

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50454430 (Nudicauline) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmacy and Pharmacology Curated by ChEMBL | Assay Description Binding affinity of Norditerpenoid Alkaloids at rat neuronal alpha7-type nicotinic acetylcholine receptor | J Med Chem 39: 4860-6 (1997) Article DOI: 10.1021/jm9604991 BindingDB Entry DOI: 10.7270/Q2BP03FP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50587087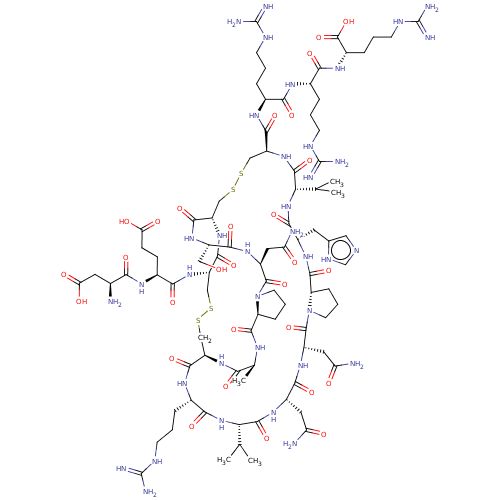 (CHEMBL5070268) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat alpha7 nAChR expressed in xenopus oocytes assessed as inhibition of Ach- induced response at -70 mV holding potential prei... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02079 BindingDB Entry DOI: 10.7270/Q2R78K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016200 (CHEMBL3262146) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016184 (CHEMBL3262131) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50587095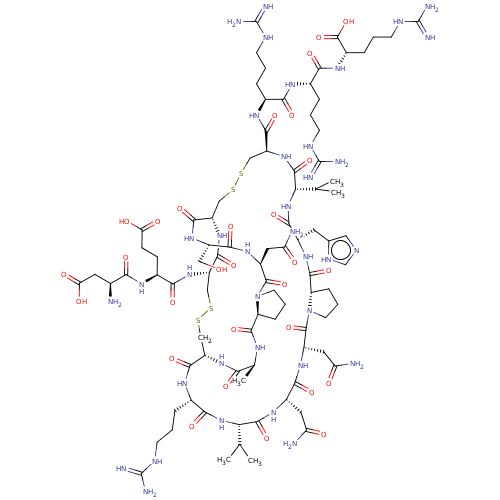 (CHEMBL5084339) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat alpha7 nAChR expressed in xenopus oocytes assessed as inhibition of Ach- induced response at -80 mV holding potential incu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02079 BindingDB Entry DOI: 10.7270/Q2R78K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016185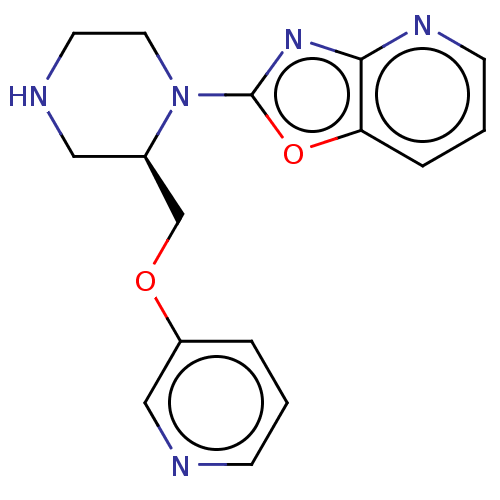 (CHEMBL3262132) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50587086 (CHEMBL5076977) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat alpha7 nAChR expressed in xenopus oocytes assessed as inhibition of Ach- induced response at -70 mV holding potential prei... