 Found 256 hits of ki for UniProtKB: P00492
Found 256 hits of ki for UniProtKB: P00492 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50467705
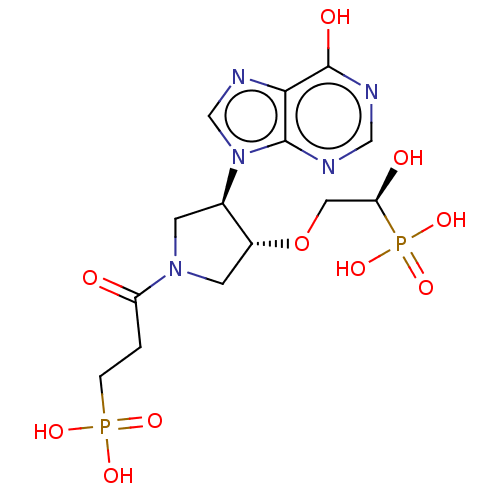
(CHEMBL4282830)Show SMILES O[C@H](CO[C@@H]1CN(C[C@H]1n1cnc2c(O)ncnc12)C(=O)CCP(O)(O)=O)P(O)(O)=O |r| Show InChI InChI=1S/C14H21N5O10P2/c20-10(1-2-30(23,24)25)18-3-8(9(4-18)29-5-11(21)31(26,27)28)19-7-17-12-13(19)15-6-16-14(12)22/h6-9,11,21H,1-5H2,(H,15,16,22)(H2,23,24,25)(H2,26,27,28)/t8-,9-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by spectrophotometric analysis |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50603703

(CHEMBL5184028)Show SMILES O[C@H](COC1CN(C[C@H]1n1cnc2c1nc[nH]c2=O)C(=O)CCP([O-])([O-])=O)P([O-])([O-])=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01881
BindingDB Entry DOI: 10.7270/Q25X2F0M |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50467705

(CHEMBL4282830)Show SMILES O[C@H](CO[C@@H]1CN(C[C@H]1n1cnc2c(O)ncnc12)C(=O)CCP(O)(O)=O)P(O)(O)=O |r| Show InChI InChI=1S/C14H21N5O10P2/c20-10(1-2-30(23,24)25)18-3-8(9(4-18)29-5-11(21)31(26,27)28)19-7-17-12-13(19)15-6-16-14(12)22/h6-9,11,21H,1-5H2,(H,15,16,22)(H2,23,24,25)(H2,26,27,28)/t8-,9-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by Hanes-plot based method |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519492
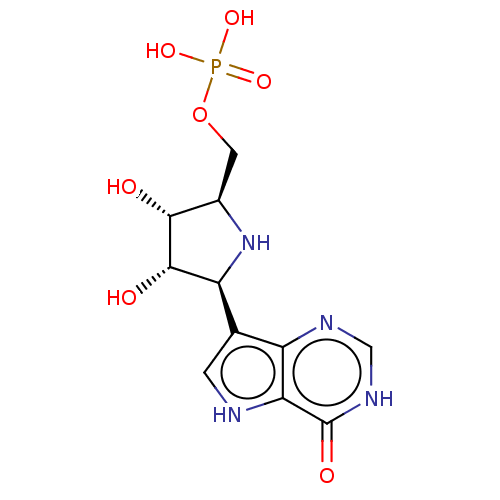
(CHEMBL1233663)Show SMILES O[C@@H]1[C@@H](COP(O)(O)=O)N[C@H]([C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H15N4O7P/c16-9-5(2-22-23(19,20)21)15-7(10(9)17)4-1-12-8-6(4)13-3-14-11(8)18/h1,3,5,7,9-10,12,15-17H,2H2,(H,13,14,18)(H2,19,20,21)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation using [5'-14C]IMP as substrate by scintilla... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50467710
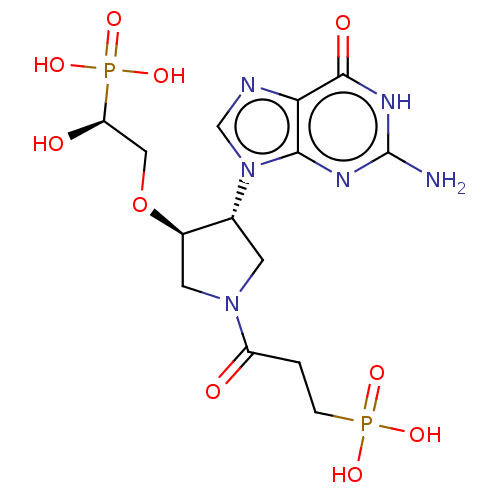
(CHEMBL4290716)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@H]1OC[C@@H](O)P(O)(O)=O)C(=O)CCP(O)(O)=O |r| Show InChI InChI=1S/C14H22N6O10P2/c15-14-17-12-11(13(23)18-14)16-6-20(12)7-3-19(9(21)1-2-31(24,25)26)4-8(7)30-5-10(22)32(27,28)29/h6-8,10,22H,1-5H2,(H2,24,25,26)(H2,27,28,29)(H3,15,17,18,23)/t7-,8-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by Hanes-plot based method |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50467710

(CHEMBL4290716)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@H]1OC[C@@H](O)P(O)(O)=O)C(=O)CCP(O)(O)=O |r| Show InChI InChI=1S/C14H22N6O10P2/c15-14-17-12-11(13(23)18-14)16-6-20(12)7-3-19(9(21)1-2-31(24,25)26)4-8(7)30-5-10(22)32(27,28)29/h6-8,10,22H,1-5H2,(H2,24,25,26)(H2,27,28,29)(H3,15,17,18,23)/t7-,8-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by Hanes plot analysis |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519500

