18,TLB:17:16:12.11:14.15,THB:8:9:12.11:14.15,(27.75,3.41,;26.42,4.2,;25.08,3.44,;23.76,4.23,;23.78,5.77,;22.46,6.56,;21.11,5.81,;21.09,4.27,;19.79,6.6,;18.44,5.84,;16.97,6.48,;15.62,5.88,;15.35,4.48,;16.71,5.11,;16.46,7.02,;16.9,8.14,;18.25,4.46,;19.01,3.12,;18.23,1.79,;16.69,1.81,;15.91,.49,;16.66,-.85,;18.21,-.86,;18.99,.46,;25.12,6.53,;26.44,5.74,)| Show InChI InChI=1S/C20H22BrN3O2/c21-16-3-5-17(6-4-16)23-20(25)26-19-15-7-10-24(11-8-15)18(19)12-14-2-1-9-22-13-14/h1-6,9,13,15,18-19H,7-8,10-12H2,(H,23,25)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MLA from human alpha7 nAChR expressed in human SH-SY5Y cells |
J Med Chem 53: 7192-201 (2010)
Article DOI: 10.1021/jm100834y
BindingDB Entry DOI: 10.7270/Q21J9C1V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from alpha7 nAChR (unknown origin) expressed in HEK cell membranes after 4 hrs by liquid scintillation counting metho... |
J Nat Prod 81: 1029-1035 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00062
BindingDB Entry DOI: 10.7270/Q2R49TF0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393247

(CHEMBL1258006)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1)c1cc2ccccc2o1 |r,wU:3.2,wD:10.12,TLB:11:10:6.5:8.9,THB:2:3:6.5:8.9,(5.71,-17.16,;5.73,-15.62,;4.4,-14.83,;3.06,-15.59,;1.59,-14.95,;.24,-15.55,;-.04,-16.95,;1.33,-16.32,;1.07,-14.41,;1.52,-13.3,;2.87,-16.97,;3.63,-18.31,;2.84,-19.64,;1.3,-19.62,;.52,-20.94,;1.28,-22.29,;2.83,-22.3,;3.61,-20.97,;7.07,-14.87,;7.24,-13.35,;8.76,-13.04,;9.54,-11.72,;11.07,-11.74,;11.83,-13.08,;11.04,-14.41,;9.51,-14.39,;8.47,-15.52,)| Show InChI InChI=1S/C22H23N3O2/c26-22(20-13-17-5-1-2-6-19(17)27-20)24-21-16-7-10-25(11-8-16)18(21)12-15-4-3-9-23-14-15/h1-6,9,13-14,16,18,21H,7-8,10-12H2,(H,24,26)/t18-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MLA from human alpha7 nAChR expressed in human SH-SY5Y cells |
J Med Chem 53: 7192-201 (2010)
Article DOI: 10.1021/jm100834y
BindingDB Entry DOI: 10.7270/Q21J9C1V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399792
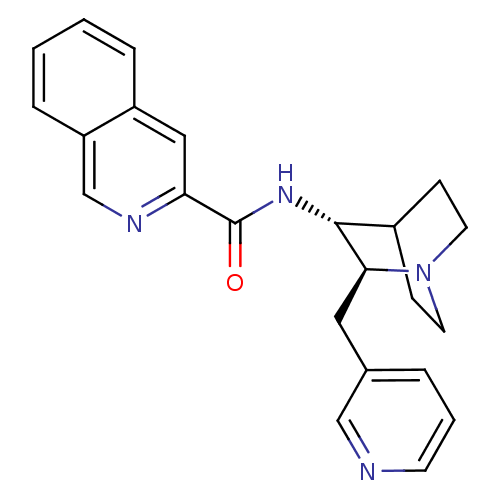
