

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAC-alpha/RAC-beta/RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM431867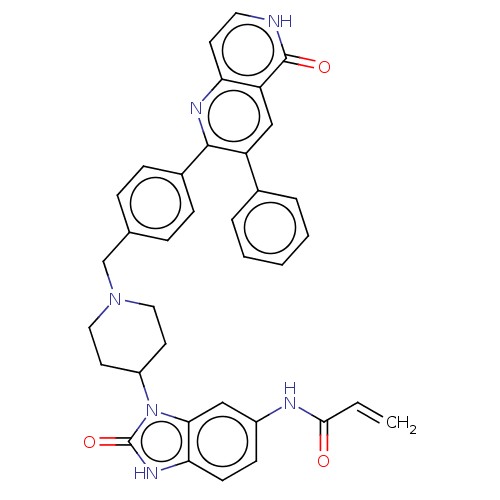 (US10550114, Compound 1a) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00073 BindingDB Entry DOI: 10.7270/Q2028WJ8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50528416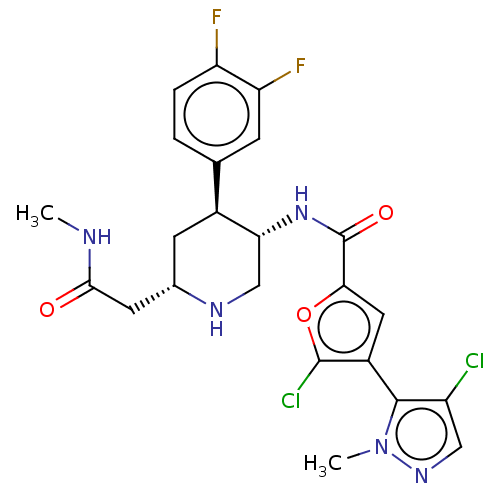 (CHEMBL4440965) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human AKT2 (120 to 481 residues) expressed in baculovirus infected Sf21 cells incubated for 1 hr in presence of A... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00815 BindingDB Entry DOI: 10.7270/Q2GF0Z92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126609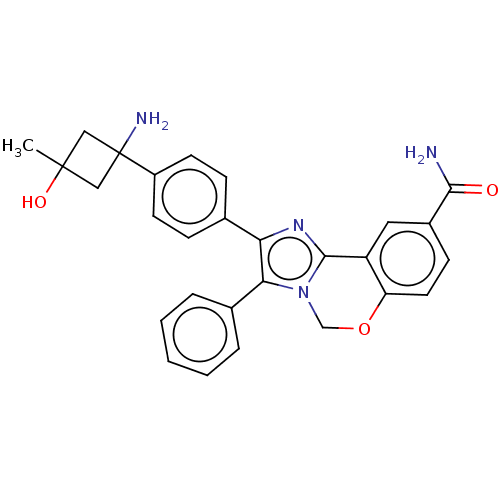 (US8772283, 53) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126581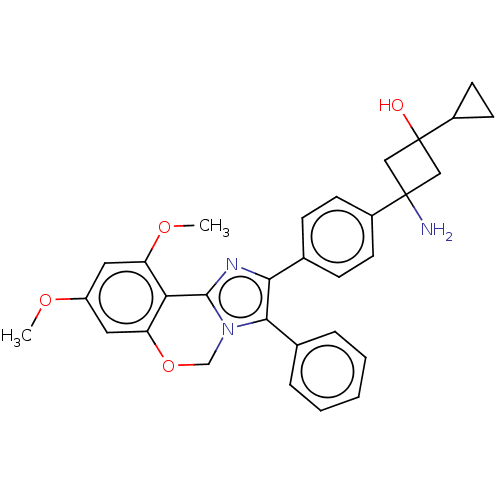 (US8772283, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126608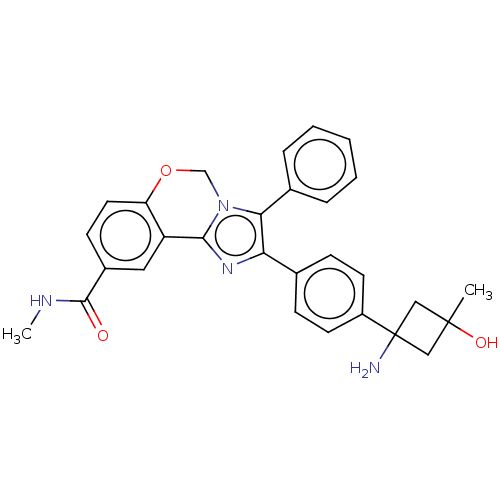 (US8772283, 52) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126557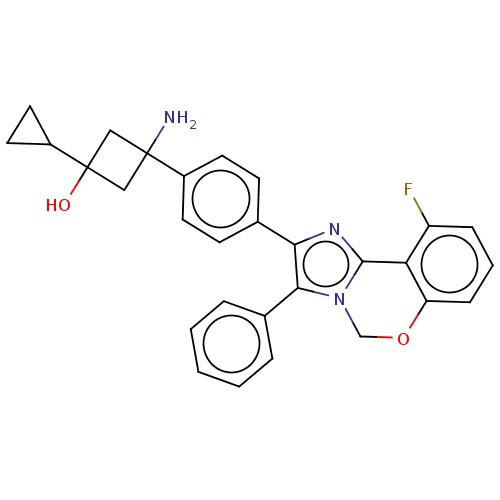 (US8772283, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126578 (US8772283, 22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126611 (US8772283, 55) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126604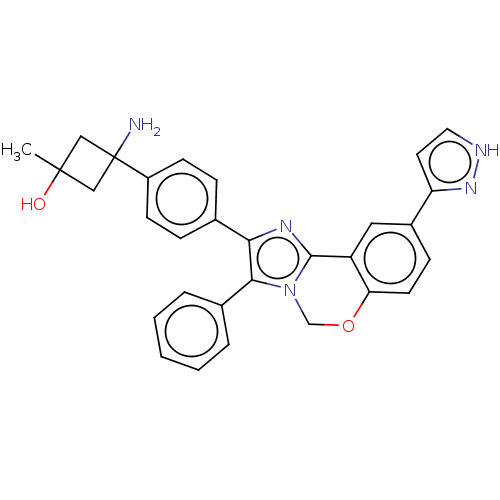 (US8772283, 48) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126572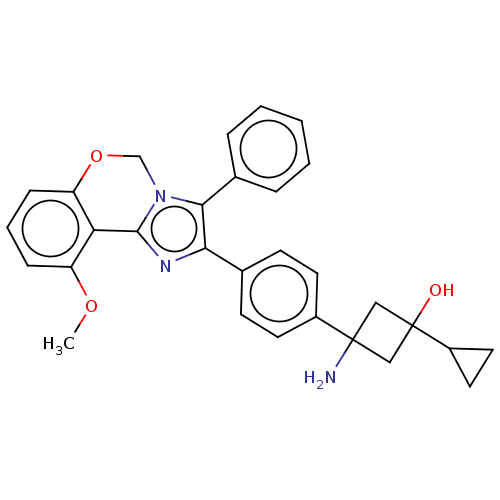 (US8772283, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126618 (US8772283, 62) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126617 (US8772283, 61) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126605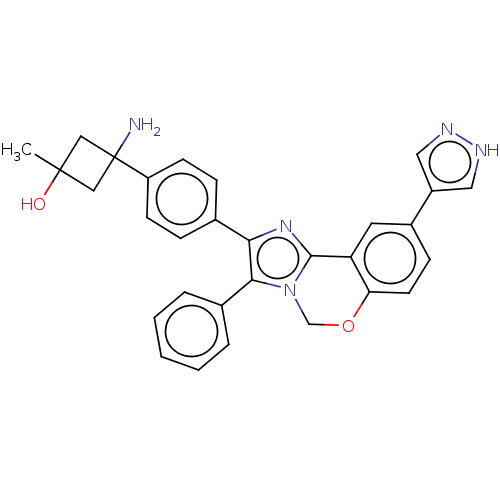 (US8772283, 49) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126591 (US8772283, 35) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126612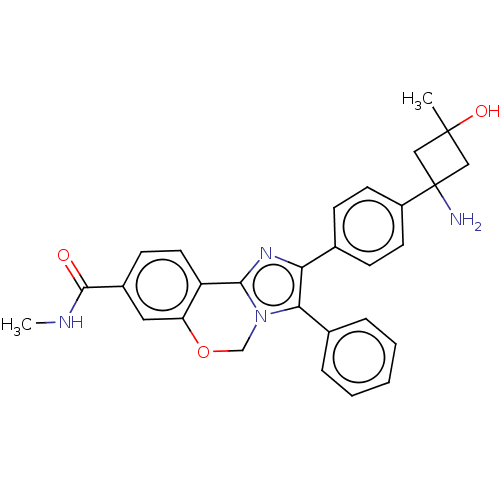 (US8772283, 56) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126558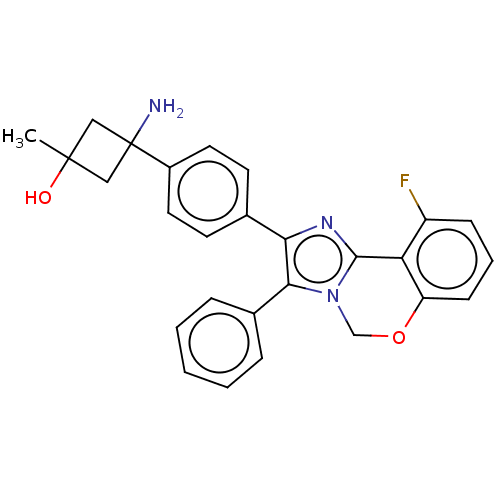 (US8772283, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM15131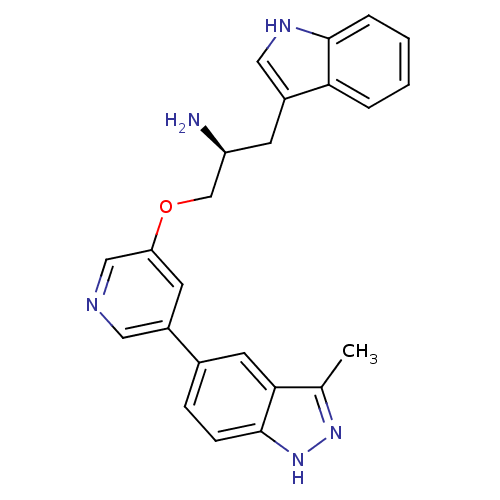 (5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Astex | Assay Description The purified PKB beta enzyme was assayed with a peptide substrate and test compound in the presence of 30 uM ATP/ [gamma-33P]ATP in 96-well plates. I... | J Mol Biol 367: 882-94 (2007) Article DOI: 10.1016/j.jmb.2007.01.004 BindingDB Entry DOI: 