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02079 BindingDB Entry DOI: 10.7270/Q2R78K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50454436 (Elatine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmacy and Pharmacology Curated by ChEMBL | Assay Description Binding affinity of Norditerpenoid Alkaloids at rat neuronal alpha7-type nicotinic acetylcholine receptor | J Med Chem 39: 4860-6 (1997) Article DOI: 10.1021/jm9604991 BindingDB Entry DOI: 10.7270/Q2BP03FP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50366779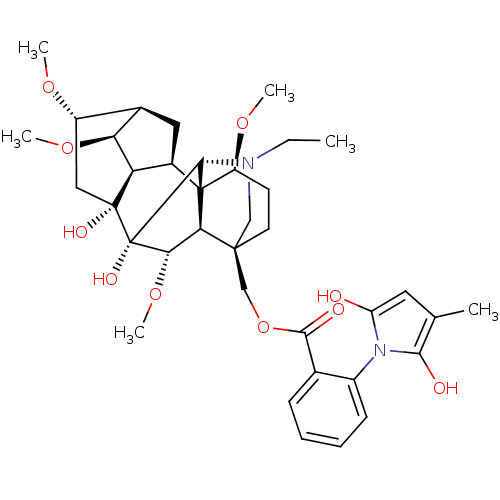 (METHYLLYCACONITINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmacy and Pharmacology Curated by ChEMBL | Assay Description Binding affinity of Norditerpenoid Alkaloids at rat neuronal alpha7-type nicotinic acetylcholine receptor | J Med Chem 39: 4860-6 (1997) Article DOI: 10.1021/jm9604991 BindingDB Entry DOI: 10.7270/Q2BP03FP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016209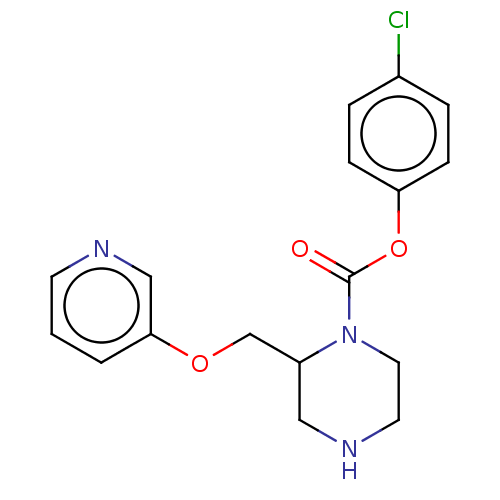 (CHEMBL3262155) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50081729 (4-{5-[1-Aza-bicyclo[2.2.2]oct-(3Z)-ylidenemethyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description The compound was tested for its binding affinity against nicotinic receptor in synaptic membrane fractions from rat cerebral cortices. | Bioorg Med Chem Lett 9: 2795-800 (1999) BindingDB Entry DOI: 10.7270/Q26H4GM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016210 (CHEMBL3262156) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50373059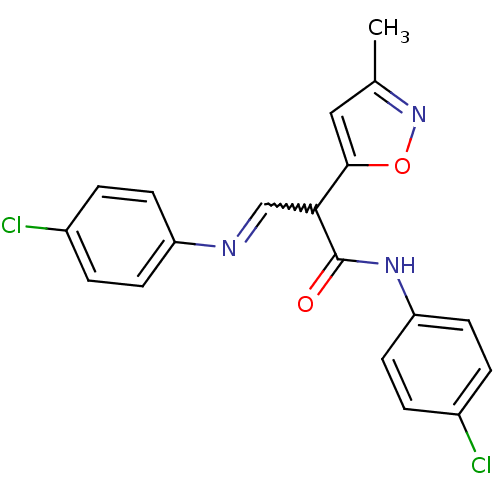 (CHEMBL429317) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Displacement of [125I]alpha-bungarotoxin from alpha-7 nAChR in rat hipocampus | Proc Natl Acad Sci USA 104: 8059-64 (2007) Article DOI: 10.1073/pnas.0701321104 BindingDB Entry DOI: 10.7270/Q2NS0VS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50373059 (CHEMBL429317) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Displacement of [125I]alpha-bungarotoxin from alpha-7 