(CHEMBL1233603)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16N5O7P/c12-11-15-5-3(1-13-7(5)10(19)16-11)6-9(18)8(17)4(14-6)2-23-24(20,21)22/h1,4,6,8-9,13-14,17-18H,2H2,(H2,20,21,22)(H3,12,15,16,19)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation using [5'-14C]IMP as substrate by scintilla... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50258940
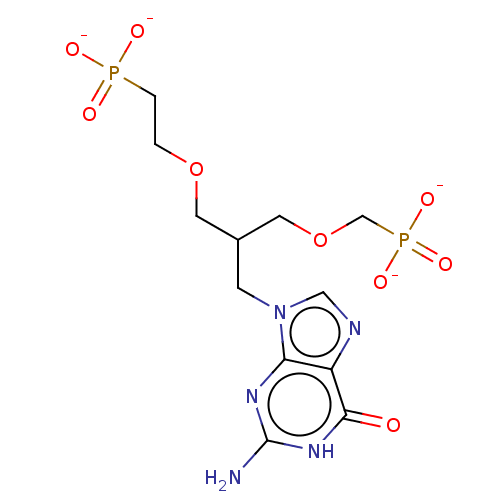
(CHEMBL4086362)Show SMILES [Na+].[Na+].[Na+].[Na+].Nc1nc2n(CC(COCCP([O-])([O-])=O)COCP([O-])([O-])=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C12H21N5O9P2.4Na/c13-12-15-10-9(11(18)16-12)14-6-17(10)3-8(5-26-7-28(22,23)24)4-25-1-2-27(19,20)21;;;;/h6,8H,1-5,7H2,(H2,19,20,21)(H2,22,23,24)(H3,13,15,16,18);;;;/q;4*+1/p-4 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using PRib-PP as the substrate by spectrophotometric method |
J Med Chem 60: 7539-7554 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00926
BindingDB Entry DOI: 10.7270/Q2J968T1 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50603704

(CHEMBL5207635)Show SMILES Nc1nc2n(C[C@@H](COCCOP([O-])([O-])=O)COCP([O-])([O-])=O)cnc2c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01881
BindingDB Entry DOI: 10.7270/Q25X2F0M |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50467712

(CHEMBL4285691)Show SMILES O[C@@H](CO[C@@H]1CN(C[C@H]1n1cnc2c(O)ncnc12)C(=O)CCP(O)(O)=O)P(O)(O)=O |r| Show InChI InChI=1S/C14H21N5O10P2/c20-10(1-2-30(23,24)25)18-3-8(9(4-18)29-5-11(21)31(26,27)28)19-7-17-12-13(19)15-6-16-14(12)22/h6-9,11,21H,1-5H2,(H,15,16,22)(H2,23,24,25)(H2,26,27,28)/t8-,9-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by spectrophotometric analysis |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50467708

(CHEMBL4277753)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@H]1OC[C@H](O)P(O)(O)=O)C(=O)CCP(O)(O)=O |r| Show InChI InChI=1S/C14H22N6O10P2/c15-14-17-12-11(13(23)18-14)16-6-20(12)7-3-19(9(21)1-2-31(24,25)26)4-8(7)30-5-10(22)32(27,28)29/h6-8,10,22H,1-5H2,(H2,24,25,26)(H2,27,28,29)(H3,15,17,18,23)/t7-,8-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by Hanes plot analysis |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50089450
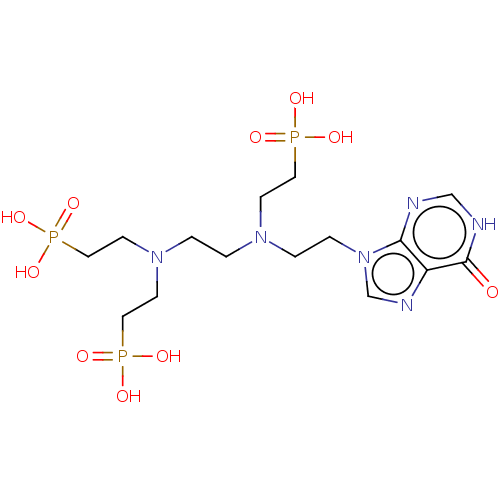
(CHEMBL3578115)Show SMILES OP(O)(=O)CCN(CCN(CCP(O)(O)=O)CCP(O)(O)=O)CCn1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C15H29N6O10P3/c22-15-13-14(16-11-17-15)21(12-18-13)4-3-19(5-8-32(23,24)25)1-2-20(6-9-33(26,27)28)7-10-34(29,30)31/h11-12H,1-10H2,(H,16,17,22)(H2,23,24,25)(H2,26,27,28)(H2,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 4822-38 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00611
BindingDB Entry DOI: 10.7270/Q2JH3NXV |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50603702

(CHEMBL5178774)Show SMILES [O-]P([O-])(=O)CCOC1CN(C[C@H]1n1cnc2c1nc[nH]c2=O)C(=O)CCP([O-])([O-])=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01881
BindingDB Entry DOI: 10.7270/Q25X2F0M |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50603705

(CHEMBL5198761)Show SMILES Nc1nc2n(CC(COCP([O-])([O-])=O)COCP([O-])([O-])=O)cnc2c(=O)[nH]1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01881
BindingDB Entry DOI: 10.7270/Q25X2F0M |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50427810
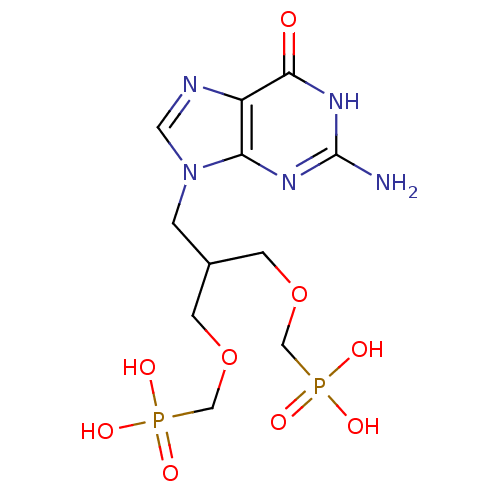
(CHEMBL2325752)Show SMILES Nc1nc2n(CC(COCP(O)(O)=O)COCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C11H19N5O9P2/c12-11-14-9-8(10(17)15-11)13-4-16(9)1-7(2-24-5-26(18,19)20)3-25-6-27(21,22)23/h4,7H,1-3,5-6H2,(H2,18,19,20)(H2,21,22,23)(H3,12,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal hexahistidine-tagged HGPRT |
J Med Chem 56: 2513-26 (2013)
Article DOI: 10.1021/jm301893b
BindingDB Entry DOI: 10.7270/Q2MW2JGT |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50089449

