 Found 1222 hits of ki for UniProtKB: P00749
Found 1222 hits of ki for UniProtKB: P00749 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147093
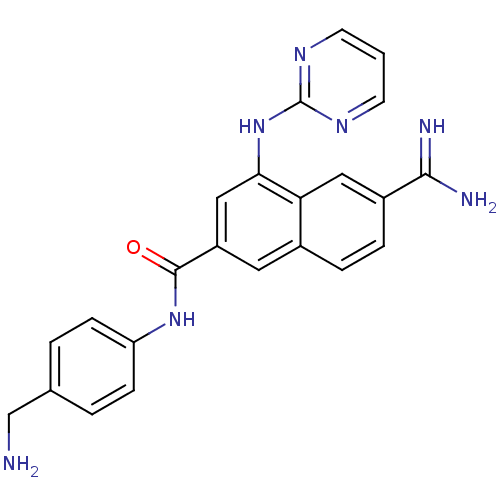
(6-Carbamimidoyl-4-(pyrimidin-2-ylamino)-naphthalen...)Show SMILES NCc1ccc(NC(=O)c2cc(Nc3ncccn3)c3cc(ccc3c2)C(N)=N)cc1 Show InChI InChI=1S/C23H21N7O/c24-13-14-2-6-18(7-3-14)29-22(31)17-10-15-4-5-16(21(25)26)11-19(15)20(12-17)30-23-27-8-1-9-28-23/h1-12H,13,24H2,(H3,25,26)(H,29,31)(H,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards Urokinase-type plasminogen activator (urokinase) |
Bioorg Med Chem Lett 14: 3063-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.030
BindingDB Entry DOI: 10.7270/Q29K49PG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147093

(6-Carbamimidoyl-4-(pyrimidin-2-ylamino)-naphthalen...)Show SMILES NCc1ccc(NC(=O)c2cc(Nc3ncccn3)c3cc(ccc3c2)C(N)=N)cc1 Show InChI InChI=1S/C23H21N7O/c24-13-14-2-6-18(7-3-14)29-22(31)17-10-15-4-5-16(21(25)26)11-19(15)20(12-17)30-23-27-8-1-9-28-23/h1-12H,13,24H2,(H3,25,26)(H,29,31)(H,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of urokinase |
J Med Chem 52: 3159-65 (2009)
Article DOI: 10.1021/jm801444x
BindingDB Entry DOI: 10.7270/Q2FF3TM8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50034582
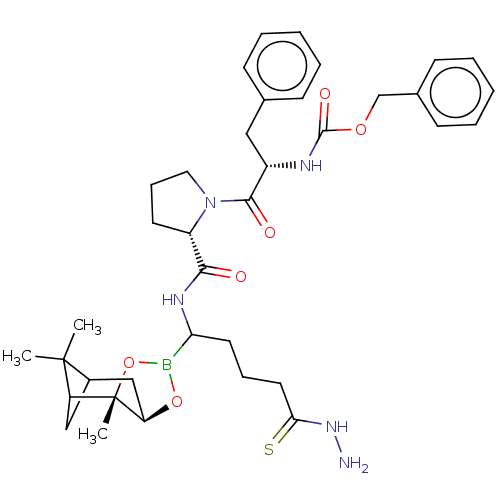
(CHEMBL2448441 | Peptide boronate)Show SMILES Br.[H][C@@]12CC3CC(C3(C)C)[C@]1(C)OB(O2)C(CCC\C(S)=N\N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |TLB:11:10:7:5,THB:12:10:7:5,14:2:7:5| Show InChI InChI=1S/C37H50BN5O6S.BrH/c1-36(2)26-21-29(36)37(3)30(22-26)48-38(49-37)31(17-10-18-32(50)42-39)41-33(44)28-16-11-19-43(28)34(45)27(20-24-12-6-4-7-13-24)40-35(46)47-23-25-14-8-5-9-15-25;/h4-9,12-15,26-31H,10-11,16-23,39H2,1-3H3,(H,40,46)(H,41,44)(H,42,50);1H/t26?,27-,28-,29?,30+,31?,37-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.848 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Urokinase plasminogen activator |
J Med Chem 38: 1511-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QR4W57 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92292
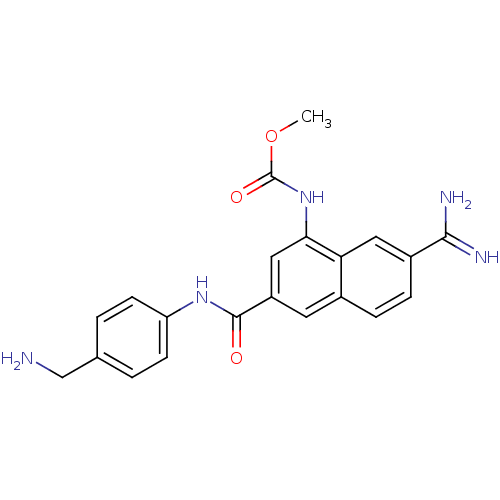
(uPa_17)Show SMILES COC(=O)Nc1cc(cc2ccc(cc12)C(N)=N)C(=O)Nc1ccc(CN)cc1 Show InChI InChI=1S/C21H21N5O3/c1-29-21(28)26-18-10-15(8-13-4-5-14(19(23)24)9-17(13)18)20(27)25-16-6-2-12(11-22)3-7-16/h2-10H,11,22H2,1H3,(H3,23,24)(H,25,27)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | -12.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16171
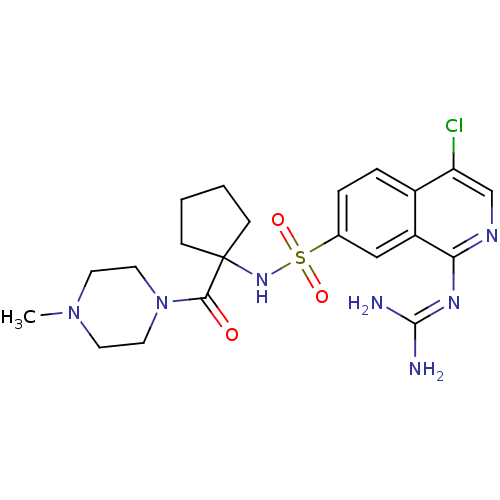
(2-[4-chloro-7-({1-[(4-methylpiperazin-1-yl)carbony...)Show SMILES [#6]-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)C1([#6]-[#6]-[#6]-[#6]1)[#7]S(=O)(=O)c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1 Show InChI InChI=1S/C21H28ClN7O3S/c1-28-8-10-29(11-9-28)19(30)21(6-2-3-7-21)27-33(31,32)14-4-5-15-16(12-14)18(26-20(23)24)25-13-17(15)22/h4-5,12-13,27H,2-3,6-11H2,1H3,(H4,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... |
J Med Chem 50: 2341-51 (2007)
Article DOI: 10.1021/jm061066t
BindingDB Entry DOI: 10.7270/Q27S7M18 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50414129