nAChR in rat amygdala | Proc Natl Acad Sci USA 104: 8059-64 (2007) Article DOI: 10.1073/pnas.0701321104 BindingDB Entry DOI: 10.7270/Q2NS0VS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50081733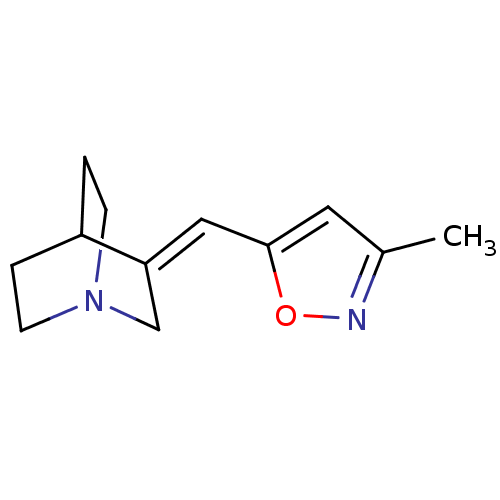 (3-(3-Methyl-isoxazol-5-ylmethylene)-1-aza-bicyclo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Compound was tested for its binding affinity against nicotinic receptor in synaptic membrane fractions from rat cerebral cortices. | Bioorg Med Chem Lett 9: 2795-800 (1999) BindingDB Entry DOI: 10.7270/Q26H4GM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016227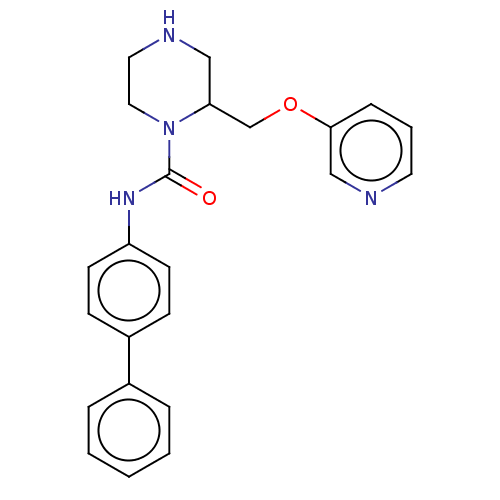 (CHEMBL3262173) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016225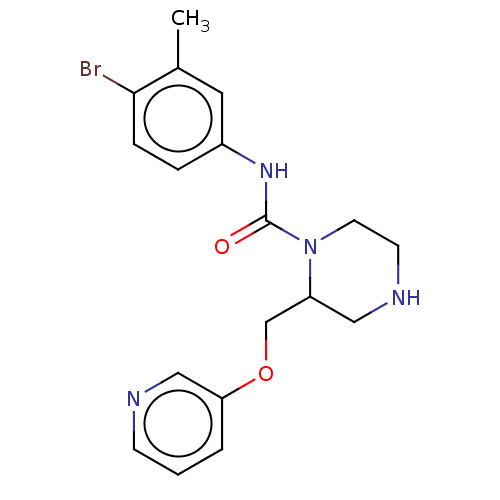 (CHEMBL3262171) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM248004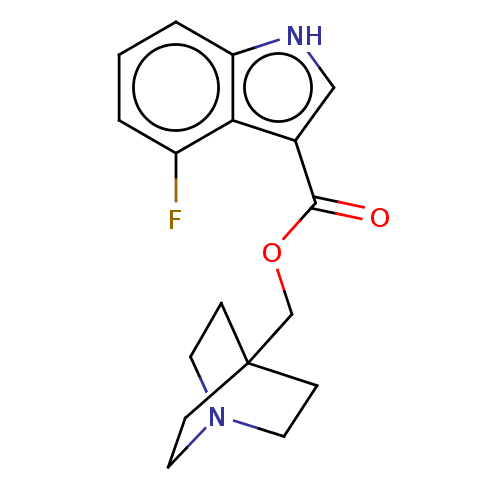 (US9434724, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.2 | -11.2 | 14.1 | n/a | n/a | n/a | n/a | 7.4 | 37 |
ALPHARMAGEN, LLC US Patent | Assay Description Protocol 1: The assay was conducted following the literature reference Meyer E. M., et al., Analysis of 3-(4-Hydroxy, 2-Methoxybenzylidene) Anabasein... | US Patent US9434724 (2016) BindingDB Entry DOI: 10.7270/Q2M32TPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50516307 (CHEMBL4445542) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Antagonist activity at rat alpha7 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of Ach-induced response at -70 mV holding potentia... | J Med Chem 62: 6262-6275 (2019) Article DOI: 10.1021/acs.jmedchem.9b00566 BindingDB Entry DOI: 10.7270/Q2GQ7231 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Compound was tested for its binding affinity against nicotinic receptor in synaptic membrane fractions from rat cerebral cortices. | Bioorg Med Chem Lett 9: 2795-800 (1999) BindingDB Entry DOI: 10.7270/Q26H4GM1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016197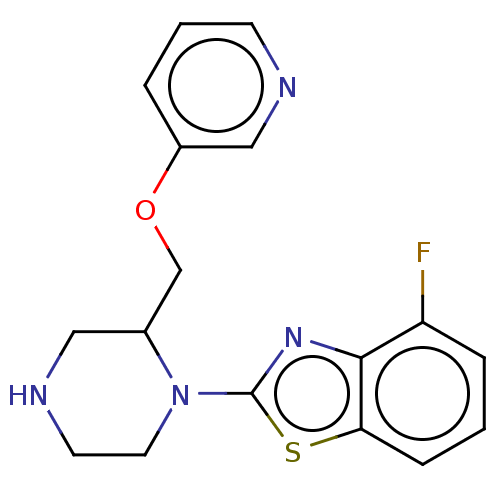 (CHEMBL3262143) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016198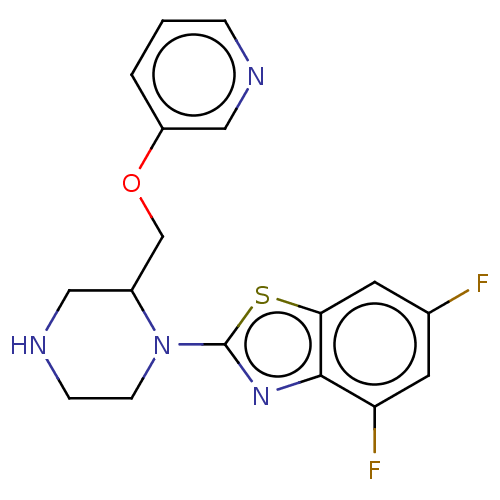 (CHEMBL3262144) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50516325 (CHEMBL4463089) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Antagonist activity at rat alpha7 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of Ach-induced response at -70 mV holding potentia... | J Med Chem 62: 6262-6275 (2019) Article DOI: 10.1021/acs.jmedchem.9b00566 BindingDB Entry DOI: 10.7270/Q2GQ7231 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016217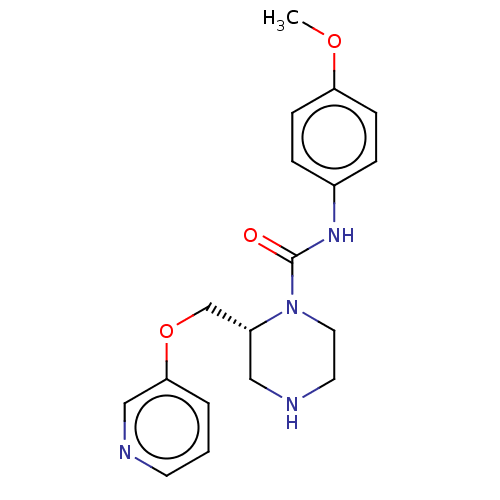 (CHEMBL3262163) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016179 (CHEMBL3262126) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50149143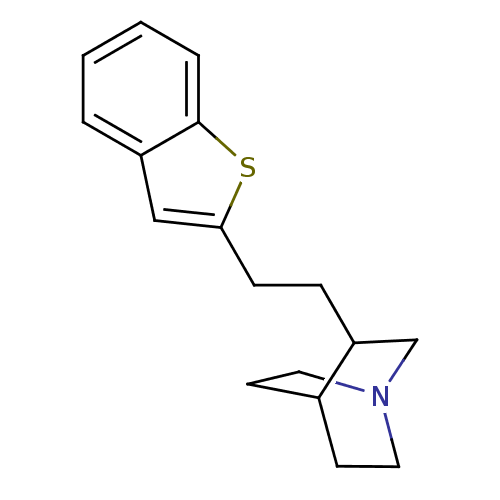 (3-(2-Benzo[b]thiophen-2-yl-ethyl)-1-aza-bicyclo[2....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Alpha 7 nicotinic acetylcholine receptor binding activity in PC12 cells | Bioorg Med Chem Lett 14: 3781-4 (2004) Article DOI: 10.1016/j.bmcl.2004.04.091 BindingDB Entry DOI: 10.7270/Q2X066G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50149143 (3-(2-Benzo[b]thiophen-2-yl-ethyl)-1-aza-bicyclo[2....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Alpha 7 nicotinic acetylcholine receptor binding activity in PC12 cells | Bioorg Med Chem Lett 14: 3781-4 (2004) Article DOI: 10.1016/j.bmcl.2004.04.091 BindingDB Entry DOI: 10.7270/Q2X066G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50149143 (3-(2-Benzo[b]thiophen-2-yl-ethyl)-1-aza-bicyclo[2....