(CHEMBL2179862)Show SMILES O=C(N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1)c1cc2ccccc2cn1 |r,wU:3.2,wD:10.12,(41.77,-32.17,;41.78,-30.63,;40.44,-29.86,;39.11,-30.62,;37.78,-29.84,;36.45,-30.62,;36.45,-32.16,;37.78,-32.92,;36.99,-31.59,;38.37,-31.09,;39.11,-32.16,;40.44,-32.93,;40.44,-34.47,;39.1,-35.24,;39.1,-36.78,;40.43,-37.55,;41.77,-36.78,;41.77,-35.24,;43.11,-29.86,;43.11,-28.33,;44.44,-27.56,;44.44,-26.03,;45.77,-25.26,;47.11,-26.03,;47.1,-27.57,;45.77,-28.34,;45.77,-29.88,;44.44,-30.64,)| Show InChI InChI=1S/C23H24N4O/c28-23(20-13-18-5-1-2-6-19(18)15-25-20)26-22-17-7-10-27(11-8-17)21(22)12-16-4-3-9-24-14-16/h1-6,9,13-15,17,21-22H,7-8,10-12H2,(H,26,28)/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM139033

(US8884017, 73)Show SMILES Nc1ccc2cc(sc2c1)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:13.14,(6.48,1.8,;4.94,1.8,;4.17,3.13,;2.63,3.13,;1.86,1.8,;.35,1.48,;.19,-.05,;1.6,-.68,;2.63,.47,;4.17,.47,;-1.14,-.82,;-1.14,-2.36,;-2.48,-.05,;-3.81,-.82,;-3.81,-2.36,;-5.14,-3.13,;-6.48,-2.36,;-6.48,-.82,;-5.14,-.05,;-4.37,-1.39,;-5.86,-1.78,)| Show InChI InChI=1S/C16H19N3OS/c17-12-2-1-11-7-15(21-14(11)8-12)16(20)18-13-9-19-5-3-10(13)4-6-19/h1-2,7-8,10,13H,3-6,9,17H2,(H,18,20)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.80 | -10.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
The [3H]-methyllycaconitine binding assay is a modification of the method described by Davies et al. in Neuropharmacol. 1999, 38, 679-690.Rat brain t... |
US Patent US8884017 (2014)
BindingDB Entry DOI: 10.7270/Q2MK6BK3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393249
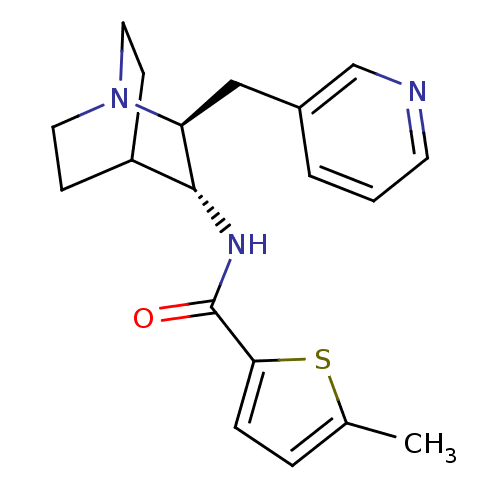
(CHEMBL2151441)Show SMILES Cc1ccc(s1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:9.9,wD:16.19,(5.74,.65,;4.2,.81,;3.44,2.15,;1.93,1.84,;1.77,.31,;3.17,-.33,;.43,-.46,;.43,-2,;-.9,.32,;-2.23,-.45,;-3.56,.33,;-4.89,-.44,;-4.89,-1.98,;-3.56,-2.75,;-4.3,-1.39,;-2.81,-1,;-2.23,-1.98,;-.9,-2.76,;-.9,-4.3,;-2.24,-5.06,;-2.24,-6.6,;-.91,-7.37,;.43,-6.6,;.43,-5.06,)| Show InChI InChI=1S/C19H23N3OS/c1-13-4-5-17(24-13)19(23)21-18-15-6-9-22(10-7-15)16(18)11-14-3-2-8-20-12-14/h2-5,8,12,15-16,18H,6-7,9-11H2,1H3,(H,21,23)/t16-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393243