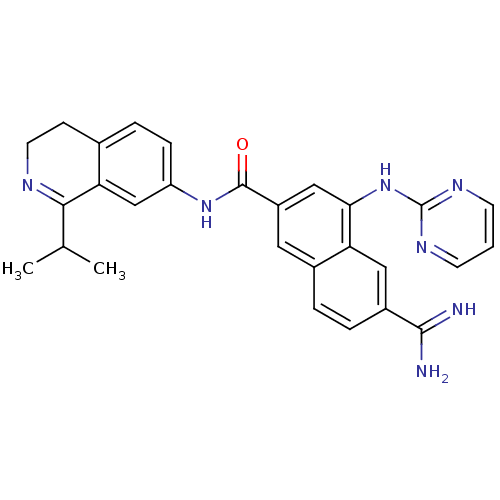
(CHEMBL562412)Show SMILES CC(C)C1=NCCc2ccc(NC(=O)c3cc(Nc4ncccn4)c4cc(ccc4c3)C(N)=N)cc12 |t:3| Show InChI InChI=1S/C28H27N7O/c1-16(2)25-23-15-21(7-6-17(23)8-11-31-25)34-27(36)20-12-18-4-5-19(26(29)30)13-22(18)24(14-20)35-28-32-9-3-10-33-28/h3-7,9-10,12-16H,8,11H2,1-2H3,(H3,29,30)(H,34,36)(H,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of urokinase |
J Med Chem 52: 3159-65 (2009)
Article DOI: 10.1021/jm801444x
BindingDB Entry DOI: 10.7270/Q2FF3TM8 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16170
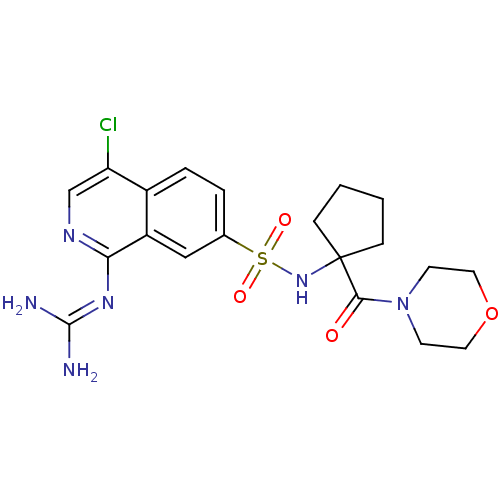
(2-(4-chloro-7-{[1-(morpholin-4-ylcarbonyl)cyclopen...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ncc(Cl)c2ccc(cc12)S(=O)(=O)[#7]C1([#6]-[#6]-[#6]-[#6]1)[#6](=O)-[#7]-1-[#6]-[#6]-[#8]-[#6]-[#6]-1 Show InChI InChI=1S/C20H25ClN6O4S/c21-16-12-24-17(25-19(22)23)15-11-13(3-4-14(15)16)32(29,30)26-20(5-1-2-6-20)18(28)27-7-9-31-10-8-27/h3-4,11-12,26H,1-2,5-10H2,(H4,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... |
J Med Chem 50: 2341-51 (2007)
Article DOI: 10.1021/jm061066t
BindingDB Entry DOI: 10.7270/Q27S7M18 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147096
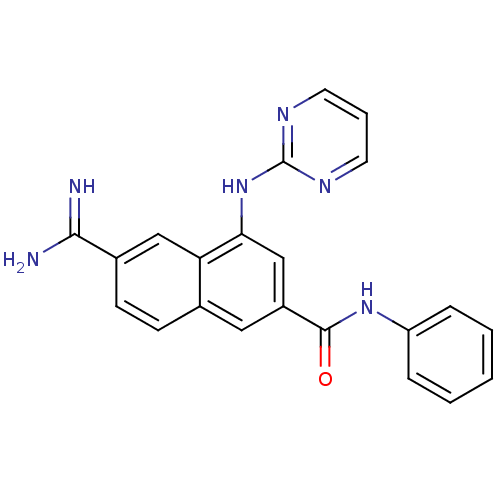
(6-Carbamimidoyl-4-(pyrimidin-2-ylamino)-naphthalen...)Show SMILES NC(=N)c1ccc2cc(cc(Nc3ncccn3)c2c1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C22H18N6O/c23-20(24)15-8-7-14-11-16(21(29)27-17-5-2-1-3-6-17)13-19(18(14)12-15)28-22-25-9-4-10-26-22/h1-13H,(H3,23,24)(H,27,29)(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards Urokinase-type plasminogen activator (urokinase) |
Bioorg Med Chem Lett 14: 3063-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.030
BindingDB Entry DOI: 10.7270/Q29K49PG |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147095
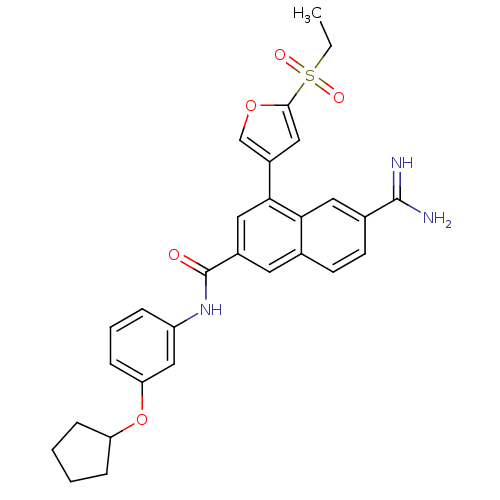
(6-Carbamimidoyl-4-(5-ethanesulfonyl-furan-3-yl)-na...)Show SMILES CCS(=O)(=O)c1cc(co1)-c1cc(cc2ccc(cc12)C(N)=N)C(=O)Nc1cccc(OC2CCCC2)c1 Show InChI InChI=1S/C29H29N3O5S/c1-2-38(34,35)27-15-21(17-36-27)26-14-20(12-18-10-11-19(28(30)31)13-25(18)26)29(33)32-22-6-5-9-24(16-22)37-23-7-3-4-8-23/h5-6,9-17,23H,2-4,7-8H2,1H3,(H3,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards Urokinase-type plasminogen activator (urokinase) |
Bioorg Med Chem Lett 14: 3063-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.030
BindingDB Entry DOI: 10.7270/Q29K49PG |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14352
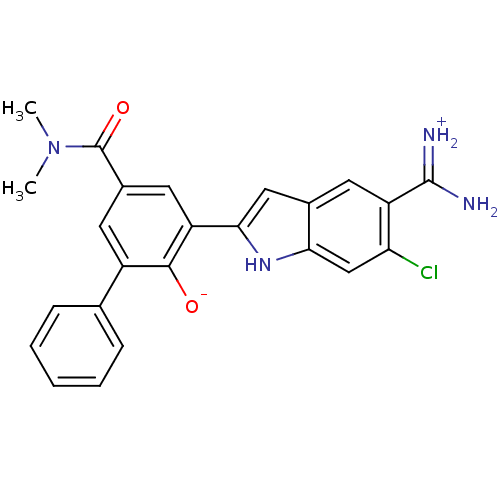
(2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...)Show SMILES CN(C)C(=O)c1cc(-c2cc3cc(C(N)=[NH2+])c(Cl)cc3[nH]2)c([O-])c(c1)-c1ccccc1 Show InChI InChI=1S/C24H21ClN4O2/c1-29(2)24(31)15-9-16(13-6-4-3-5-7-13)22(30)18(10-15)21-11-14-8-17(23(26)27)19(25)12-20(14)28-21/h3-12,28,30H,1-2H3,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14351

(2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...)Show SMILES COc1cc(-c2cc3cc(C(N)=[NH2+])c(Cl)cc3[nH]2)c([O-])c(c1)-c1ccccc1 Show InChI InChI=1S/C22H18ClN3O2/c1-28-14-9-15(12-5-3-2-4-6-12)21(27)17(10-14)20-8-13-7-16(22(24)25)18(23)11-19(13)26-20/h2-11,26-27H,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16165