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Alpha 7 nicotinic acetylcholine receptor binding activity in PC12 cells | Bioorg Med Chem Lett 14: 3781-4 (2004) Article DOI: 10.1016/j.bmcl.2004.04.091 BindingDB Entry DOI: 10.7270/Q2X066G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM247960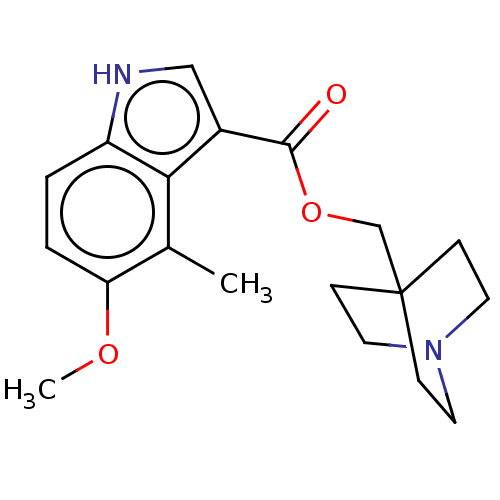 (US9434724, 34) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20.4 | -10.9 | 23.6 | n/a | n/a | n/a | n/a | 7.4 | 37 |
ALPHARMAGEN, LLC US Patent | Assay Description Protocol 1: The assay was conducted following the literature reference Meyer E. M., et al., Analysis of 3-(4-Hydroxy, 2-Methoxybenzylidene) Anabasein... | US Patent US9434724 (2016) BindingDB Entry DOI: 10.7270/Q2M32TPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM248024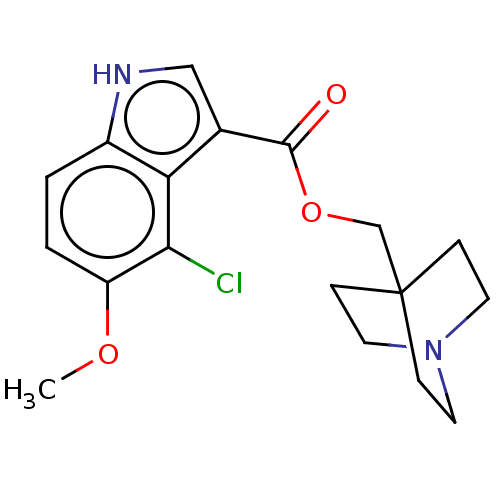 (US9434724, 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20.6 | -10.9 | 23.9 | n/a | n/a | n/a | n/a | 7.4 | 37 |
ALPHARMAGEN, LLC US Patent | Assay Description Protocol 1: The assay was conducted following the literature reference Meyer E. M., et al., Analysis of 3-(4-Hydroxy, 2-Methoxybenzylidene) Anabasein... | US Patent US9434724 (2016) BindingDB Entry DOI: 10.7270/Q2M32TPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50149146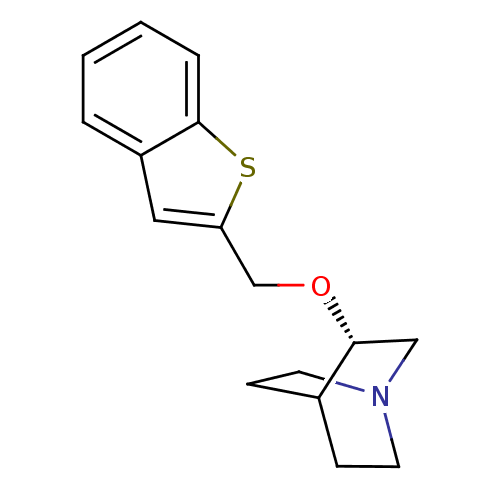 ((S)-3-(Benzo[b]thiophen-2-ylmethoxy)-1-aza-bicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Alpha 7 nicotinic acetylcholine receptor binding activity in PC12 cells | Bioorg Med Chem Lett 14: 3781-4 (2004) Article DOI: 10.1016/j.bmcl.2004.04.091 BindingDB Entry DOI: 10.7270/Q2X066G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM247988 (US9434724, 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 22.9 | -10.8 | 26.6 | n/a | n/a | n/a | n/a | 7.4 | 37 |