(CHEMBL2151437)Show SMILES C1c2cccnc2O[C@]11CN2CCC1CC2 |r,wU:8.8,(7.8,-.19,;8.94,.84,;10.46,.68,;11.37,1.92,;10.74,3.32,;9.22,3.48,;8.32,2.25,;6.79,2.09,;6.47,.58,;6.47,-.96,;5.13,-1.72,;3.8,-.96,;3.8,.59,;5.13,1.37,;5.89,.03,;4.39,-.37,)| Show InChI InChI=1S/C13H16N2O/c1-2-10-8-13(16-12(10)14-5-1)9-15-6-3-11(13)4-7-15/h1-2,5,11H,3-4,6-9H2/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]alpha-bungarotoxin from human alpha7 nAChR expressed in HEK293 cell membranes after 2 hrs by topcount scintillation counting an... |
Bioorg Med Chem Lett 27: 5002-5005 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.009
BindingDB Entry DOI: 10.7270/Q24Q7XJ3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393243

(CHEMBL2151437)Show SMILES C1c2cccnc2O[C@]11CN2CCC1CC2 |r,wU:8.8,(7.8,-.19,;8.94,.84,;10.46,.68,;11.37,1.92,;10.74,3.32,;9.22,3.48,;8.32,2.25,;6.79,2.09,;6.47,.58,;6.47,-.96,;5.13,-1.72,;3.8,-.96,;3.8,.59,;5.13,1.37,;5.89,.03,;4.39,-.37,)| Show InChI InChI=1S/C13H16N2O/c1-2-10-8-13(16-12(10)14-5-1)9-15-6-3-11(13)4-7-15/h1-2,5,11H,3-4,6-9H2/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human alpha7 nAchR |
J Med Chem 54: 7943-61 (2011)
Article DOI: 10.1021/jm2007672
BindingDB Entry DOI: 10.7270/Q29P32QP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50173944

(5'-phenyl-(2'R)-spiro[4-azabicyclo[2.2.2]octane-2,...)Show SMILES C1c2cc(cnc2O[C@]11CN2CCC1CC2)-c1ccccc1 |wD:8.8,TLB:7:8:14.15:12.11,THB:0:8:14.15:12.11,(2.37,-1.18,;2.91,-2.63,;4.33,-3.19,;4.54,-4.71,;3.34,-5.67,;1.92,-5.1,;1.69,-3.58,;.41,-2.72,;.83,-1.24,;.55,.16,;-.81,.77,;-2.28,.12,;-2.08,-1.26,;-.54,-.59,;-.28,1.3,;-.74,2.41,;5.99,-5.27,;7.18,-4.31,;8.61,-4.87,;8.85,-6.41,;7.63,-7.37,;6.2,-6.8,)| Show InChI InChI=1S/C19H20N2O/c1-2-4-14(5-3-1)16-10-15-11-19(22-18(15)20-12-16)13-21-8-6-17(19)7-9-21/h1-5,10,12,17H,6-9,11,13H2/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human nicotinic acetylcholine receptor alpha 7 expressed in GH4C1 cell using [3H]methyllycaconitine |
Bioorg Med Chem Lett 15: 4727-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.070
BindingDB Entry DOI: 10.7270/Q28K79W0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM139027
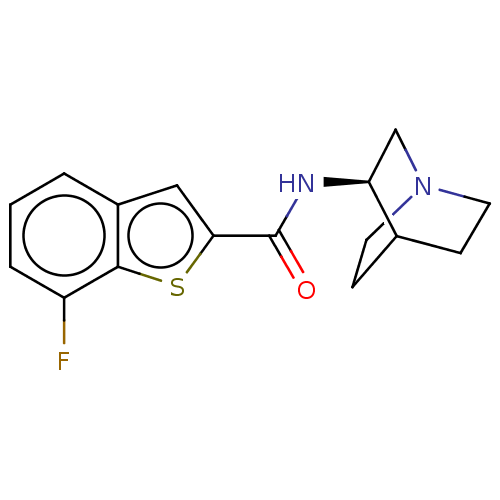