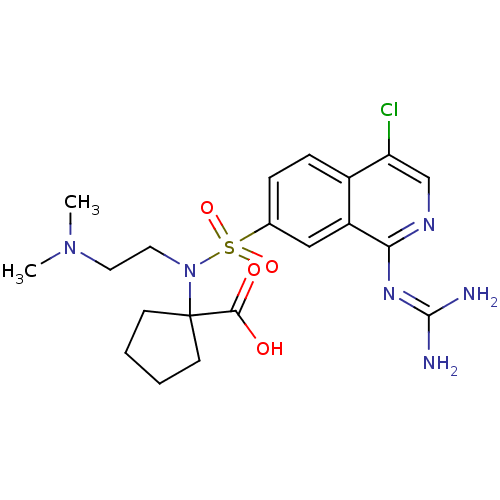
(1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...)Show SMILES [#6]-[#7](-[#6])-[#6]-[#6]-[#7](C1([#6]-[#6]-[#6]-[#6]1)[#6](-[#8])=O)S(=O)(=O)c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1 Show InChI InChI=1S/C20H27ClN6O4S/c1-26(2)9-10-27(20(18(28)29)7-3-4-8-20)32(30,31)13-5-6-14-15(11-13)17(25-19(22)23)24-12-16(14)21/h5-6,11-12H,3-4,7-10H2,1-2H3,(H,28,29)(H4,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... |
J Med Chem 50: 2341-51 (2007)
Article DOI: 10.1021/jm061066t
BindingDB Entry DOI: 10.7270/Q27S7M18 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
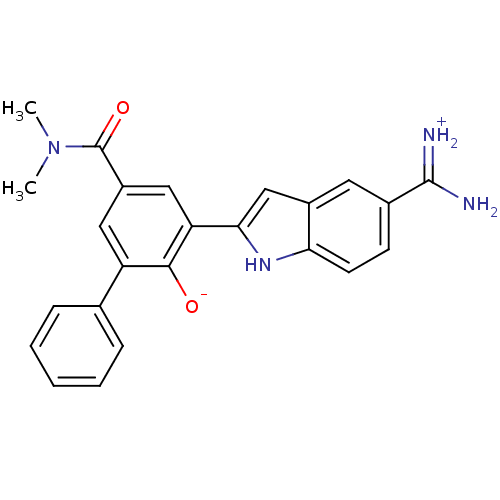
(Homo sapiens (Human)) | BDBM14354
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-(di...)Show SMILES CN(C)C(=O)c1cc(-c2cc3cc(ccc3[nH]2)C(N)=[NH2+])c([O-])c(c1)-c1ccccc1 Show InChI InChI=1S/C24H22N4O2/c1-28(2)24(30)17-11-18(14-6-4-3-5-7-14)22(29)19(12-17)21-13-16-10-15(23(25)26)8-9-20(16)27-21/h3-13,27,29H,1-2H3,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50034585
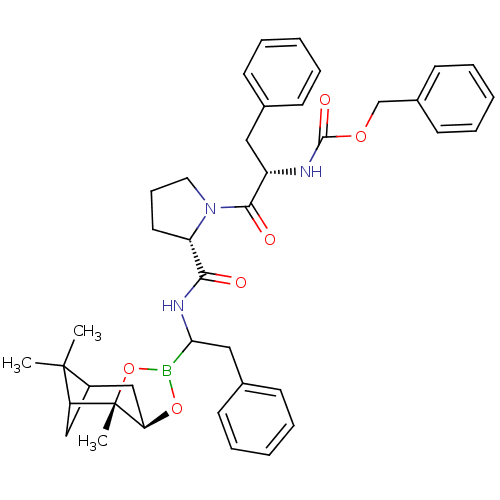
(CHEMBL285285 | Peptide boronate)Show SMILES CC1(C)C2CC1[C@]1(C)OB(O[C@@H]1C2)C(Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C40H48BN3O6/c1-39(2)30-24-33(39)40(3)34(25-30)49-41(50-40)35(23-28-16-9-5-10-17-28)43-36(45)32-20-13-21-44(32)37(46)31(22-27-14-7-4-8-15-27)42-38(47)48-26-29-18-11-6-12-19-29/h4-12,14-19,30-35H,13,20-26H2,1-3H3,(H,42,47)(H,43,45)/t30?,31-,32-,33?,34+,35?,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Urokinase plasminogen activator |
J Med Chem 38: 1511-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QR4W57 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50157087
(6-(2-Phenyl-cyclopropyl)-8-(pyrimidin-2-ylamino)-n...)Show SMILES NC(=N)c1ccc2cc(cc(Nc3ncccn3)c2c1)C1CC1c1ccccc1 Show InChI InChI=1S/C24H21N5/c25-23(26)17-8-7-16-11-18(20-14-19(20)15-5-2-1-3-6-15)13-22(21(16)12-17)29-24-27-9-4-10-28-24/h1-13,19-20H,14H2,(H3,25,26)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity value against urokinase plasminogen activator |
Bioorg Med Chem Lett 15: 93-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.026
BindingDB Entry DOI: 10.7270/Q2R210W5 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50157087

(6-(2-Phenyl-cyclopropyl)-8-(pyrimidin-2-ylamino)-n...)Show SMILES NC(=N)c1ccc2cc(cc(Nc3ncccn3)c2c1)C1CC1c1ccccc1 Show InChI InChI=1S/C24H21N5/c25-23(26)17-8-7-16-11-18(20-14-19(20)15-5-2-1-3-6-15)13-22(21(16)12-17)29-24-27-9-4-10-28-24/h1-13,19-20H,14H2,(H3,25,26)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of urokinase |
J Med Chem 52: 3159-65 (2009)
Article DOI: 10.1021/jm801444x
BindingDB Entry DOI: 10.7270/Q2FF3TM8 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16154
(1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ncc(Cl)c2ccc(cc12)S(=O)(=O)[#7]C1([#6]-[#6]-[#6]-[#6]1)[#6](-[#8])=O Show InChI InChI=1S/C16H18ClN5O4S/c17-12-8-20-13(21-15(18)19)11-7-9(3-4-10(11)12)27(25,26)22-16(14(23)24)5-1-2-6-16/h3-4,7-8,22H,1-2,5-6H2,(H,23,24)(H4,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... |
J Med Chem 50: 2341-51 (2007)
