

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366426 (CHEMBL4161819) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50158914 (3-(1-Benzyl-1H-imidazol-4-yl)-pyridine | CHEMBL178...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration value against human cytochrome P-450 2E1 | J Med Chem 48: 224-39 (2005) Article DOI: 10.1021/jm049696n BindingDB Entry DOI: 10.7270/Q2T154DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50010231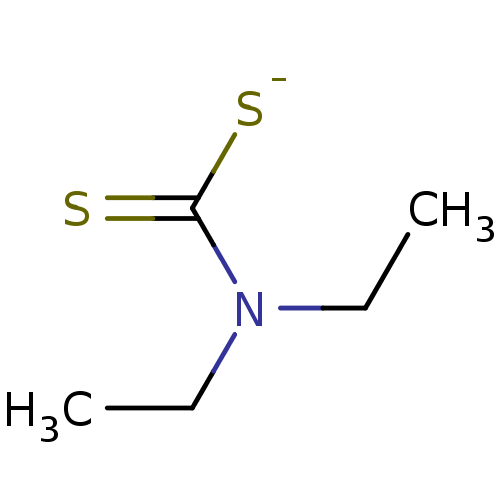 (CHEMBL107217 | Ditiocarb sodium | Sodium salt of D...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50110231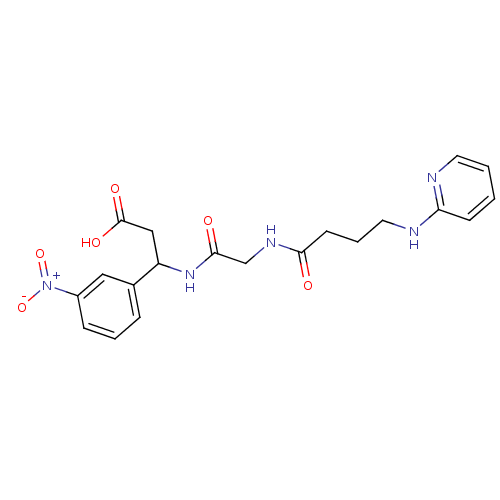 (3-(3-Nitro-phenyl)-3-{2-[4-(pyridin-2-ylamino)-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50158925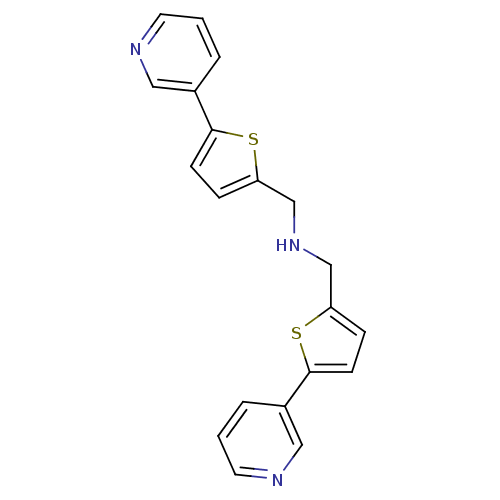 (Bis-(5-pyridin-3-yl-thiophen-2-ylmethyl)-amine | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration value against human cytochrome P-450 2E1 | J Med Chem 48: 224-39 (2005) Article DOI: 10.1021/jm049696n BindingDB Entry DOI: 10.7270/Q2T154DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366334 (CHEMBL4173133) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366407 (CHEMBL4169324) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366379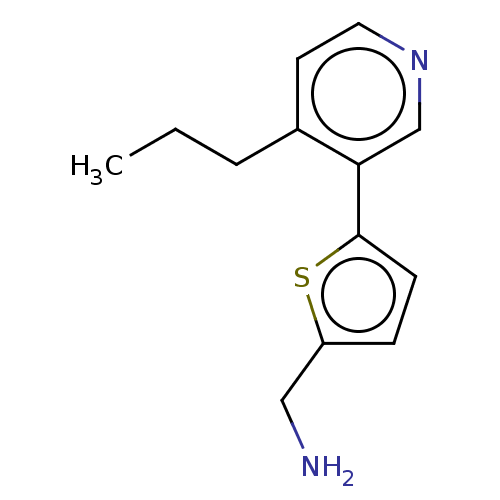 (CHEMBL4165249) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366401 (CHEMBL4169721) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366410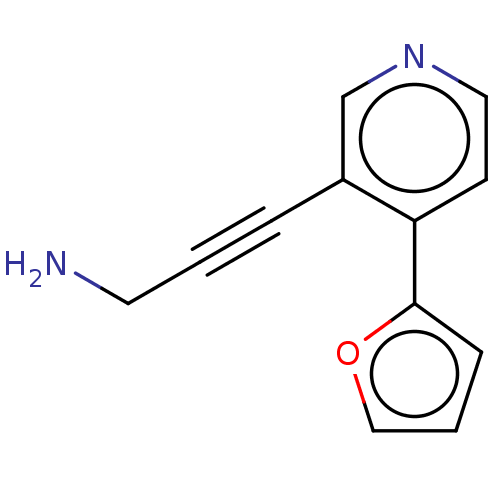 (CHEMBL4163694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366393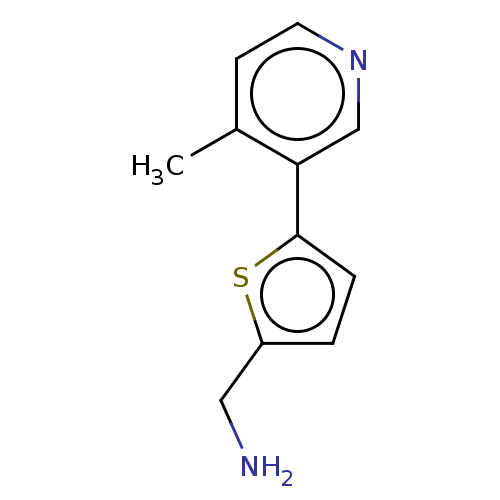 (CHEMBL4176100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366399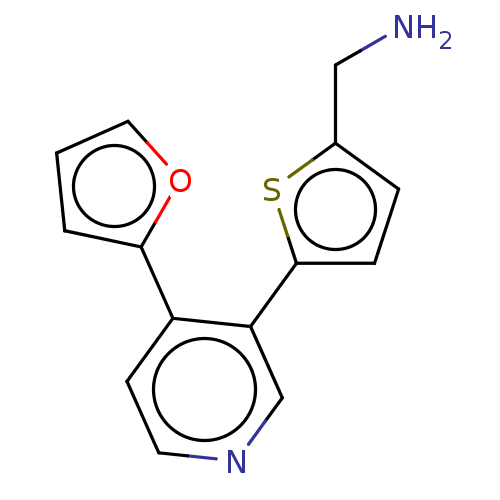 (CHEMBL4168669) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM12352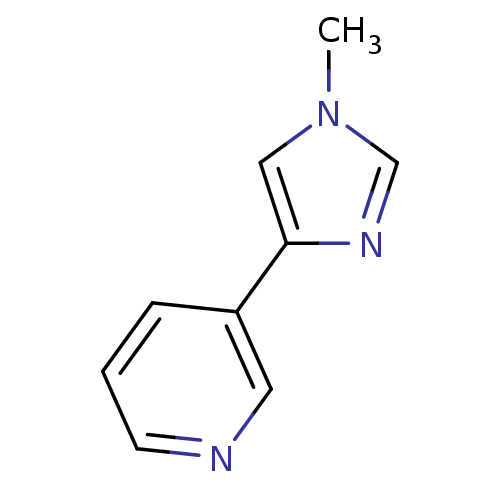 (3-(1-methyl-1H-imidazol-4-yl)pyridine | CHEMBL3609...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration value against human cytochrome P-450 2E1 | J Med Chem 48: 224-39 (2005) Article DOI: 10.1021/jm049696n BindingDB Entry DOI: 10.7270/Q2T154DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50158919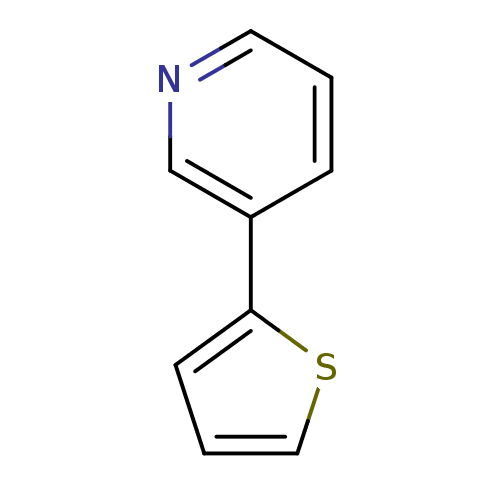 (3-Thiophen-2-yl-pyridine | CHEMBL179618 | US860970...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration value against human cytochrome P-450 2E1 | J Med Chem 48: 224-39 (2005) Article DOI: 10.1021/jm049696n BindingDB Entry DOI: 10.7270/Q2T154DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50158920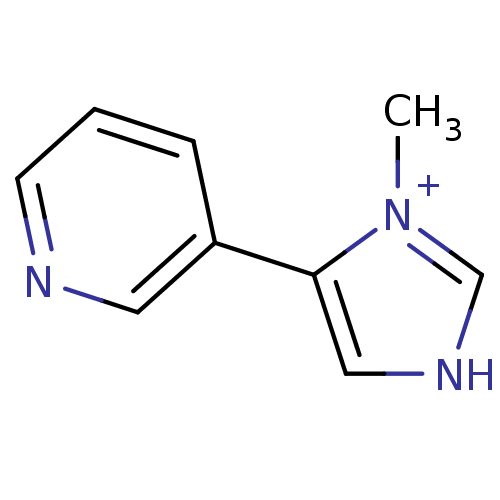 (3-(3-Methyl-1H-imidazol-4-yl)-pyridine | CHEMBL179...