(CHEMBL3578114)Show SMILES Nc1nc2n(CCN(CCN(CCP(O)(O)=O)CCP(O)(O)=O)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C15H30N7O10P3/c16-15-18-13-12(14(23)19-15)17-11-22(13)4-3-20(5-8-33(24,25)26)1-2-21(6-9-34(27,28)29)7-10-35(30,31)32/h11H,1-10H2,(H2,24,25,26)(H2,27,28,29)(H2,30,31,32)(H3,16,18,19,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 4822-38 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00611
BindingDB Entry DOI: 10.7270/Q2JH3NXV |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50089444
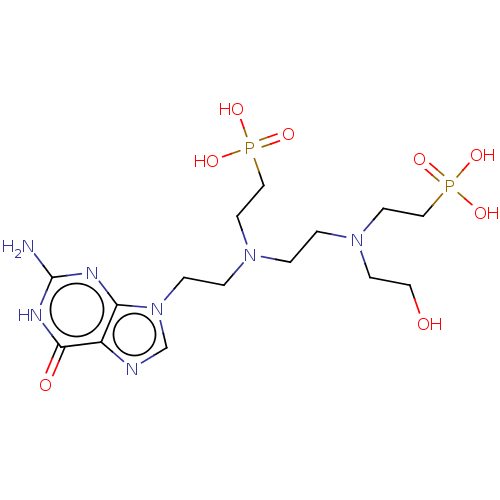
(CHEMBL3578110)Show SMILES Nc1nc2n(CCN(CCN(CCO)CCP(O)(O)=O)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C15H29N7O8P2/c16-15-18-13-12(14(24)19-15)17-11-22(13)4-3-20(6-9-31(25,26)27)1-2-21(5-8-23)7-10-32(28,29)30/h11,23H,1-10H2,(H2,25,26,27)(H2,28,29,30)(H3,16,18,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 4822-38 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00611
BindingDB Entry DOI: 10.7270/Q2JH3NXV |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50059910
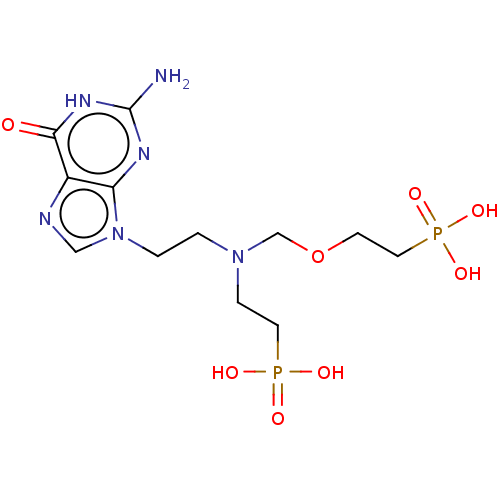
(CHEMBL3394315)Show SMILES Nc1nc2n(CCN(CCP(O)(O)=O)COCCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C12H22N6O8P2/c13-12-15-10-9(11(19)16-12)14-7-18(10)2-1-17(3-5-27(20,21)22)8-26-4-6-28(23,24)25/h7H,1-6,8H2,(H2,20,21,22)(H2,23,24,25)(H3,13,15,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 827-46 (2015)
Article DOI: 10.1021/jm501416t
BindingDB Entry DOI: 10.7270/Q2HM5B3V |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50467707

(CHEMBL4280490)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1OCCP(O)(O)=O)C(=O)CCP(O)(O)=O |r| Show InChI InChI=1S/C14H22N6O9P2/c15-14-17-12-11(13(22)18-14)16-7-20(12)8-5-19(10(21)1-3-30(23,24)25)6-9(8)29-2-4-31(26,27)28/h7-9H,1-6H2,(H2,23,24,25)(H2,26,27,28)(H3,15,17,18,22)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by spectrophotometric analysis |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50059908

(CHEMBL3394327)Show SMILES Nc1nc2n(CCN(CCOCCP(O)(O)=O)CCP(O)(O)=O)c(Br)nc2c(=O)[nH]1 Show InChI InChI=1S/C13H23BrN6O8P2/c14-12-16-9-10(17-13(15)18-11(9)21)20(12)2-1-19(4-7-29(22,23)24)3-5-28-6-8-30(25,26)27/h1-8H2,(H2,22,23,24)(H2,25,26,27)(H3,15,17,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 827-46 (2015)
Article DOI: 10.1021/jm501416t
BindingDB Entry DOI: 10.7270/Q2HM5B3V |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50089446

(CHEMBL3578112)Show SMILES Nc1nc2n(CCN(CCN(CCCO)CCP(O)(O)=O)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C16H31N7O8P2/c17-16-19-14-13(15(25)20-16)18-12-23(14)6-5-22(8-11-33(29,30)31)4-3-21(2-1-9-24)7-10-32(26,27)28/h12,24H,1-11H2,(H2,26,27,28)(H2,29,30,31)(H3,17,19,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 4822-38 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00611
BindingDB Entry DOI: 10.7270/Q2JH3NXV |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519500