Article DOI: 10.1021/jm061066t
BindingDB Entry DOI: 10.7270/Q27S7M18 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138673
(6-Carbamimidoyl-naphthalene-2-carboxylic acid (4-e...)Show SMILES CCC1CNCc2ccc(NC(=O)c3ccc4cc(ccc4c3)C(N)=N)cc12 Show InChI InChI=1S/C23H24N4O/c1-2-14-12-26-13-19-7-8-20(11-21(14)19)27-23(28)18-6-4-15-9-17(22(24)25)5-3-16(15)10-18/h3-11,14,26H,2,12-13H2,1H3,(H3,24,25)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human urokinase-type plasminogen activator (microPa). |
J Med Chem 47: 303-24 (2004)
Article DOI: 10.1021/jm0300072
BindingDB Entry DOI: 10.7270/Q2BR8RMH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138673

(6-Carbamimidoyl-naphthalene-2-carboxylic acid (4-e...)Show SMILES CCC1CNCc2ccc(NC(=O)c3ccc4cc(ccc4c3)C(N)=N)cc12 Show InChI InChI=1S/C23H24N4O/c1-2-14-12-26-13-19-7-8-20(11-21(14)19)27-23(28)18-6-4-15-9-17(22(24)25)5-3-16(15)10-18/h3-11,14,26H,2,12-13H2,1H3,(H3,24,25)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of urokinase |
J Med Chem 52: 3159-65 (2009)
Article DOI: 10.1021/jm801444x
BindingDB Entry DOI: 10.7270/Q2FF3TM8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16153
(1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ncc(Cl)c2ccc(cc12)S(=O)(=O)[#7]C1([#6]-[#6]-[#6]1)[#6](-[#8])=O Show InChI InChI=1S/C15H16ClN5O4S/c16-11-7-19-12(20-14(17)18)10-6-8(2-3-9(10)11)26(24,25)21-15(13(22)23)4-1-5-15/h2-3,6-7,21H,1,4-5H2,(H,22,23)(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... |
J Med Chem 50: 2341-51 (2007)
Article DOI: 10.1021/jm061066t
BindingDB Entry DOI: 10.7270/Q27S7M18 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16156
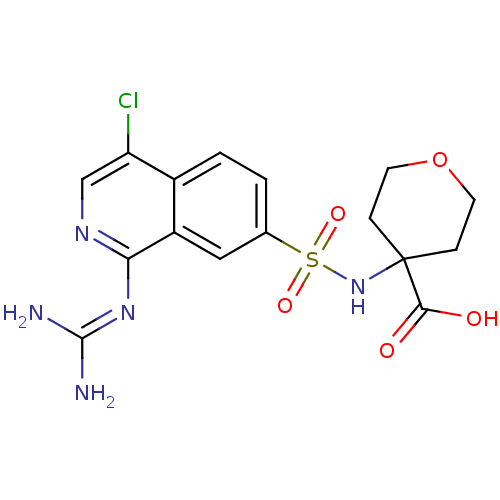
(4-({4-chloro-1-[(diaminomethylidene)amino]isoquino...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ncc(Cl)c2ccc(cc12)S(=O)(=O)[#7]C1([#6]-[#6]-[#8]-[#6]-[#6]1)[#6](-[#8])=O Show InChI InChI=1S/C16H18ClN5O5S/c17-12-8-20-13(21-15(18)19)11-7-9(1-2-10(11)12)28(25,26)22-16(14(23)24)3-5-27-6-4-16/h1-2,7-8,22H,3-6H2,(H,23,24)(H4,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... |
J Med Chem 50: 2341-51 (2007)
Article DOI: 10.1021/jm061066t
BindingDB Entry DOI: 10.7270/Q27S7M18 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110025
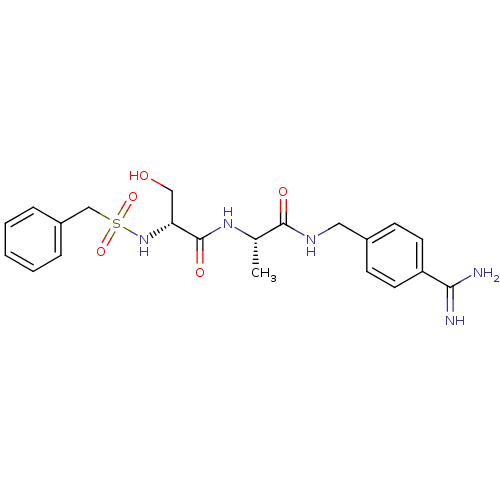
(CHEMBL158405 | N-(BENZYLSULFONYL)-D-SERYL-N-{4-[AM...)Show SMILES C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C21H27N5O5S/c1-14(20(28)24-11-15-7-9-17(10-8-15)19(22)23)25-21(29)18(12-27)26-32(30,31)13-16-5-3-2-4-6-16/h2-10,14,18,26-27H,11-13H2,1H3,(H3,22,23)(H,24,28)(H,25,29)/t14-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA using pyroGlu-Gly-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins by spectrop... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110025

(CHEMBL158405 | N-(BENZYLSULFONYL)-D-SERYL-N-{4-[AM...)Show SMILES C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C21H27N5O5S/c1-14(20(28)24-11-15-7-9-17(10-8-15)19(22)23)25-21(29)18(12-27)26-32(30,31)13-16-5-3-2-4-6-16/h2-10,14,18,26-27H,11-13H2,1H3,(H3,22,23)(H,24,28)(H,25,29)/t14-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102780