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration value against human cytochrome P-450 2E1 | J Med Chem 48: 224-39 (2005) Article DOI: 10.1021/jm049696n BindingDB Entry DOI: 10.7270/Q2T154DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50158923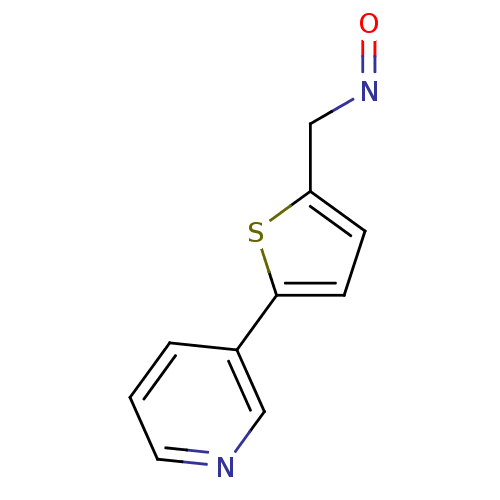 (5-Pyridin-3-yl-thiophene-2-carbaldehyde oxime | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration value against human cytochrome P-450 2E1 | J Med Chem 48: 224-39 (2005) Article DOI: 10.1021/jm049696n BindingDB Entry DOI: 10.7270/Q2T154DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366406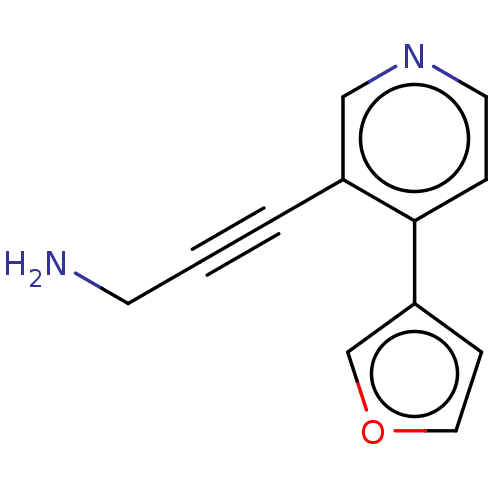 (CHEMBL4174365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366402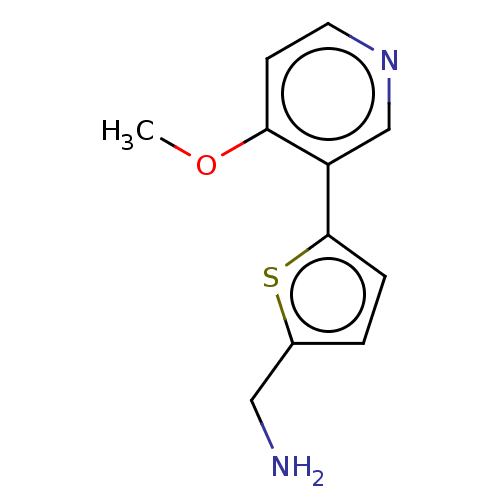 (CHEMBL4172462) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM19188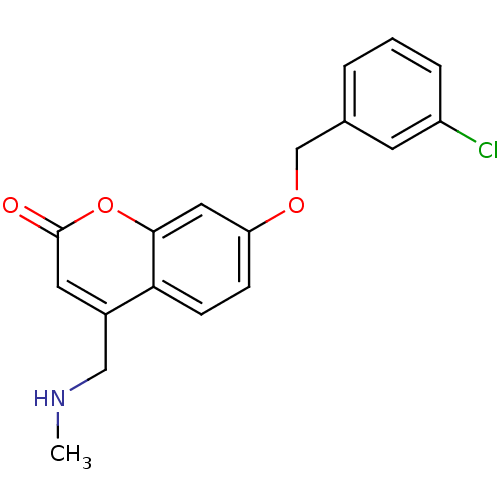 (7-(3-chlorobenzyloxy)-4-(methylamino)methyl-coumar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2E1 | J Med Chem 52: 6685-706 (2009) Article DOI: 10.1021/jm9010127 BindingDB Entry DOI: 10.7270/Q2DR2VJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366411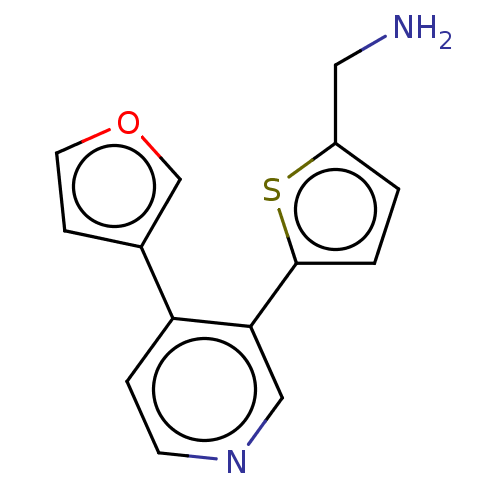 (CHEMBL4160749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366404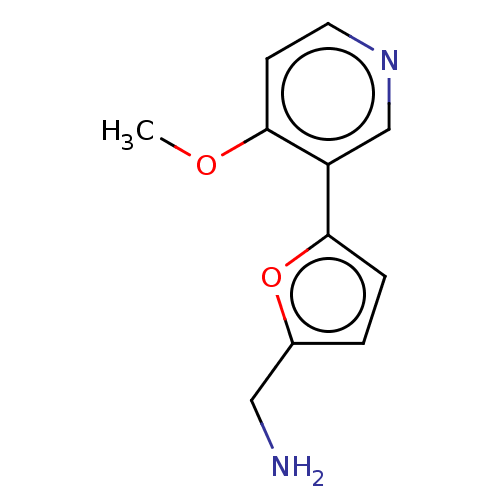 (CHEMBL4162449) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM12358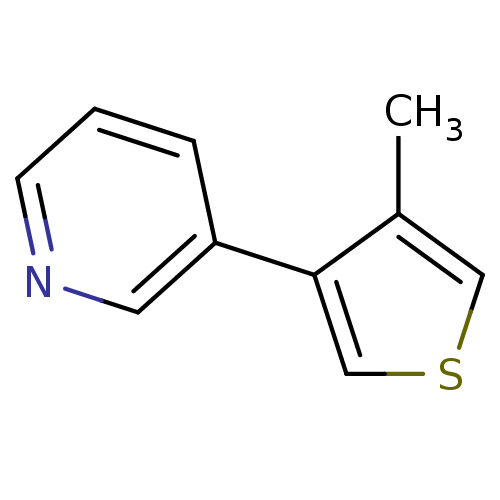 (3-(4-methylthiophen-3-yl)pyridine | CHEMBL179704 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration value against human cytochrome P-450 2E1 | J Med