(CHEMBL1233603)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16N5O7P/c12-11-15-5-3(1-13-7(5)10(19)16-11)6-9(18)8(17)4(14-6)2-23-24(20,21)22/h1,4,6,8-9,13-14,17-18H,2H2,(H2,20,21,22)(H3,12,15,16,19)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT assessed as inhibitor constant for enzyme-inhibitor complex formation using [5'-14C]IMP as substrate by scintillation count... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392261
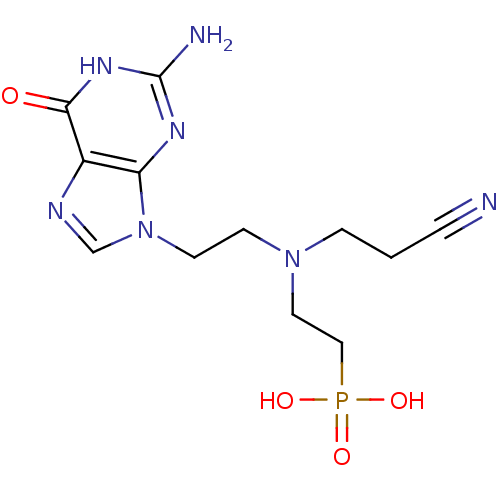
(CHEMBL2153480)Show SMILES Nc1nc2n(CCN(CCC#N)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C12H18N7O4P/c13-2-1-3-18(6-7-24(21,22)23)4-5-19-8-15-9-10(19)16-12(14)17-11(9)20/h8H,1,3-7H2,(H2,21,22,23)(H3,14,16,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 827-46 (2015)
Article DOI: 10.1021/jm501416t
BindingDB Entry DOI: 10.7270/Q2HM5B3V |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392261

(CHEMBL2153480)Show SMILES Nc1nc2n(CCN(CCC#N)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C12H18N7O4P/c13-2-1-3-18(6-7-24(21,22)23)4-5-19-8-15-9-10(19)16-12(14)17-11(9)20/h8H,1,3-7H2,(H2,21,22,23)(H3,14,16,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM194501
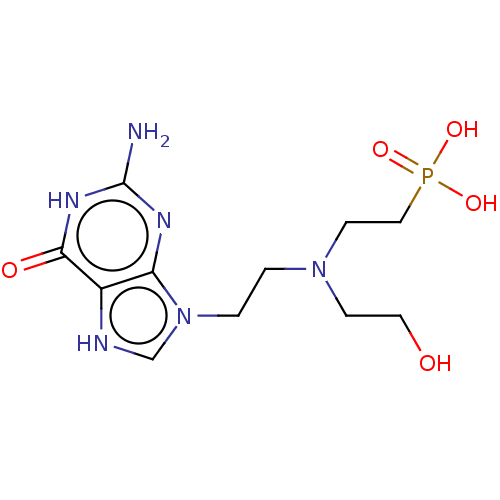
(US9200020, Table 3 compound 10)Show SMILES Nc1nc2n(CCN(CCO)CCP(O)(O)=O)c[nH]c2c(=O)[nH]1 Show InChI InChI=1S/C7H19BNO4P/c8-2-4-9(5-6-10)3-1-7-14(11,12)13/h10H,1-8H2,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I.
US Patent
| Assay Description
The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... |
US Patent US9200020 (2015)
BindingDB Entry DOI: 10.7270/Q2TM78XB |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392263
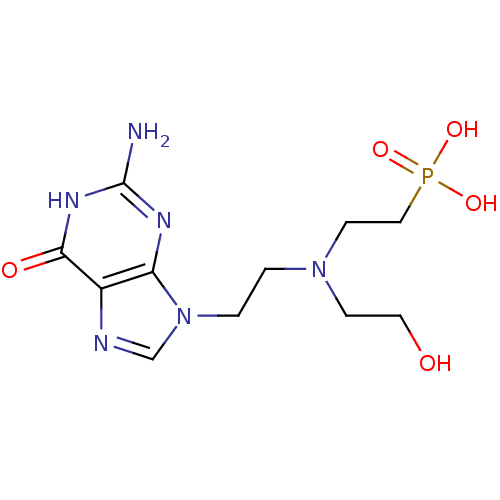
(CHEMBL2153497)Show InChI InChI=1S/C11H19N6O5P/c12-11-14-9-8(10(19)15-11)13-7-17(9)2-1-16(3-5-18)4-6-23(20,21)22/h7,18H,1-6H2,(H2,20,21,22)(H3,12,14,15,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50603701
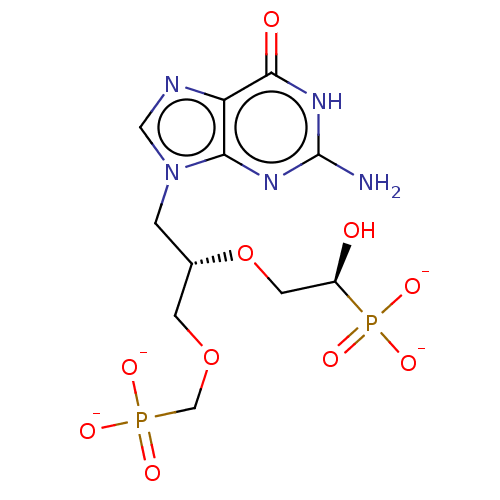
(CHEMBL5185227)Show SMILES Nc1nc2n(C[C@@H](COCP([O-])([O-])=O)OC[C@@H](O)P([O-])([O-])=O)cnc2c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01881
BindingDB Entry DOI: 10.7270/Q25X2F0M |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM194496
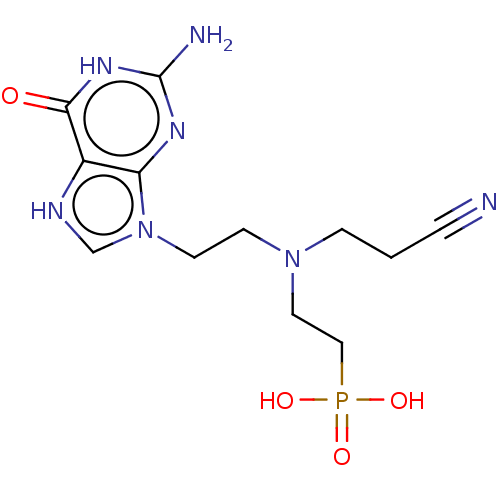
(US9200020, Table 3 compound 5)Show SMILES Nc1nc2n(CCN(CCC#N)CCP(O)(O)=O)c[nH]c2c(=O)[nH]1 Show InChI InChI=1S/C8H18BN2O3P/c9-3-7-11(5-1-4-10)6-2-8-15(12,13)14/h1-3,5-9H2,(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I.
US Patent
| Assay Description
The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... |
US Patent US9200020 (2015)
BindingDB Entry DOI: 10.7270/Q2TM78XB |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50059909
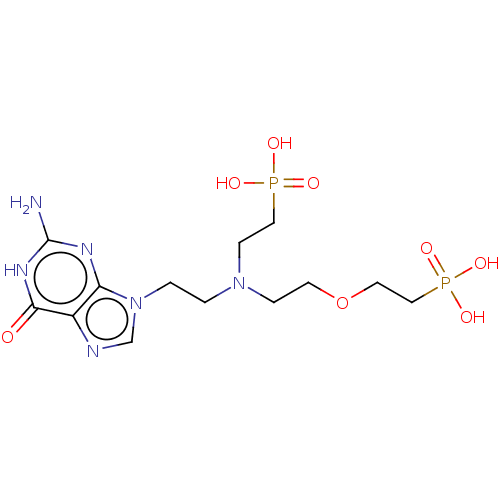
(CHEMBL3394316)Show SMILES Nc1nc2n(CCN(CCOCCP(O)(O)=O)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C13H24N6O8P2/c14-13-16-11-10(12(20)17-13)15-9-19(11)2-1-18(4-7-28(21,22)23)3-5-27-6-8-29(24,25)26/h9H,1-8H2,(H2,21,22,23)(H2,24,25,26)(H3,14,16,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 827-46 (2015)
Article DOI: 10.1021/jm501416t
BindingDB Entry DOI: 10.7270/Q2HM5B3V |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50059909