(2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of human urokinase type plasminogen activator (microPa) |
J Med Chem 44: 3856-71 (2001)
BindingDB Entry DOI: 10.7270/Q22R3QXR |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102780

(2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16155
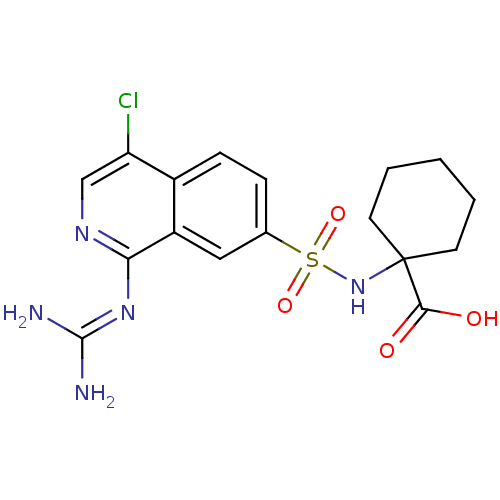
(1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ncc(Cl)c2ccc(cc12)S(=O)(=O)[#7]C1([#6]-[#6]-[#6]-[#6]-[#6]1)[#6](-[#8])=O Show InChI InChI=1S/C17H20ClN5O4S/c18-13-9-21-14(22-16(19)20)12-8-10(4-5-11(12)13)28(26,27)23-17(15(24)25)6-2-1-3-7-17/h4-5,8-9,23H,1-3,6-7H2,(H,24,25)(H4,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... |
J Med Chem 50: 2341-51 (2007)
Article DOI: 10.1021/jm061066t
BindingDB Entry DOI: 10.7270/Q27S7M18 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115874
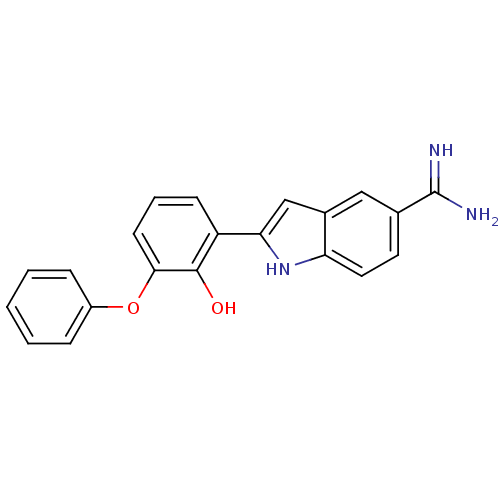
(2-(2-Hydroxy-3-phenoxy-phenyl)-1H-indole-5-carboxa...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(Oc2ccccc2)c1O Show InChI InChI=1S/C21H17N3O2/c22-21(23)13-9-10-17-14(11-13)12-18(24-17)16-7-4-8-19(20(16)25)26-15-5-2-1-3-6-15/h1-12,24-25H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14142
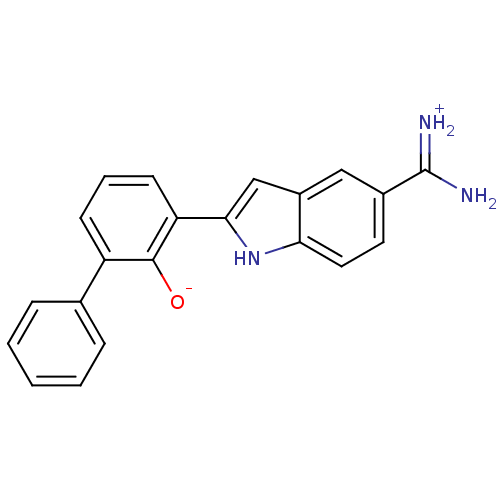
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1[O-] Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 8 | -10.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147089
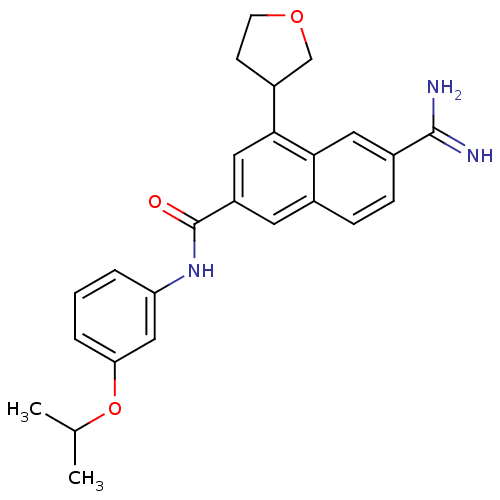
(6-Carbamimidoyl-4-(tetrahydro-furan-3-yl)-naphthal...)Show SMILES CC(C)Oc1cccc(NC(=O)c2cc(C3CCOC3)c3cc(ccc3c2)C(N)=N)c1 Show InChI InChI=1S/C25H27N3O3/c1-15(2)31-21-5-3-4-20(13-21)28-25(29)19-10-16-6-7-17(24(26)27)11-22(16)23(12-19)18-8-9-30-14-18/h3-7,10-13,15,18H,8-9,14H2,1-2H3,(H3,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards Urokinase-type plasminogen activator (urokinase) |
Bioorg Med Chem Lett 14: 3063-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.030
BindingDB Entry DOI: 10.7270/Q29K49PG |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147416

(3-(4-Chloro-1-guanidino-isoquinolin-7-yl)-4-methox...)Show SMILES [#6]-[#8]-c1ccc(cc1-c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1)-[#6](-[#8])=O Show InChI InChI=1S/C18H15ClN4O3/c1-26-15-5-3-10(17(24)25)7-12(15)9-2-4-11-13(6-9)16(23-18(20)21)22-8-14(11)19/h2-8H,1H3,(H,24,25)(H4,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92321
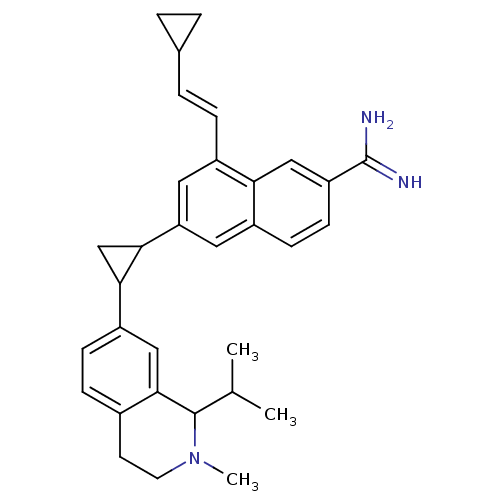
(uPa_43)Show SMILES CC(C)C1N(C)CCc2ccc(cc12)C1CC1c1cc(\C=C\C2CC2)c2cc(ccc2c1)C(N)=N Show InChI InChI=1S/C32H37N3/c1-19(2)31-30-16-24(9-8-21(30)12-13-35(31)3)28-18-29(28)26-14-22(7-6-20-4-5-20)27-17-25(32(33)34)11-10-23(27)15-26/h6-11,14-17,19-20,28-29,31H,4-5,12-13,18H2,1-3H3,(H3,33,34)/b7-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | -11.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50106240
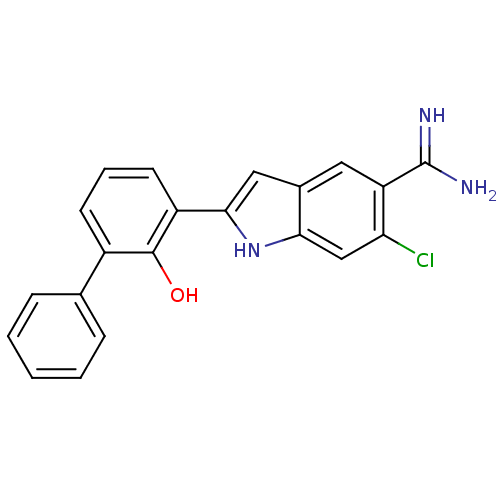
(6-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...)Show SMILES NC(=N)c1cc2cc([nH]c2cc1Cl)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16ClN3O/c22-17-11-18-13(9-16(17)21(23)24)10-19(25-18)15-8-4-7-14(20(15)26)12-5-2-1-3-6-12/h1-11,25-26H,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of human urokinase type plasminogen activator (microPa) |