ALPHARMAGEN, LLC US Patent | Assay Description Protocol 1: The assay was conducted following the literature reference Meyer E. M., et al., Analysis of 3-(4-Hydroxy, 2-Methoxybenzylidene) Anabasein... | US Patent US9434724 (2016) BindingDB Entry DOI: 10.7270/Q2M32TPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50516336 (CHEMBL4465982) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat alpha7 nAChR expressed in xenopus oocytes assessed as inhibition of Ach- induced response at -70 mV holding potential prei... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02079 BindingDB Entry DOI: 10.7270/Q2R78K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50373059 (CHEMBL429317) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Displacement of [125I]alpha-bungarotoxin from alpha-7 nAChR in rat cortex | Proc Natl Acad Sci USA 104: 8059-64 (2007) Article DOI: 10.1073/pnas.0701321104 BindingDB Entry DOI: 10.7270/Q2NS0VS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50587088 (CHEMBL5082749) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat alpha7 nAChR expressed in xenopus oocytes assessed as inhibition of Ach- induced response at -70 mV holding potential prei... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02079 BindingDB Entry DOI: 10.7270/Q2R78K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM247999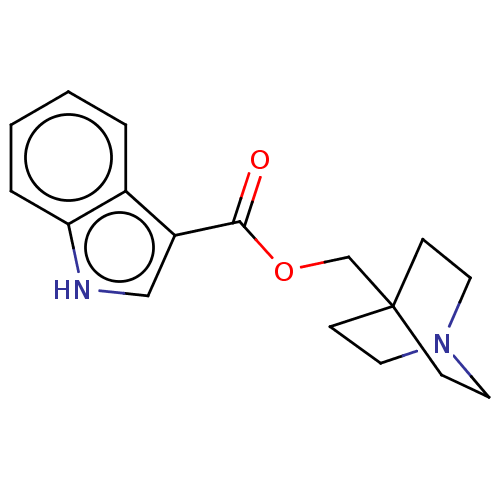 (US9434724, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 27.6 | -10.7 | 31.8 | n/a | n/a | n/a | n/a | 7.4 | 37 |
ALPHARMAGEN, LLC US Patent | Assay Description Protocol 1: The assay was conducted following the literature reference Meyer E. M., et al., Analysis of 3-(4-Hydroxy, 2-Methoxybenzylidene) Anabasein... | US Patent US9434724 (2016) BindingDB Entry DOI: 10.7270/Q2M32TPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM247980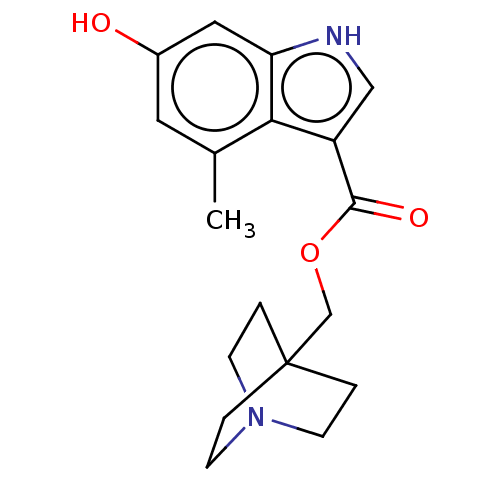 (US9434724, 83) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 27.9 | -10.7 | 31.8 | n/a | n/a | n/a | n/a | 7.4 | 37 |