(CHEMBL3394316)Show SMILES Nc1nc2n(CCN(CCOCCP(O)(O)=O)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C13H24N6O8P2/c14-13-16-11-10(12(20)17-13)15-9-19(11)2-1-18(4-7-28(21,22)23)3-5-27-6-8-29(24,25)26/h9H,1-8H2,(H2,21,22,23)(H2,24,25,26)(H3,14,16,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HGPRT using PRib-PP as substrate by Hanes-plot based method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113416
BindingDB Entry DOI: 10.7270/Q2T43XWD |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50603699

(CHEMBL5208021)Show SMILES Nc1nc2n(C[C@@H](COCP([O-])([O-])=O)OCC(O)P([O-])([O-])=O)cnc2c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01881
BindingDB Entry DOI: 10.7270/Q25X2F0M |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50603700

(CHEMBL5171024)Show SMILES Nc1nc2n(C[C@@H](COCP([O-])([O-])=O)OC[C@H](O)P([O-])([O-])=O)cnc2c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01881
BindingDB Entry DOI: 10.7270/Q25X2F0M |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392266

(CHEMBL2153484)Show SMILES Nc1nc2n(CCN(CCCC(O)=O)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C13H21N6O6P/c14-13-16-11-10(12(22)17-13)15-8-19(11)5-4-18(3-1-2-9(20)21)6-7-26(23,24)25/h8H,1-7H2,(H,20,21)(H2,23,24,25)(H3,14,16,17,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392260
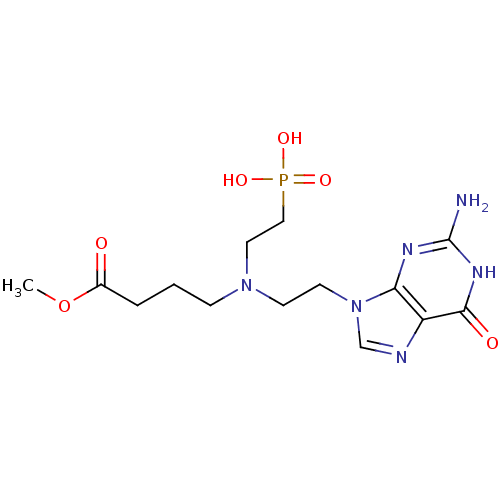
(CHEMBL2153478)Show SMILES COC(=O)CCCN(CCn1cnc2c1nc(N)[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C14H23N6O6P/c1-26-10(21)3-2-4-19(7-8-27(23,24)25)5-6-20-9-16-11-12(20)17-14(15)18-13(11)22/h9H,2-8H2,1H3,(H2,23,24,25)(H3,15,17,18,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392258