J Med Chem 44: 3856-71 (2001)
BindingDB Entry DOI: 10.7270/Q22R3QXR |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14152
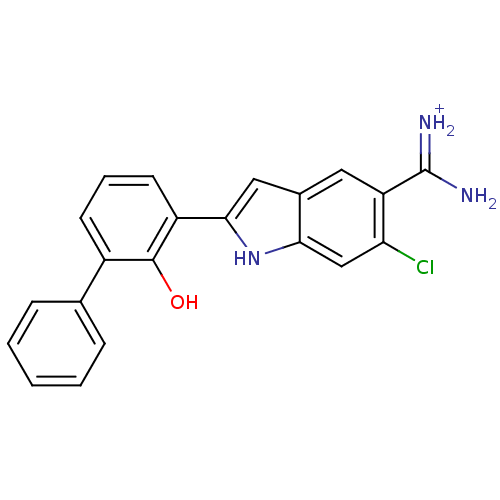
(6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-C...)Show SMILES NC(=[NH2+])c1cc2cc([nH]c2cc1Cl)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16ClN3O/c22-17-11-18-13(9-16(17)21(23)24)10-19(25-18)15-8-4-7-14(20(15)26)12-5-2-1-3-6-12/h1-11,25-26H,(H3,23,24)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 9 | -10.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16159
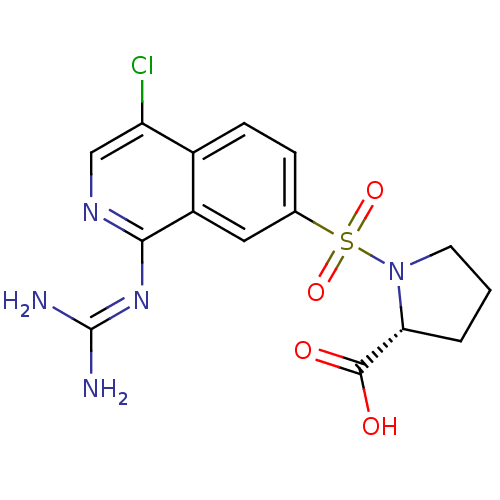
((2R)-1-({4-chloro-1-[(diaminomethylidene)amino]iso...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ncc(Cl)c2ccc(cc12)S(=O)(=O)[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](-[#8])=O |r| Show InChI InChI=1S/C15H16ClN5O4S/c16-11-7-19-13(20-15(17)18)10-6-8(3-4-9(10)11)26(24,25)21-5-1-2-12(21)14(22)23/h3-4,6-7,12H,1-2,5H2,(H,22,23)(H4,17,18,19,20)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... |
J Med Chem 50: 2341-51 (2007)
Article DOI: 10.1021/jm061066t
BindingDB Entry DOI: 10.7270/Q27S7M18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16152
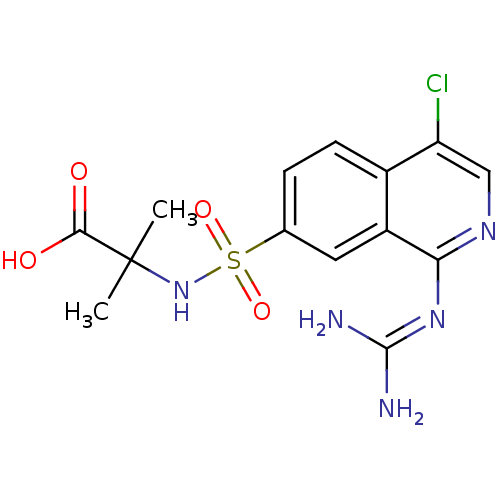
(2-({4-chloro-1-[(diaminomethylidene)amino]isoquino...)Show SMILES [#6]C([#6])([#7]S(=O)(=O)c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1)[#6](-[#8])=O Show InChI InChI=1S/C14H16ClN5O4S/c1-14(2,12(21)22)20-25(23,24)7-3-4-8-9(5-7)11(19-13(16)17)18-6-10(8)15/h3-6,20H,1-2H3,(H,21,22)(H4,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA)
Curated by ChEMBL
| Assay Description
Inhibition of human uPA using S-2444 as substrate |
J Med Chem 58: 9238-57 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01171
BindingDB Entry DOI: 10.7270/Q241713M |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50157094
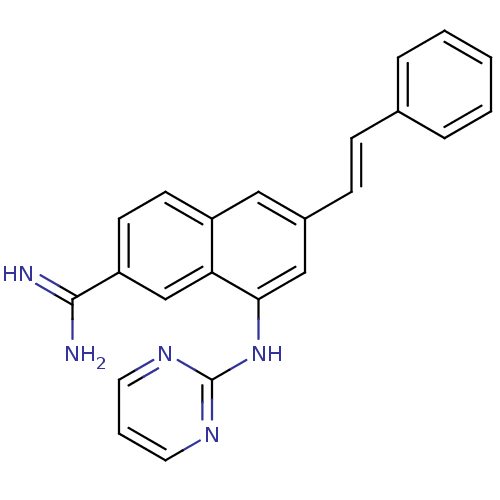
(8-(Pyrimidin-2-ylamino)-6-((E)-styryl)-naphthalene...)Show SMILES NC(=N)c1ccc2cc(\C=C\c3ccccc3)cc(Nc3ncccn3)c2c1 Show InChI InChI=1S/C23H19N5/c24-22(25)19-10-9-18-13-17(8-7-16-5-2-1-3-6-16)14-21(20(18)15-19)28-23-26-11-4-12-27-23/h1-15H,(H3,24,25)(H,26,27,28)/b8-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity value against urokinase plasminogen activator |
Bioorg Med Chem Lett 15: 93-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.026
BindingDB Entry DOI: 10.7270/Q2R210W5 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16169
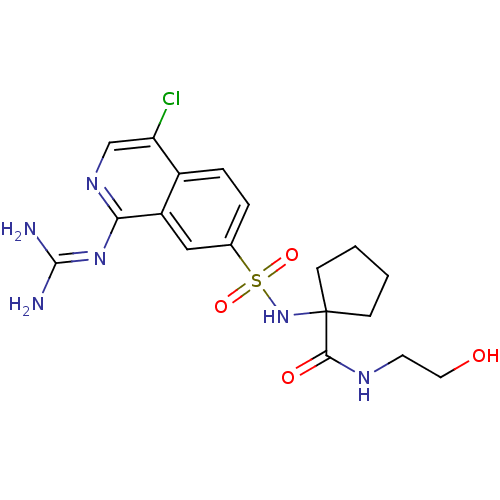
(1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ncc(Cl)c2ccc(cc12)S(=O)(=O)[#7]C1([#6]-[#6]-[#6]-[#6]1)[#6](=O)-[#7]-[#6]-[#6]-[#8] Show InChI InChI=1S/C18H23ClN6O4S/c19-14-10-23-15(24-17(20)21)13-9-11(3-4-12(13)14)30(28,29)25-18(5-1-2-6-18)16(27)22-7-8-26/h3-4,9-10,25-26H,1-2,5-8H2,(H,22,27)(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... |
J Med Chem 50: 2341-51 (2007)
Article DOI: 10.1021/jm061066t
BindingDB Entry DOI: 10.7270/Q27S7M18 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50003738
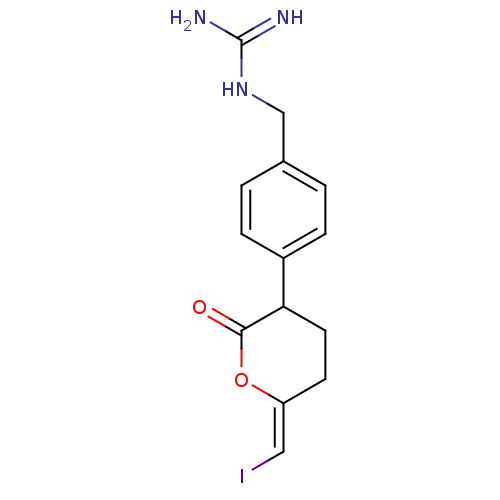