(US8884017, 58)Show SMILES Fc1cccc2cc(sc12)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:13.14,(5.71,-.87,;4.94,.47,;5.71,1.8,;4.94,3.13,;3.4,3.13,;2.63,1.8,;1.12,1.48,;.96,-.05,;2.37,-.68,;3.4,.47,;-.37,-.82,;-.37,-2.36,;-1.71,-.05,;-3.04,-.82,;-3.04,-2.36,;-4.37,-3.13,;-5.71,-2.36,;-5.71,-.82,;-4.37,-.05,;-3.6,-1.39,;-5.14,-1.8,)| Show InChI InChI=1S/C16H17FN2OS/c17-12-3-1-2-11-8-14(21-15(11)12)16(20)18-13-9-19-6-4-10(13)5-7-19/h1-3,8,10,13H,4-7,9H2,(H,18,20)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3.10 | -10.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
The [3H]-methyllycaconitine binding assay is a modification of the method described by Davies et al. in Neuropharmacol. 1999, 38, 679-690.Rat brain t... |
US Patent US8884017 (2014)
BindingDB Entry DOI: 10.7270/Q2MK6BK3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50309888
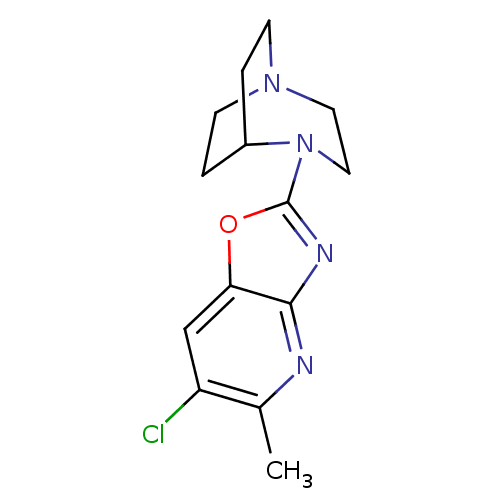
(4-(6-Chloro-5-methyloxazolo[4,5-b]pyridin-2-yl)-1,...)Show SMILES Cc1nc2nc(oc2cc1Cl)N1CCN2CCC1CC2 |TLB:5:11:16.15:18.19,(1.04,-10.22,;-.49,-10.46,;-1.05,-11.9,;-2.58,-12.13,;-3.43,-13.41,;-4.92,-13,;-4.98,-11.47,;-3.54,-10.92,;-2.99,-9.49,;-1.46,-9.25,;-.9,-7.79,;-6.25,-13.78,;-5.89,-15.24,;-6.89,-14.52,;-8.27,-14.59,;-8.34,-12.89,;-7.73,-11.74,;-7.66,-13.15,;-8.96,-13.86,;-9.26,-15.3,)| Show InChI InChI=1S/C14H17ClN4O/c1-9-11(15)8-12-13(16-9)17-14(20-12)19-7-6-18-4-2-10(19)3-5-18/h8,10H,2-7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to alpha7 nAChR |
J Med Chem 54: 7943-61 (2011)
Article DOI: 10.1021/jm2007672
BindingDB Entry DOI: 10.7270/Q29P32QP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50398850
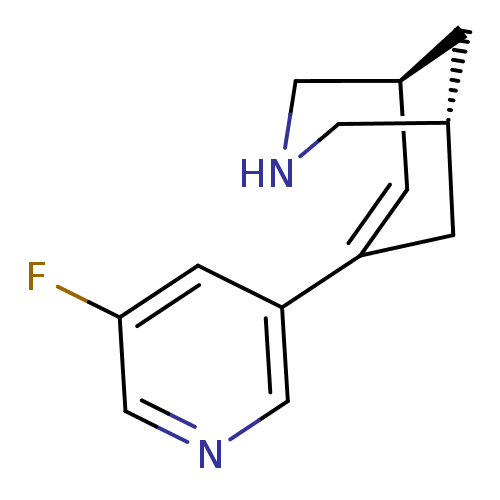
(CHEMBL2177552)Show SMILES Fc1cncc(c1)C1=C[C@H]2CNC[C@H](C2)C1 |r,t:8| Show InChI InChI=1S/C13H15FN2/c14-13-4-12(7-16-8-13)11-2-9-1-10(3-11)6-15-5-9/h2,4,7-10,15H,1,3,5-6H2/t9-,10+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha7 nAChR expressed in human HEK/RIC3 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50173942