Chem 48: 224-39 (2005) Article DOI: 10.1021/jm049696n BindingDB Entry DOI: 10.7270/Q2T154DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366395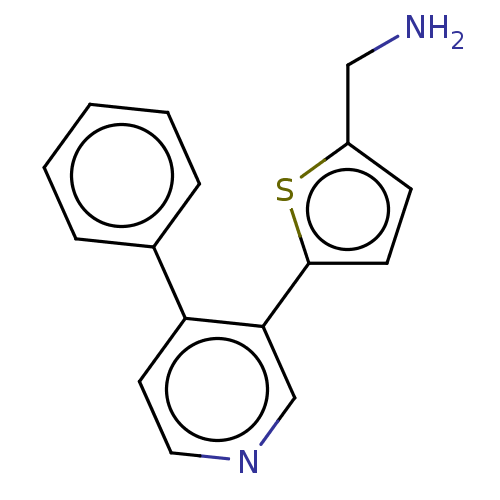 (CHEMBL4175869) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM12351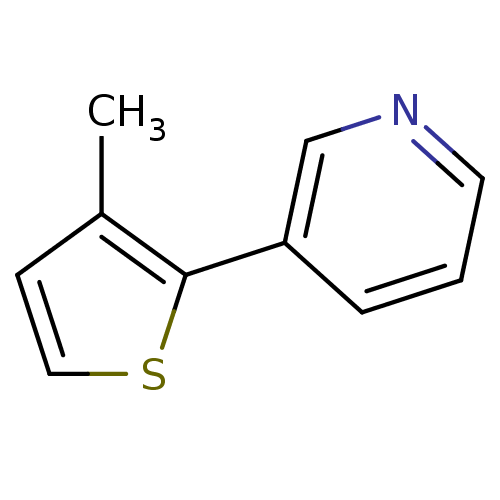 (3-(3-methylthiophen-2-yl)pyridine | CHEMBL179669 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration value against human cytochrome P-450 2E1 | J Med Chem 48: 224-39 (2005) Article DOI: 10.1021/jm049696n BindingDB Entry DOI: 10.7270/Q2T154DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366394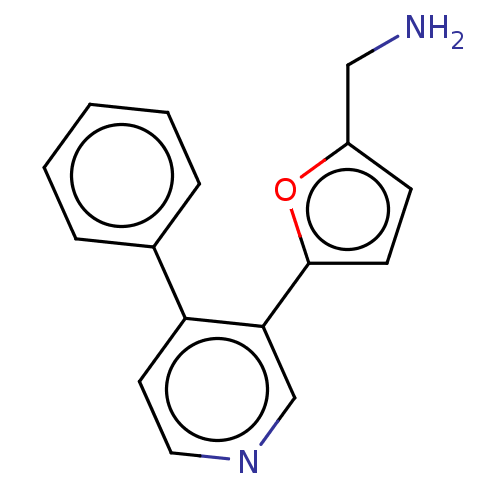 (CHEMBL4159065) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50158913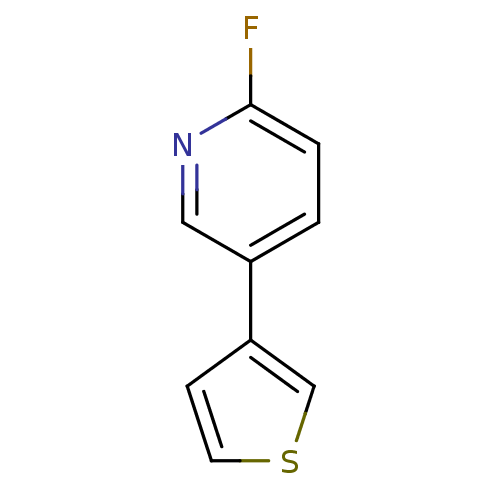 (2-Fluoro-5-thiophen-3-yl-pyridine | CHEMBL179005 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration value against human cytochrome P-450 2E1 | J Med Chem 48: 224-39 (2005) Article DOI: 10.1021/jm049696n BindingDB Entry DOI: 10.7270/Q2T154DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM189322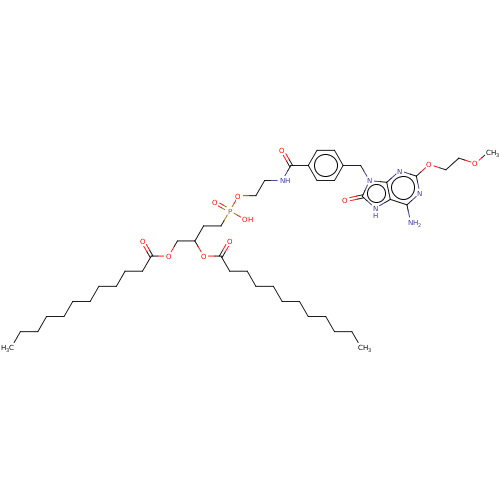 (US9173935, SC12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM190404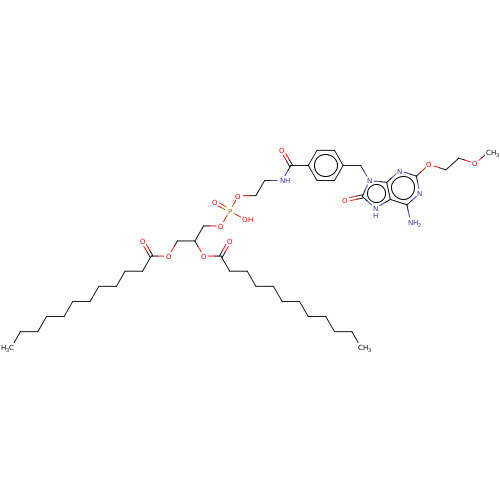 (US9180183, SC12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366435 (CHEMBL4161400) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50017203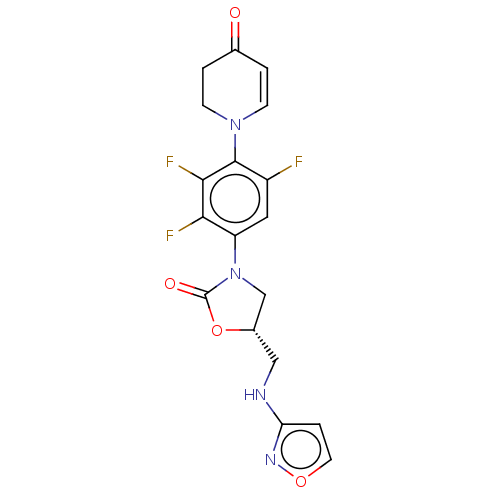 (CHEMBL3287379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MicuRx Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2E1 (unknown origin) | J Med Chem 57: 4487-97 (2014) Article DOI: 10.1021/jm401931e BindingDB Entry DOI: 10.7270/Q2M90B64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50566788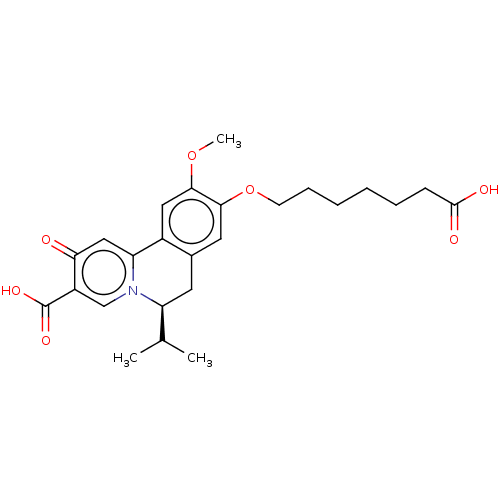 (CHEMBL4850531) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by NADPH addition by LC-MS/MS analys... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00228 BindingDB Entry DOI: 10.7270/Q2WS8Z07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50341410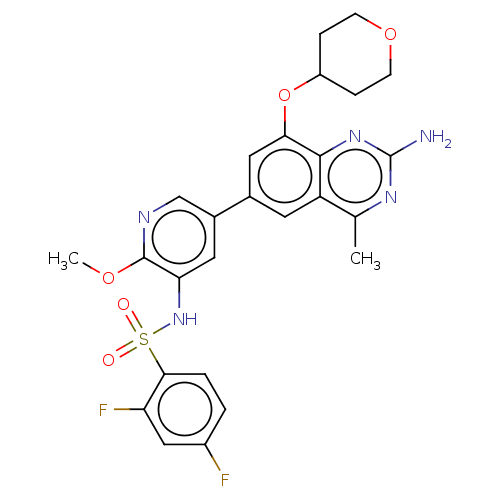 (CHEMBL4166144 | US11534443, Example 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes assessed as reduction in 6-Hydroxychlorzoxazone formation using chlorzoxazone as substrate after 10 to... | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50549522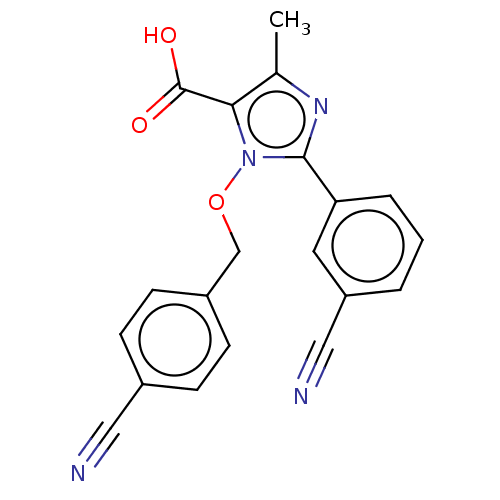 (CHEMBL4786038) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by NADPH generating system addition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01524 BindingDB Entry DOI: 10.7270/Q2GH9NJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50341408 (CHEMBL4166594 | US11534443, Example 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes assessed as reduction in 6-Hydroxychlorzoxazone