((lactone 2)N-[4-(6-Iodomethylene-2-oxo-tetrahydro-...)Show InChI InChI=1S/C14H16IN3O2/c15-7-11-5-6-12(13(19)20-11)10-3-1-9(2-4-10)8-18-14(16)17/h1-4,7,12H,5-6,8H2,(H4,16,17,18)/b11-7- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against the enzyme Urokinase-type plasminogen activator |
J Med Chem 35: 4297-305 (1992)
BindingDB Entry DOI: 10.7270/Q2TD9W9D |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16152

(2-({4-chloro-1-[(diaminomethylidene)amino]isoquino...)Show SMILES [#6]C([#6])([#7]S(=O)(=O)c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1)[#6](-[#8])=O Show InChI InChI=1S/C14H16ClN5O4S/c1-14(2,12(21)22)20-25(23,24)7-3-4-8-9(5-7)11(19-13(16)17)18-6-10(8)15/h3-6,20H,1-2H3,(H,21,22)(H4,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... |
J Med Chem 50: 2341-51 (2007)
Article DOI: 10.1021/jm061066t
BindingDB Entry DOI: 10.7270/Q27S7M18 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81608

(Isocoumarin, 9b)Show SMILES NC(=N)SCCCOc1oc(=O)c2cc(NC(=O)c3ccccc3)ccc2c1Cl Show InChI InChI=1S/C20H18ClN3O4S/c21-16-14-8-7-13(24-17(25)12-5-2-1-3-6-12)11-15(14)18(26)28-19(16)27-9-4-10-29-20(22)23/h1-3,5-8,11H,4,9-10H2,(H3,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
| Assay Description
Inhibition of human urokinase-type plasminogen activator (uPA). |
BMC Chem Biol 6: 1 (2006)
Article DOI: 10.1186/1472-6769-6-1
BindingDB Entry DOI: 10.7270/Q20C4T8F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115868
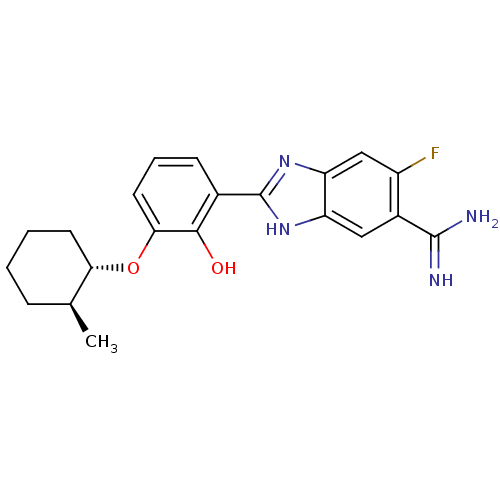
(2-{5-[AMINO(IMINIO)METHYL]-6-FLUORO-1H-BENZIMIDAZO...)Show SMILES C[C@H]1CCCC[C@@H]1Oc1cccc(-c2nc3cc(F)c(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C21H23FN4O2/c1-11-5-2-3-7-17(11)28-18-8-4-6-12(19(18)27)21-25-15-9-13(20(23)24)14(22)10-16(15)26-21/h4,6,8-11,17,27H,2-3,5,7H2,1H3,(H3,23,24)(H,25,26)/t11-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
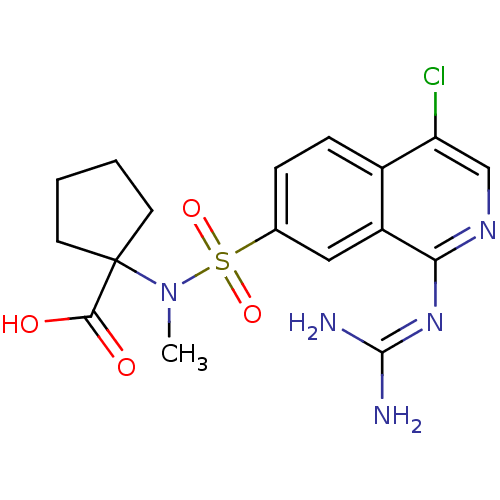
(Homo sapiens (Human)) | BDBM16164
(1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...)Show SMILES [#6]-[#7](C1([#6]-[#6]-[#6]-[#6]1)[#6](-[#8])=O)S(=O)(=O)c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1 Show InChI InChI=1S/C17H20ClN5O4S/c1-23(17(15(24)25)6-2-3-7-17)28(26,27)10-4-5-11-12(8-10)14(22-16(19)20)21-9-13(11)18/h4-5,8-9H,2-3,6-7H2,1H3,(H,24,25)(H4,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... |
J Med Chem 50: 2341-51 (2007)
Article DOI: 10.1021/jm061066t
BindingDB Entry DOI: 10.7270/Q27S7M18 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16157
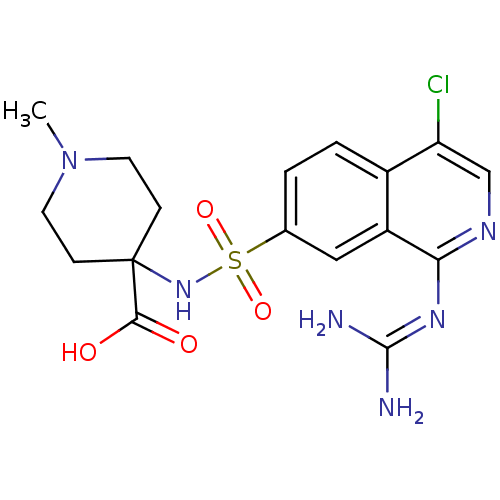
(4-({4-chloro-1-[(diaminomethylidene)amino]isoquino...)Show SMILES [#6]-[#7]-1-[#6]-[#6]C([#6]-[#6]-1)([#7]S(=O)(=O)c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1)[#6](-[#8])=O Show InChI InChI=1S/C17H21ClN6O4S/c1-24-6-4-17(5-7-24,15(25)26)23-29(27,28)10-2-3-11-12(8-10)14(22-16(19)20)21-9-13(11)18/h2-3,8-9,23H,4-7H2,1H3,(H,25,26)(H4,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... |
J Med Chem 50: 2341-51 (2007)
Article DOI: 10.1021/jm061066t
BindingDB Entry DOI: 10.7270/Q27S7M18 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14149
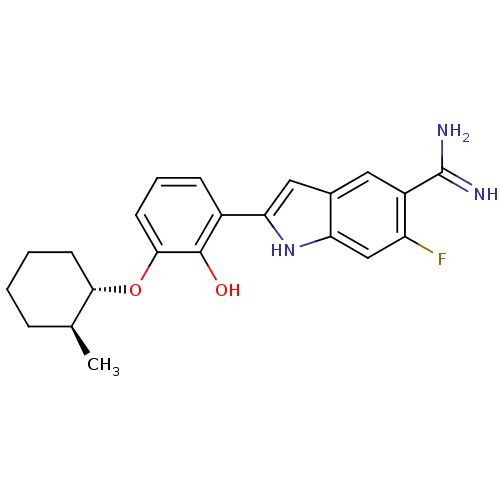