(CHEMBL195329 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc(s1)-c1ccccc1 |wD:3.2,THB:2:3:9.10:7.6,(.22,-.91,;-.34,-2.35,;-1.87,-2.56,;-2.82,-1.34,;-3.11,.06,;-4.46,.67,;-4.4,2.31,;-3.94,1.19,;-4.2,-.71,;-5.74,-1.36,;-5.94,.02,;.62,-3.55,;.19,-5.03,;1.48,-5.9,;2.69,-4.94,;2.15,-3.49,;4.17,-5.36,;4.17,-6.88,;5.49,-7.67,;6.84,-6.91,;6.84,-5.36,;5.5,-4.59,)| Show InChI InChI=1S/C18H20N2OS/c21-18(19-15-12-20-10-8-13(15)9-11-20)17-7-6-16(22-17)14-4-2-1-3-5-14/h1-7,13,15H,8-12H2,(H,19,21)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human nicotinic acetylcholine receptor alpha 7 expressed in GH4C1 cell using [3H]methyllycaconitine |
Bioorg Med Chem Lett 15: 4727-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.070
BindingDB Entry DOI: 10.7270/Q28K79W0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50173943
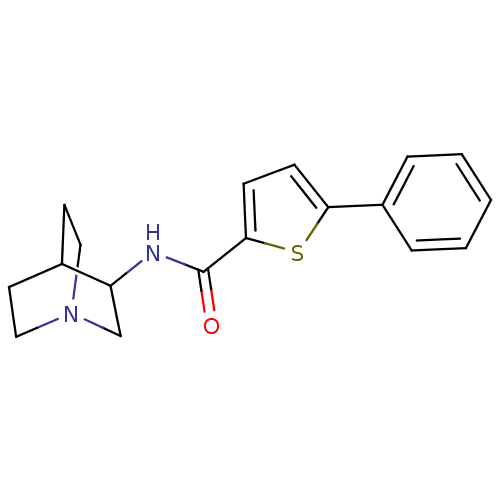
(5-Phenyl-thiophene-2-carboxylic acid (1-aza-bicycl...)Show SMILES O=C(NC1CN2CCC1CC2)c1ccc(s1)-c1ccccc1 |THB:2:3:9.10:7.6,(5.48,-.12,;4.92,-1.57,;3.39,-1.79,;2.44,-.57,;2.15,.83,;.8,1.44,;.87,3.08,;1.32,1.96,;1.06,.07,;-.48,-.59,;-.68,.79,;5.88,-2.77,;5.46,-4.26,;6.74,-5.12,;7.95,-4.16,;7.41,-2.71,;9.43,-4.58,;9.43,-6.11,;10.76,-6.88,;12.1,-6.13,;12.1,-4.58,;10.77,-3.81,)| Show InChI InChI=1S/C18H20N2OS/c21-18(19-15-12-20-10-8-13(15)9-11-20)17-7-6-16(22-17)14-4-2-1-3-5-14/h1-7,13,15H,8-12H2,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human nicotinic acetylcholine receptor alpha 7 expressed in GH4C1 cell using [3H]methyllycaconitine |
Bioorg Med Chem Lett 15: 4727-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.070
BindingDB Entry DOI: 10.7270/Q28K79W0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50398849

(CHEMBL2177553)Show SMILES Fc1cncc(c1)C1=C[C@@H]2CNC[C@@H](C2)C1 |r,t:8| Show InChI InChI=1S/C13H15FN2/c14-13-4-12(7-16-8-13)11-2-9-1-10(3-11)6-15-5-9/h2,4,7-10,15H,1,3,5-6H2/t9-,10+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha7 nAChR expressed in human HEK/RIC3 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM139020
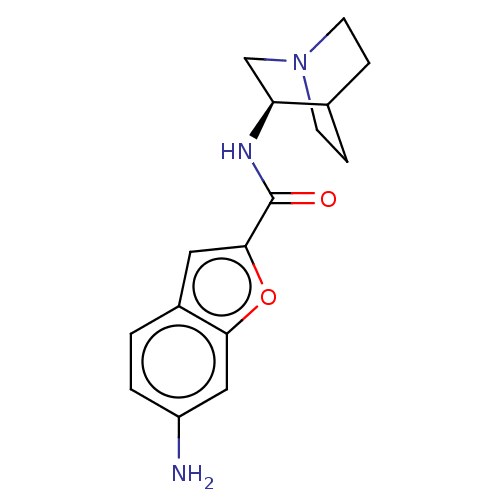