formation using chlorzoxazone as substrate after 10 to... | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50236754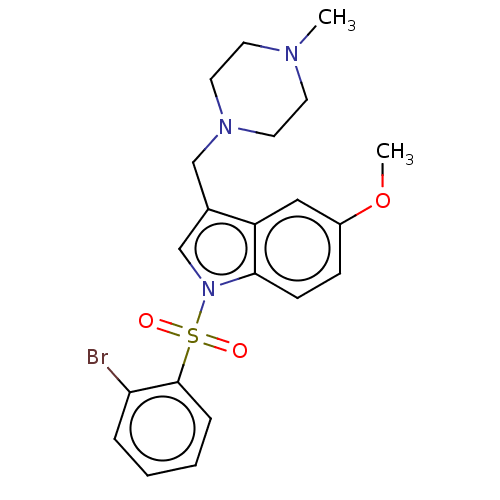 (CHEMBL4082473) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Inhibitory concentration against Herpes simplex virus type 1 thymidine kinase(HSV-1 TK) | J Med Chem 60: 1843-1859 (2017) Article DOI: 10.1021/acs.jmedchem.6b01662 BindingDB Entry DOI: 10.7270/Q27S7R1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50341416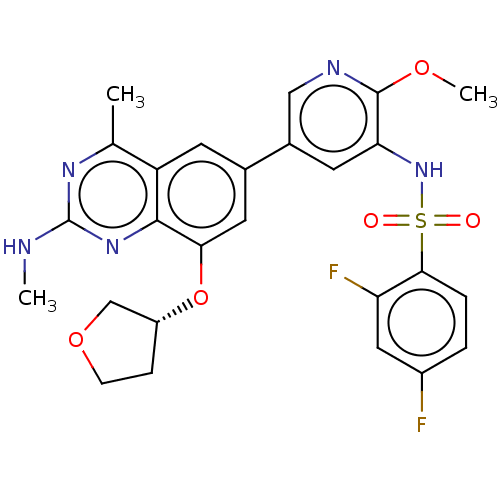 (CHEMBL4174909 | US11534443, Example 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes assessed as reduction in 6-Hydroxychlorzoxazone formation using chlorzoxazone as substrate after 10 to... | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50341409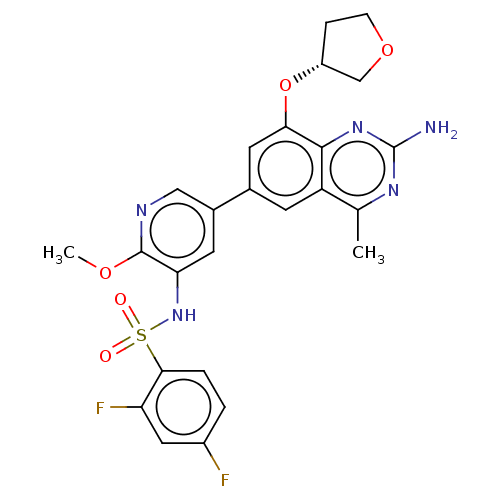 (CHEMBL4176771 | US11534443, Example 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes assessed as reduction in 6-Hydroxychlorzoxazone formation using chlorzoxazone as substrate after 10 to... | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50517497 (CHEMBL4593911) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Inhibition of CYP2E1 (unknown origin) | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50452361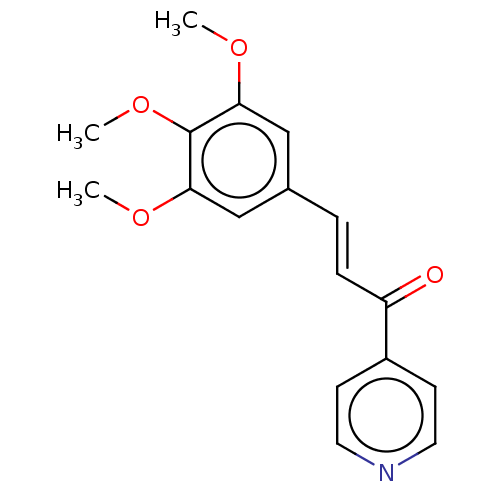 (CHEMBL4218749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