(6-fluoro-2-(2-hydroxy-3-{[(1S,2S)-2-methylcyclohex...)Show SMILES C[C@H]1CCCC[C@@H]1Oc1cccc(-c2cc3cc(C(N)=N)c(F)cc3[nH]2)c1O |r| Show InChI InChI=1S/C22H24FN3O2/c1-12-5-2-3-7-19(12)28-20-8-4-6-14(21(20)27)18-10-13-9-15(22(24)25)16(23)11-17(13)26-18/h4,6,8-12,19,26-27H,2-3,5,7H2,1H3,(H3,24,25)/t12-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 11 | -10.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14152

(6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-C...)Show SMILES NC(=[NH2+])c1cc2cc([nH]c2cc1Cl)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16ClN3O/c22-17-11-18-13(9-16(17)21(23)24)10-19(25-18)15-8-4-7-14(20(15)26)12-5-2-1-3-6-12/h1-11,25-26H,(H3,23,24)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50110023
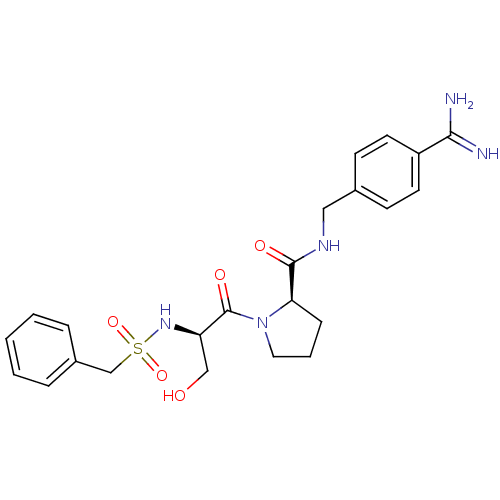
(2-(3-Hydroxy-2-phenylmethanesulfonylamino-propiony...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H]2CCCN2C(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C23H29N5O5S/c24-21(25)18-10-8-16(9-11-18)13-26-22(30)20-7-4-12-28(20)23(31)19(14-29)27-34(32,33)15-17-5-2-1-3-6-17/h1-3,5-6,8-11,19-20,27,29H,4,7,12-15H2,(H3,24,25)(H,26,30)/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Jena
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasminogen activator urokinase. |
Bioorg Med Chem Lett 12: 645-8 (2002)
BindingDB Entry DOI: 10.7270/Q2NZ86XS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14152

(6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-C...)Show SMILES NC(=[NH2+])c1cc2cc([nH]c2cc1Cl)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16ClN3O/c22-17-11-18-13(9-16(17)21(23)24)10-19(25-18)15-8-4-7-14(20(15)26)12-5-2-1-3-6-12/h1-11,25-26H,(H3,23,24)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 344: 527-47 (2004)
Article DOI: 10.1016/j.jmb.2004.09.032
BindingDB Entry DOI: 10.7270/Q2V40SF3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16166
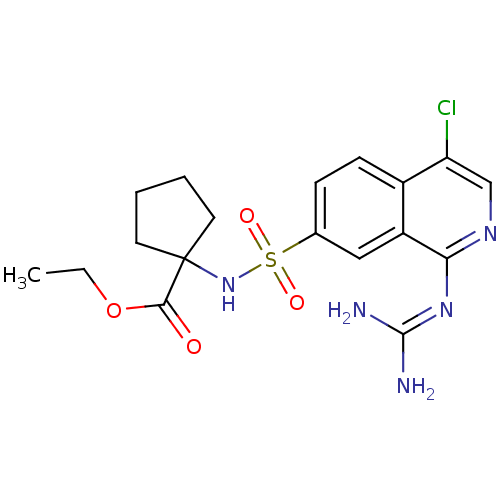
(cycloleucine deriv. 40 | ethyl 1-({4-chloro-1-[(di...)Show SMILES [#6]-[#6]-[#8]-[#6](=O)C1([#6]-[#6]-[#6]-[#6]1)[#7]S(=O)(=O)c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1 Show InChI InChI=1S/C18H22ClN5O4S/c1-2-28-16(25)18(7-3-4-8-18)24-29(26,27)11-5-6-12-13(9-11)15(23-17(20)21)22-10-14(12)19/h5-6,9-10,24H,2-4,7-8H2,1H3,(H4,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... |
J Med Chem 50: 2341-51 (2007)
Article DOI: 10.1021/jm061066t
BindingDB Entry DOI: 10.7270/Q27S7M18 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50034579
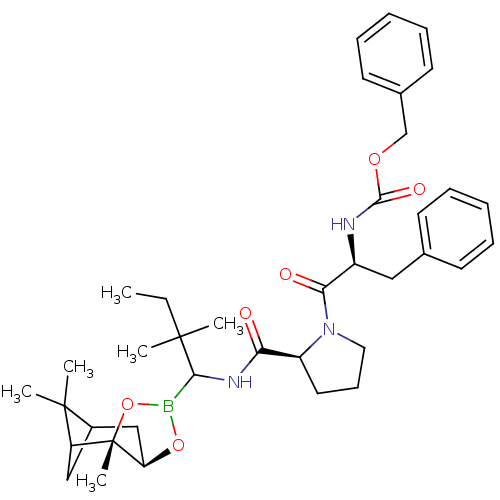
(CHEMBL290577 | Peptide boronate)Show SMILES CCC(C)(C)C(NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1 Show InChI InChI=1S/C38H52BN3O6/c1-7-36(2,3)34(39-47-31-23-27-22-30(37(27,4)5)38(31,6)48-39)41-32(43)29-19-14-20-42(29)33(44)28(21-25-15-10-8-11-16-25)40-35(45)46-24-26-17-12-9-13-18-26/h8-13,15-18,27-31,34H,7,14,19-24H2,1-6H3,(H,40,45)(H,41,43)/t27?,28-,29-,30?,31+,34?,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Urokinase plasminogen activator |
J Med Chem 38: 1511-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QR4W57 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data