(US8884017, 35)Show SMILES Nc1ccc2cc(oc2c1)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:13.14,(6.48,1.8,;4.94,1.8,;4.17,3.13,;2.63,3.13,;1.86,1.8,;.35,1.48,;.19,-.05,;1.6,-.68,;2.63,.47,;4.17,.47,;-1.14,-.82,;-1.14,-2.36,;-2.48,-.05,;-3.81,-.82,;-3.81,-2.36,;-5.14,-3.13,;-6.48,-2.36,;-6.48,-.82,;-5.14,-.05,;-4.4,-1.27,;-5.91,-1.8,)| Show InChI InChI=1S/C16H19N3O2/c17-12-2-1-11-7-15(21-14(11)8-12)16(20)18-13-9-19-5-3-10(13)4-6-19/h1-2,7-8,10,13H,3-6,9,17H2,(H,18,20)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3.70 | -10.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
The [3H]-methyllycaconitine binding assay is a modification of the method described by Davies et al. in Neuropharmacol. 1999, 38, 679-690.Rat brain t... |
US Patent US8884017 (2014)
BindingDB Entry DOI: 10.7270/Q2MK6BK3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50399775
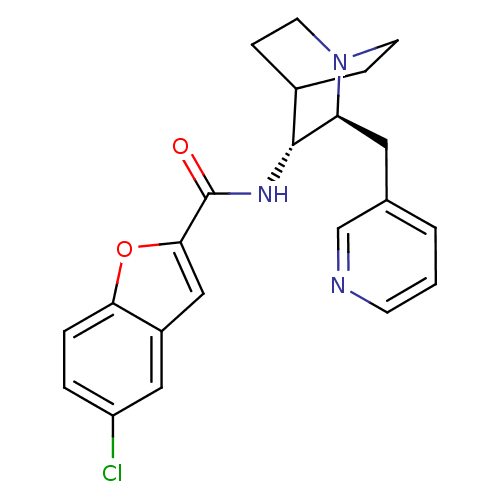
(CHEMBL2179879)Show SMILES Clc1ccc2oc(cc2c1)C(=O)N[C@@H]1C2CCN(CC2)[C@H]1Cc1cccnc1 |r,wU:13.14,wD:20.24,(12,-44.81,;11.37,-46.21,;12.27,-47.46,;11.63,-48.86,;10.11,-49.01,;9.21,-50.25,;7.75,-49.76,;7.75,-48.23,;9.22,-47.76,;9.85,-46.37,;6.59,-50.8,;6.59,-52.34,;5.26,-50.02,;3.92,-50.79,;2.59,-50.01,;1.26,-50.79,;1.26,-52.33,;2.59,-53.1,;1.8,-51.76,;3.18,-51.26,;3.92,-52.33,;5.25,-53.11,;5.25,-54.65,;3.91,-55.42,;3.91,-56.96,;5.25,-57.73,;6.59,-56.95,;6.58,-55.42,)| Show InChI InChI=1S/C22H22ClN3O2/c23-17-3-4-19-16(11-17)12-20(28-19)22(27)25-21-15-5-8-26(9-6-15)18(21)10-14-2-1-7-24-13-14/h1-4,7,11-13,15,18,21H,5-6,8-10H2,(H,25,27)/t18-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM139026

(US8884017, 51)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccccc2s1 |r,wU:3.2,(-.37,-2.36,;-.37,-.82,;-1.71,-.05,;-3.04,-.82,;-3.04,-2.36,;-4.37,-3.13,;-5.71,-2.36,;-5.71,-.82,;-4.37,-.05,;-3.6,-1.39,;-5.09,-1.78,;.96,-.05,;1.12,1.48,;2.63,1.8,;3.4,3.13,;4.94,3.13,;5.71,1.8,;4.94,.47,;3.4,.47,;2.37,-.68,)| Show InChI InChI=1S/C16H18N2OS/c19-16(15-9-12-3-1-2-4-14(12)20-15)17-13-10-18-7-5-11(13)6-8-18/h1-4,9,11,13H,5-8,10H2,(H,17,19)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3.90 | -10.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
The [3H]-methyllycaconitine binding assay is a modification of the method described by Davies et al. in Neuropharmacol. 1999, 38, 679-690.Rat brain t... |
US Patent US8884017 (2014)
BindingDB Entry DOI: 10.7270/Q2MK6BK3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data