De Montfort University Curated by ChEMBL | Assay Description Inhibition of human CYP2E1 expressed in yeast cells | Bioorg Med Chem Lett 27: 5409-5414 (2017) Article DOI: 10.1016/j.bmcl.2017.11.009 BindingDB Entry DOI: 10.7270/Q2MP55TT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50396352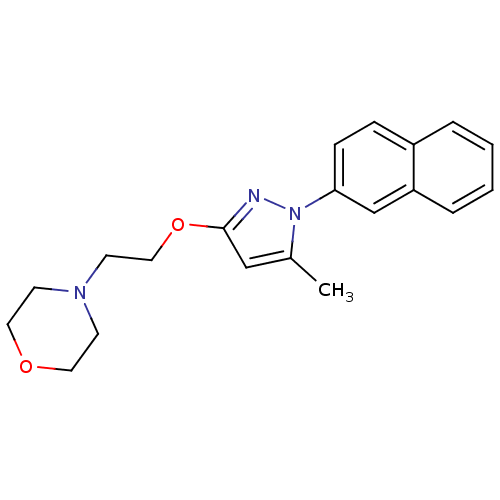 (CHEMBL2170062) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Esteve Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2E1 | J Med Chem 55: 8211-24 (2012) Article DOI: 10.1021/jm3007323 BindingDB Entry DOI: 10.7270/Q2Z89DJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50396352 (CHEMBL2170062) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Esteve Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes | J Med Chem 55: 8211-24 (2012) Article DOI: 10.1021/jm3007323 BindingDB Entry DOI: 10.7270/Q2Z89DJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366392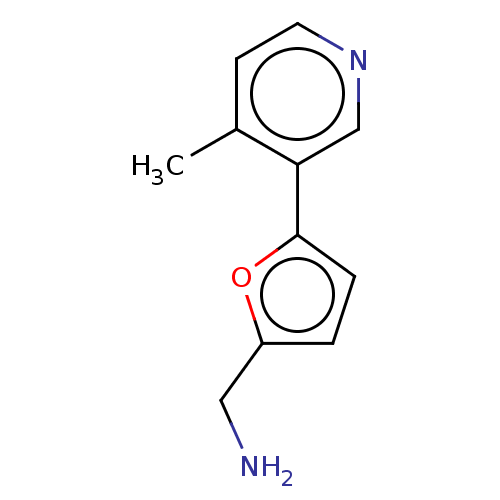 (CHEMBL4172417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50536234 (CHEMBL4580214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of CYP2E1 (unknown origin) | J Med Chem 59: 6293-302 (2016) Article DOI: 10.1021/acs.jmedchem.6b00541 BindingDB Entry DOI: 10.7270/Q23T9MQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366405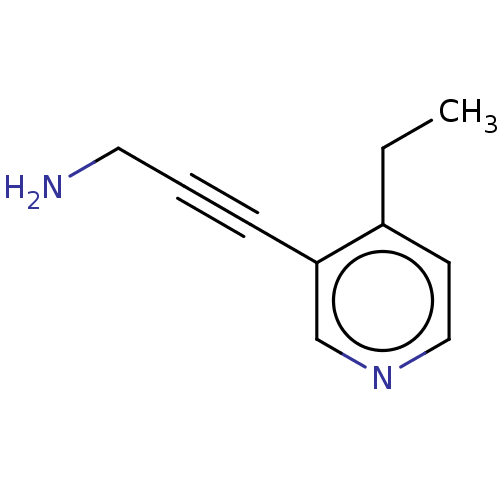 (CHEMBL4175429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366398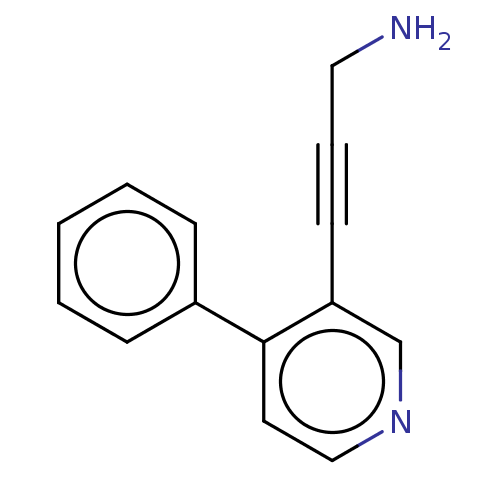 (CHEMBL4164113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366337 (CHEMBL4167560) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM12348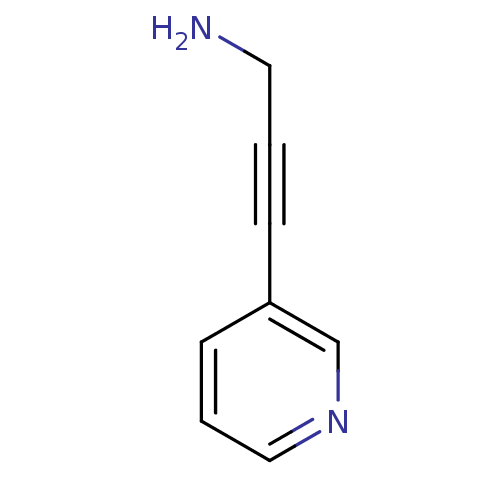 (3-(pyridin-3-yl)prop-2-yn-1-amine | CHEMBL360541 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50366391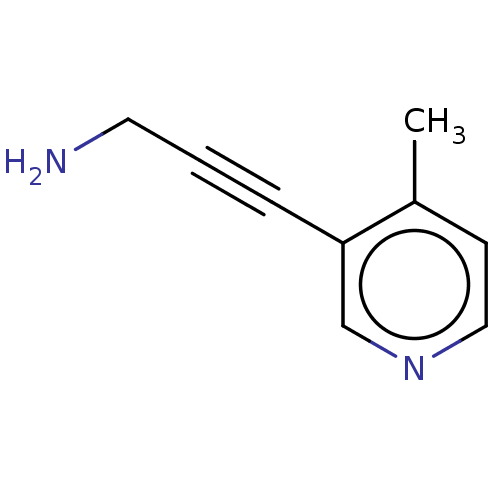 (CHEMBL4173088) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 5 mins followed by addition of NADPH-regenerating sy... | J Med Chem 61: 7065-7086 (2018) Article DOI: 10.1021/acs.jmedchem.8b00084 BindingDB Entry DOI: 10.7270/Q2QN69B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50548260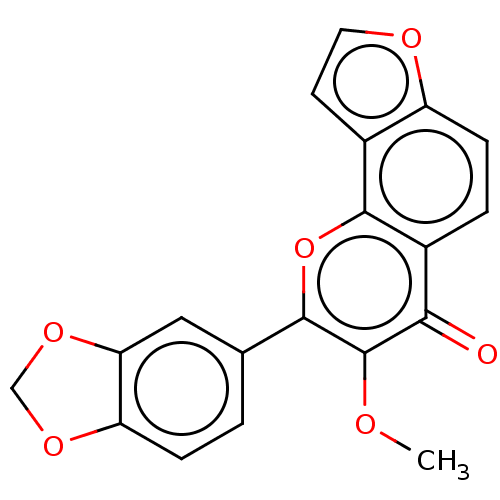 (Pongapin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CYP2E1 expressed in Sacchrosomes by fluorescence based assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.11.013 BindingDB Entry DOI: 10.7270/Q20V8HDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50438846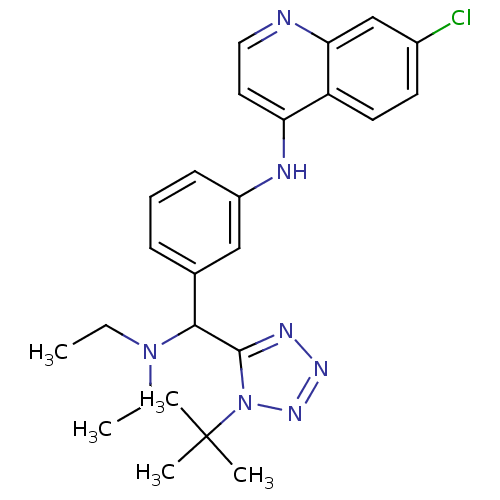 (CHEMBL2413881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes assessed as chlorzoxazone 6-hydroxylation after 20 mins by LC-MS analysis | Bioorg Med Chem 21: 4904-13 (2013) Article DOI: 10.1016/j.bmc.2013.06.067 BindingDB Entry DOI: 10.7270/Q2WH2RD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 149 total ) | Next | Last >> |