(CHEMBL2153476)Show SMILES CCOC(=O)CN(CCn1cnc2c1nc(N)[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C13H21N6O6P/c1-2-25-9(20)7-18(5-6-26(22,23)24)3-4-19-8-15-10-11(19)16-13(14)17-12(10)21/h8H,2-7H2,1H3,(H2,22,23,24)(H3,14,16,17,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439836
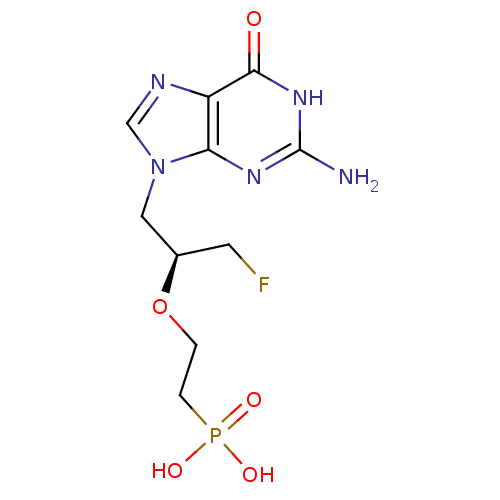
(CHEMBL2420072)Show SMILES Nc1nc2n(C[C@@H](CF)OCCP(O)(O)=O)cnc2c(=O)[nH]1 |r| Show InChI InChI=1S/C10H15FN5O5P/c11-3-6(21-1-2-22(18,19)20)4-16-5-13-7-8(16)14-10(12)15-9(7)17/h5-6H,1-4H2,(H2,18,19,20)(H3,12,14,15,17)/t6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HGPRT expressed in Escherichia coli Sphi606 cells by spectrophotometric analysis |
Eur J Med Chem 67: 81-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.032
BindingDB Entry DOI: 10.7270/Q20Z74Q0 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50268142
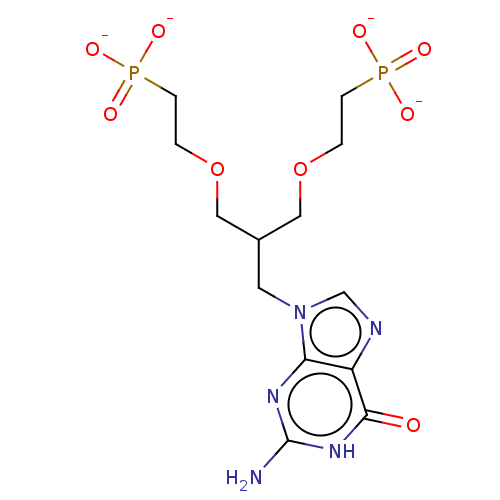
(CHEMBL4098313)Show SMILES [Na+].[Na+].[Na+].[Na+].Nc1nc2n(CC(COCCP([O-])([O-])=O)COCCP([O-])([O-])=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C13H23N5O9P2/c14-13-16-11-10(12(19)17-13)15-8-18(11)5-9(6-26-1-3-28(20,21)22)7-27-2-4-29(23,24)25/h8-9H,1-7H2,(H2,20,21,22)(H2,23,24,25)(H3,14,16,17,19)/p-4 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences, Flemingovo nám. 2, CZ-16610 Prague 6, Czech Republic.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal hexa-histidine-tagged human HGPRT using PRib-PP as substrate in presence of guanine by Hanes plot analysis |
Bioorg Med Chem 25: 4008-4030 (2017)
Article DOI: 10.1016/j.bmc.2017.05.048
BindingDB Entry DOI: 10.7270/Q2NS0XCJ |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50258937
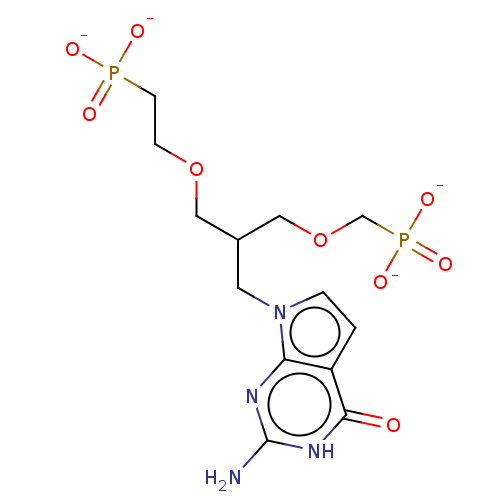
(CHEMBL4067618)Show SMILES [Na+].[Na+].[Na+].[Na+].Nc1nc2n(CC(COCCP([O-])([O-])=O)COCP([O-])([O-])=O)ccc2c(=O)[nH]1 Show InChI InChI=1S/C13H22N4O9P2.4Na/c14-13-15-11-10(12(18)16-13)1-2-17(11)5-9(7-26-8-28(22,23)24)6-25-3-4-27(19,20)21;;;;/h1-2,9H,3-8H2,(H2,19,20,21)(H2,22,23,24)(H3,14,15,16,18);;;;/q;4*+1/p-4 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using PRib-PP as the substrate by spectrophotometric method |
J Med Chem 60: 7539-7554 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00926
BindingDB Entry DOI: 10.7270/Q2J968T1 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50304140
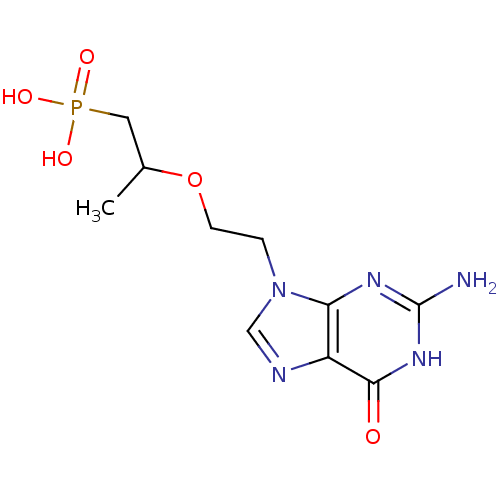
(9-[2-(1-Phosphonopropan-2-yloxy)ethyl]guanine | CH...)Show InChI InChI=1S/C10H16N5O5P/c1-6(4-21(17,18)19)20-3-2-15-5-12-7-8(15)13-10(11)14-9(7)16/h5-6H,2-4H2,1H3,(H2,17,18,19)(H3,11,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT expressed in Escherichia coli by spectrophotometric assay |
Bioorg Med Chem 17: 6218-32 (2009)
Article DOI: 10.1016/j.bmc.2009.07.044
BindingDB Entry DOI: 10.7270/Q23778TP |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50258941
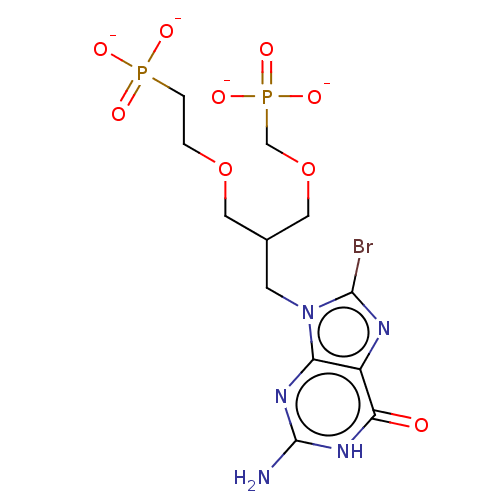
(CHEMBL4078513)Show SMILES [Na+].[Na+].[Na+].[Na+].Nc1nc2n(CC(COCCP([O-])([O-])=O)COCP([O-])([O-])=O)c(Br)nc2c(=O)[nH]1 Show InChI InChI=1S/C12H20BrN5O9P2.4Na/c13-11-15-8-9(16-12(14)17-10(8)19)18(11)3-7(5-27-6-29(23,24)25)4-26-1-2-28(20,21)22;;;;/h7H,1-6H2,(H2,20,21,22)(H2,23,24,25)(H3,14,16,17,19);;;;/q;4*+1/p-4 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using PRib-PP as the substrate by spectrophotometric method |
J Med Chem 60: 7539-7554 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00926
BindingDB Entry DOI: 10.7270/Q2J968T1 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519497

