

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50501173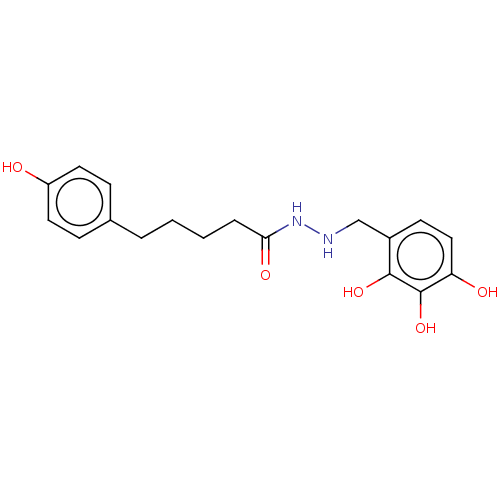 (CHEMBL3968123) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Coxsackievirus B3 3C protease expressed in Escherichia coli BL21 (DE3) preincubated with protein followed by ad... | Eur J Med Chem 120: 202-16 (2016) Article DOI: 10.1016/j.ejmech.2016.03.085 BindingDB Entry DOI: 10.7270/Q25B05HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM92521 (TG-0205486) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | -8.72 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM92520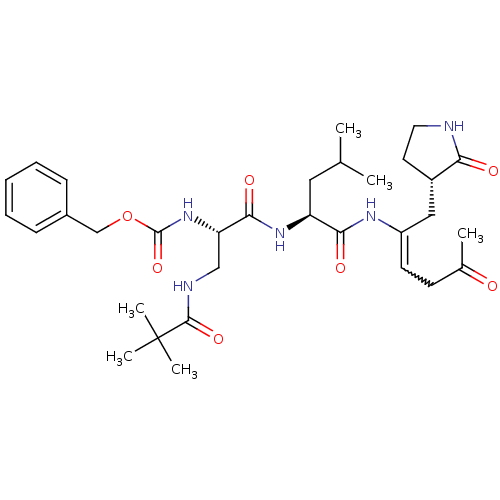 (TG-0204998) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | -8.31 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50501161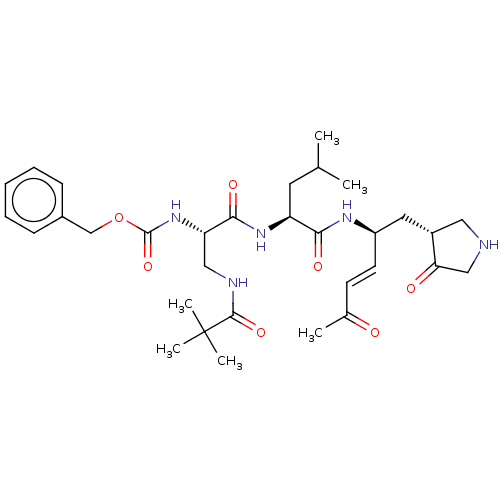 (CHEMBL3911377) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Inhibition of recombinant Coxsackievirus B3 C-terminal 6His-tagged 3C protease expressed in Escherichia coli BL21 preincubated for 20 mins followed b... | Eur J Med Chem 120: 202-16 (2016) Article DOI: 10.1016/j.ejmech.2016.03.085 BindingDB Entry DOI: 10.7270/Q25B05HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM11232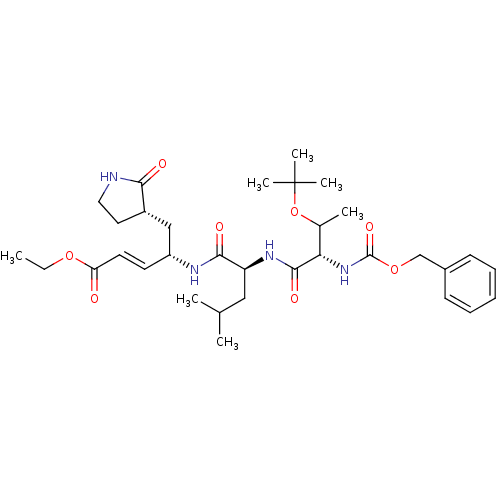 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+3 | -7.94 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+3 | -7.64 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50501750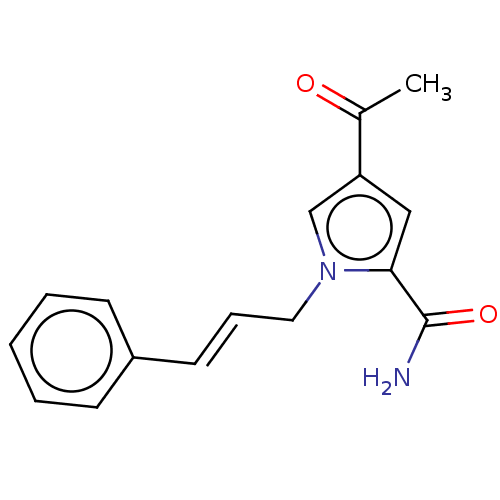 (CHEMBL4096676) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.97E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Reversible inhibition of Coxsackievirus B3 C-terminal His6-tagged protease 3C expressed in Escherichia coli BL21(DE3) using N-Dabcyl-KTLEALFQGPPVYE-(... | J Med Chem 61: 1218-1230 (2018) Article DOI: 10.1021/acs.jmedchem.7b01440 BindingDB Entry DOI: 10.7270/Q2N58QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50501751 (CHEMBL4096233) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.72E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Reversible inhibition of Coxsackievirus B3 C-terminal His6-tagged protease 3C expressed in Escherichia coli BL21(DE3) using N-Dabcyl-KTLEALFQGPPVYE-(... | J Med Chem 61: 1218-1230 (2018) Article DOI: 10.1021/acs.jmedchem.7b01440 BindingDB Entry DOI: 10.7270/Q2N58QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50501749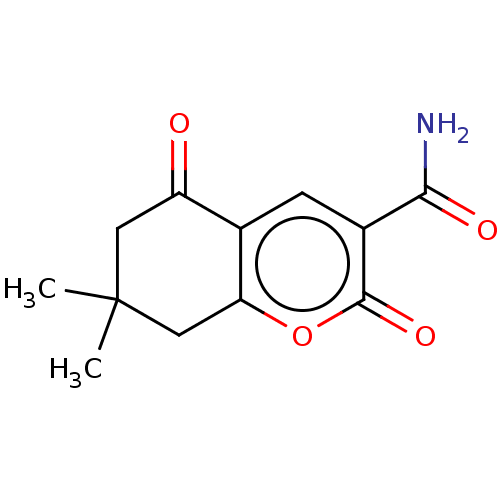 (CHEMBL4068292) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 C-terminal His6-tagged protease 3C expressed in Escherichia coli BL21(DE3) using N-Dabcyl-KTLEALFQGPPVYE-(Edans)-NH2 ... | J Med Chem 61: 1218-1230 (2018) Article DOI: 10.1021/acs.jmedchem.7b01440 BindingDB Entry DOI: 10.7270/Q2N58QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||