ALPHARMAGEN, LLC US Patent | Assay Description Protocol 1: The assay was conducted following the literature reference Meyer E. M., et al., Analysis of 3-(4-Hydroxy, 2-Methoxybenzylidene) Anabasein... | US Patent US9434724 (2016) BindingDB Entry DOI: 10.7270/Q2M32TPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50516308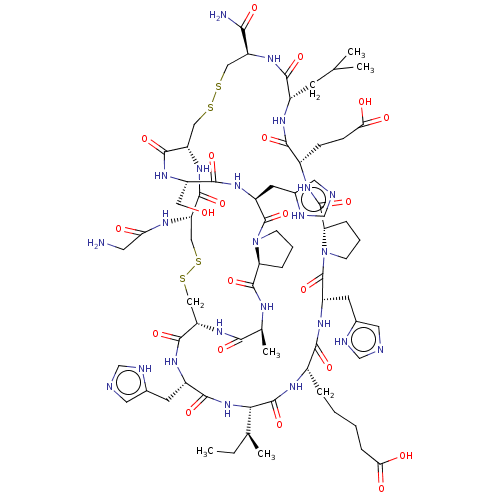 (CHEMBL4469829) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Antagonist activity at rat alpha7 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of Ach-induced response at -70 mV holding potentia... | J Med Chem 62: 6262-6275 (2019) Article DOI: 10.1021/acs.jmedchem.9b00566 BindingDB Entry DOI: 10.7270/Q2GQ7231 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016199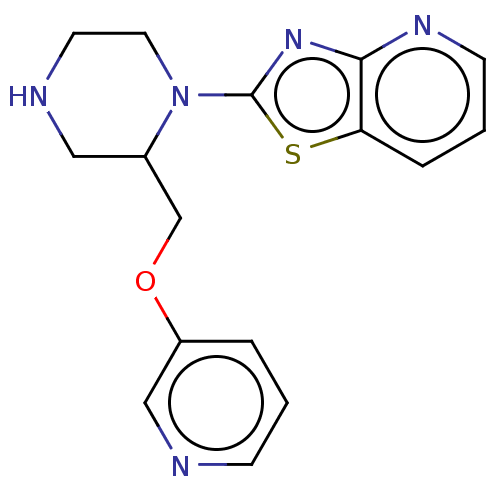 (CHEMBL3262145) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM248009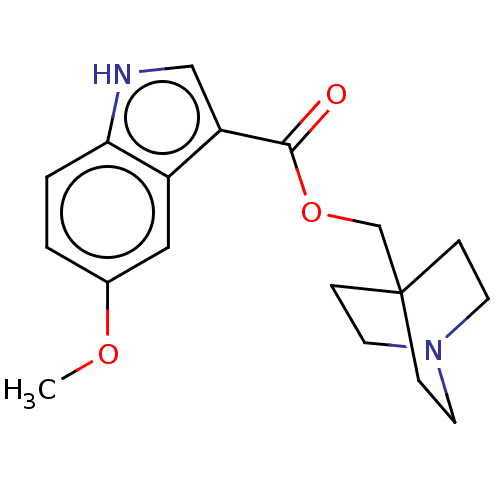 (US9434724, 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 28.3 | -10.7 | 32.6 | n/a | n/a | n/a | n/a | 7.4 | 37 |
ALPHARMAGEN, LLC US Patent | Assay Description Protocol 1: The assay was conducted following the literature reference Meyer E. M., et al., Analysis of 3-(4-Hydroxy, 2-Methoxybenzylidene) Anabasein... | US Patent US9434724 (2016) BindingDB Entry DOI: 10.7270/Q2M32TPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM247967 (US9434724, 47) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 28.5 | -10.7 | 33.3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
ALPHARMAGEN, LLC US Patent | Assay Description Protocol 1: The assay was conducted following the literature reference Meyer E. M., et al., Analysis of 3-(4-Hydroxy, 2-Methoxybenzylidene) Anabasein... | US Patent US9434724 (2016) BindingDB Entry DOI: 10.7270/Q2M32TPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM247990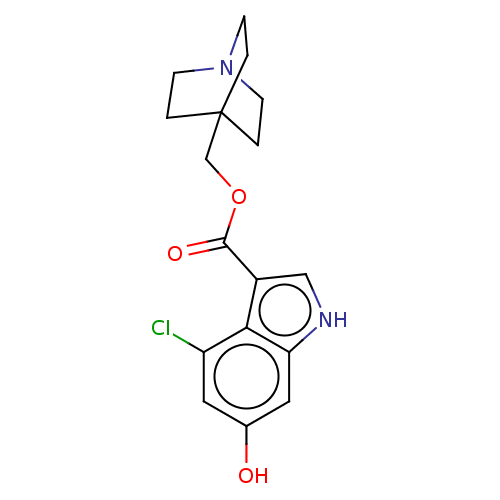 (US9434724, 46) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 28.6 | -10.7 | 33.4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