(CHEMBL4546337)Show SMILES Nc1nc2n(C[C@H](CO)n3cc(nn3)P(O)(O)=O)cnc2c(=O)[nH]1 |r| Show InChI InChI=1S/C10H13N8O5P/c11-10-13-8-7(9(20)14-10)12-4-17(8)1-5(3-19)18-2-6(15-16-18)24(21,22)23/h2,4-5,19H,1,3H2,(H2,21,22,23)(H3,11,13,14,20)/t5-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by Hanes-plot based method |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM194500
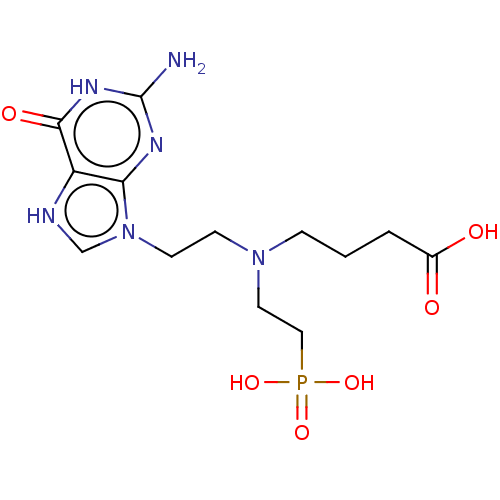
(US9200020, Table 3 compound 9)Show SMILES Nc1nc2n(CCN(CCCC(O)=O)CCP(O)(O)=O)c[nH]c2c(=O)[nH]1 Show InChI InChI=1S/C9H21BNO5P/c10-4-7-11(5-1-3-9(12)13)6-2-8-17(14,15)16/h1-8,10H2,(H,12,13)(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I.
US Patent
| Assay Description
The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... |
US Patent US9200020 (2015)
BindingDB Entry DOI: 10.7270/Q2TM78XB |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM194494