ALPHARMAGEN, LLC US Patent | Assay Description Protocol 1: The assay was conducted following the literature reference Meyer E. M., et al., Analysis of 3-(4-Hydroxy, 2-Methoxybenzylidene) Anabasein... | US Patent US9434724 (2016) BindingDB Entry DOI: 10.7270/Q2M32TPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50516329 (CHEMBL4467363) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Antagonist activity at rat alpha7 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of Ach-induced response at -70 mV holding potentia... | J Med Chem 62: 6262-6275 (2019) Article DOI: 10.1021/acs.jmedchem.9b00566 BindingDB Entry DOI: 10.7270/Q2GQ7231 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016208 (CHEMBL3262154) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016212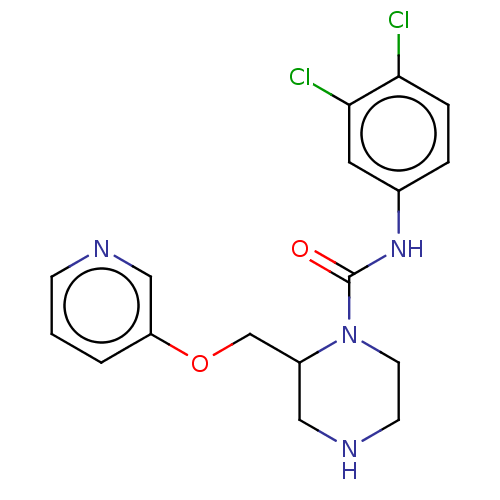 (CHEMBL3262158) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016228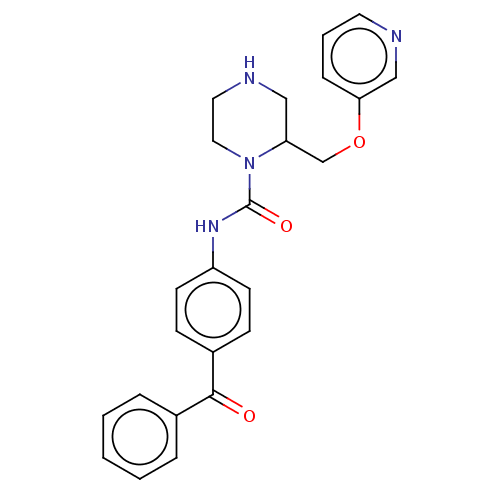 (CHEMBL3262174) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016226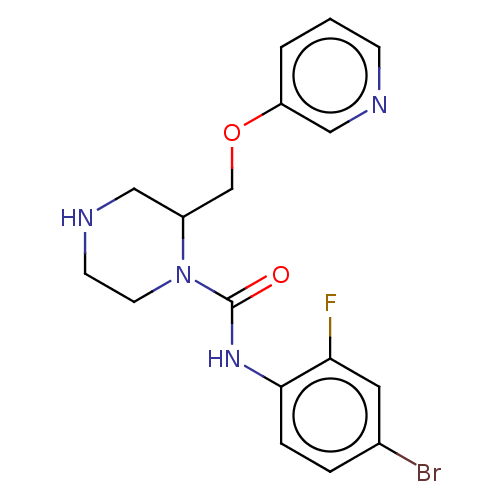 (CHEMBL3262172) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016222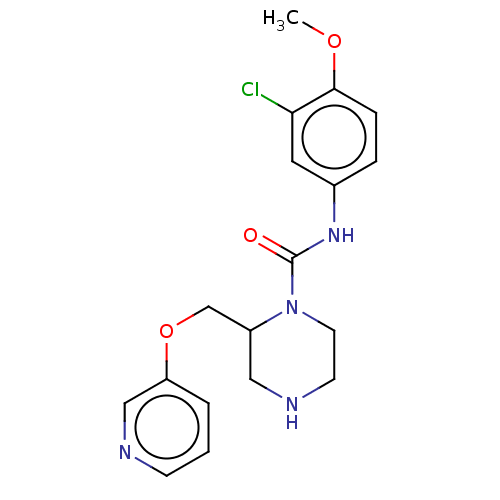 (CHEMBL3262168) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50016189 (CHEMBL3262135) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM248023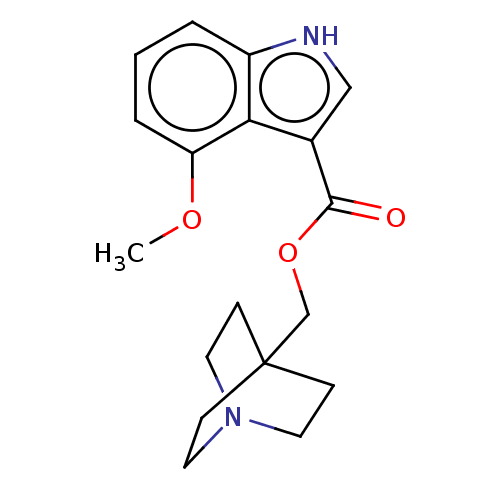 (US9434724, 36) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 42.3 | -10.5 | 48.9 | n/a | n/a | n/a | n/a | 7.4 | 37 |
ALPHARMAGEN, LLC US Patent | Assay Description Protocol 1: The assay was conducted following the literature reference Meyer E. M., et al., Analysis of 3-(4-Hydroxy, 2-Methoxybenzylidene) Anabasein... | US Patent US9434724 (2016) BindingDB Entry DOI: 10.7270/Q2M32TPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 274 total ) | Next | Last >> |