(US9200020, Table 3 compound 3)Show SMILES COC(=O)CCCN(CCn1c[nH]c2c1nc(N)[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C10H23BNO5P/c1-17-10(13)4-2-6-12(8-5-11)7-3-9-18(14,15)16/h2-9,11H2,1H3,(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I.
US Patent
| Assay Description
The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... |
US Patent US9200020 (2015)
BindingDB Entry DOI: 10.7270/Q2TM78XB |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM194492
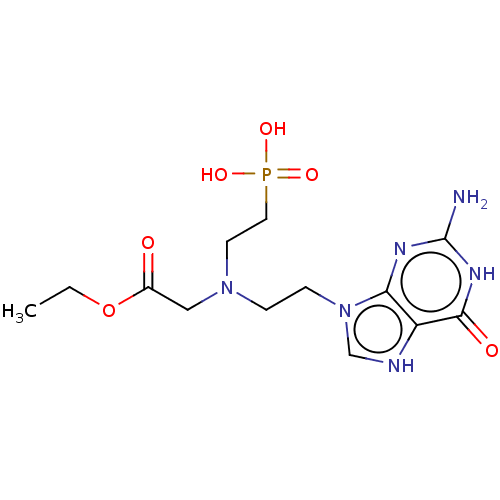
(US9200020, Table 3 compound 1)Show SMILES CCOC(=O)CN(CCn1c[nH]c2c1nc(N)[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C9H21BNO5P/c1-2-16-9(12)8-11(6-4-10)5-3-7-17(13,14)15/h2-8,10H2,1H3,(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I.
US Patent
| Assay Description
The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... |
US Patent US9200020 (2015)
BindingDB Entry DOI: 10.7270/Q2TM78XB |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392265
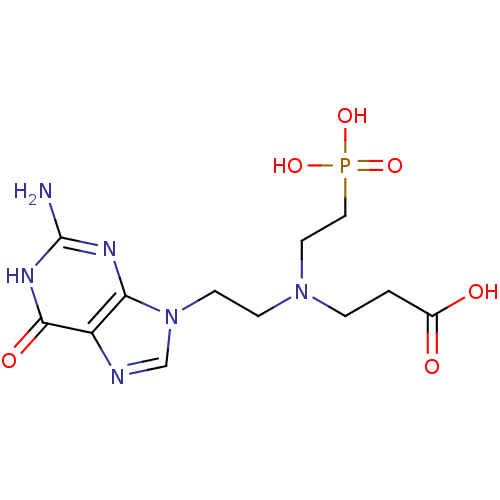
(CHEMBL2153483)Show SMILES Nc1nc2n(CCN(CCC(O)=O)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C12H19N6O6P/c13-12-15-10-9(11(21)16-12)14-7-18(10)4-3-17(2-1-8(19)20)5-6-25(22,23)24/h7H,1-6H2,(H,19,20)(H2,22,23,24)(H3,13,15,16,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM194499
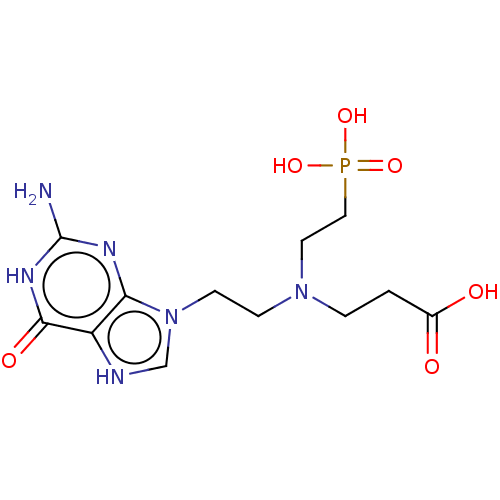
(US9200020, Table 3 compound 8)Show SMILES Nc1nc2n(CCN(CCC(O)=O)CCP(O)(O)=O)c[nH]c2c(=O)[nH]1 Show InChI InChI=1S/C8H19BNO5P/c9-3-6-10(5-2-8(11)12)4-1-7-16(13,14)15/h1-7,9H2,(H,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I.
US Patent
| Assay Description
The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... |
US Patent US9200020 (2015)
BindingDB Entry DOI: 10.7270/Q2TM78XB |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50569742
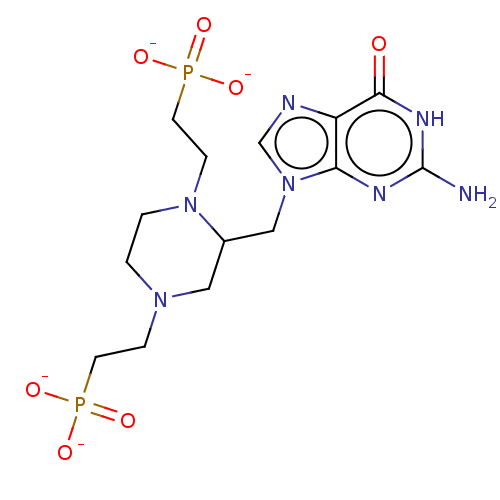
(CHEMBL4846343)Show SMILES Nc1nc2n(CC3CN(CCP([O-])([O-])=O)CCN3CCP([O-])([O-])=O)cnc2c(=O)[nH]1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HGPRT using PRib-PP as substrate by Hanes-plot based method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113416
BindingDB Entry DOI: 10.7270/Q2T43XWD |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50059913
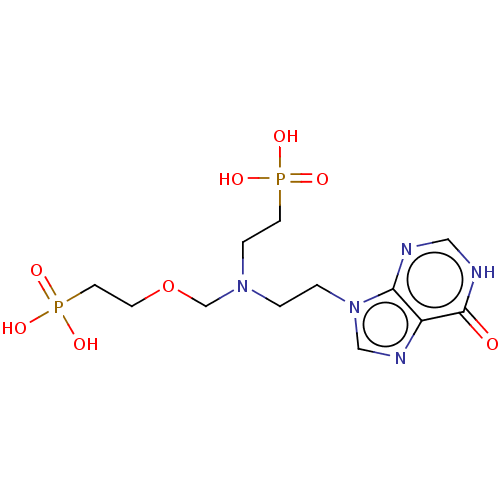
(CHEMBL3394312)Show SMILES OP(O)(=O)CCOCN(CCn1cnc2c1nc[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C12H21N5O8P2/c18-12-10-11(13-7-14-12)17(8-15-10)2-1-16(3-5-26(19,20)21)9-25-4-6-27(22,23)24/h7-8H,1-6,9H2,(H,13,14,18)(H2,19,20,21)(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 827-46 (2015)
Article DOI: 10.1021/jm501416t
BindingDB Entry DOI: 10.7270/Q2HM5B3V |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM194502
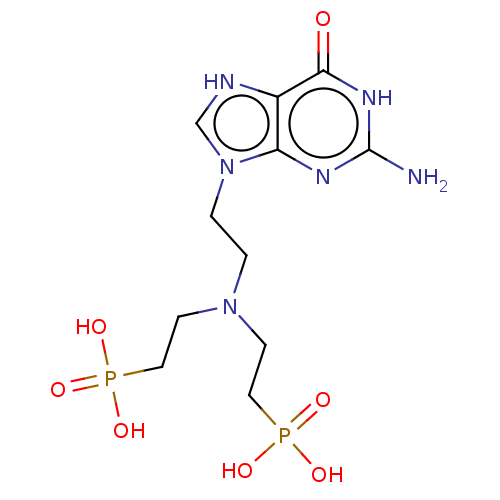
(US9200020, Table 3 compound 11)Show SMILES Nc1nc2n(CCN(CCP(O)(O)=O)CCP(O)(O)=O)c[nH]c2c(=O)[nH]1 Show InChI InChI=1S/C7H20BNO6P2/c8-2-4-9(5-7-17(13,14)15)3-1-6-16(10,11)12/h1-8H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE UNIVERSITY OF QUEENSLAND; INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY ASCR, V.V.I.
US Patent
| Assay Description
The Ki values were determined using a spectrophotometric assay at 25° C., 0.1 M Tris-HCl, 10 mM MgCl2, pH 7.4 (Keough, D. T.; Ng, A. L.; Winzor, D. J... |
US Patent US9200020 (2015)
BindingDB Entry DOI: 10.7270/Q2TM78XB |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50603687

(CHEMBL5186759)Show SMILES Nc1nc2n(CCOCC(O)P([O-])([O-])=O)cnc2c(=O)[nH]1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01881
BindingDB Entry DOI: 10.7270/Q25X2F0M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data