10.7270/Q29Z934H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126615 (US8772283, 59) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50528409 (CHEMBL4441825) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human Akt2 by mobile shift assay | J Med Chem 62: 7264-7288 (2019) Article DOI: 10.1021/acs.jmedchem.9b00891 BindingDB Entry DOI: 10.7270/Q2XW4P7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126600 (US8772283, 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126616 (US8772283, 60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126574 (US8772283, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50528415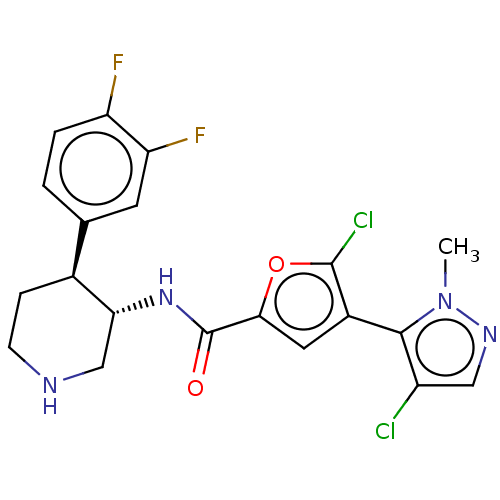 (CHEMBL4457064) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human Akt2 by mobile shift assay | J Med Chem 62: 7264-7288 (2019) Article DOI: 10.1021/acs.jmedchem.9b00891 BindingDB Entry DOI: 10.7270/Q2XW4P7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126614 (US8772283, 58) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126560 (US8772283, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126592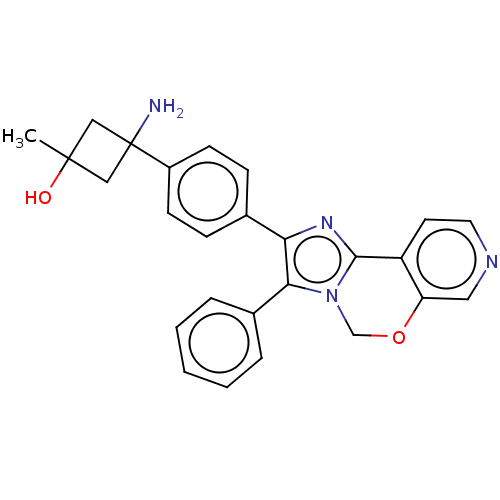 (US8772283, 36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126576 (US8772283, 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126587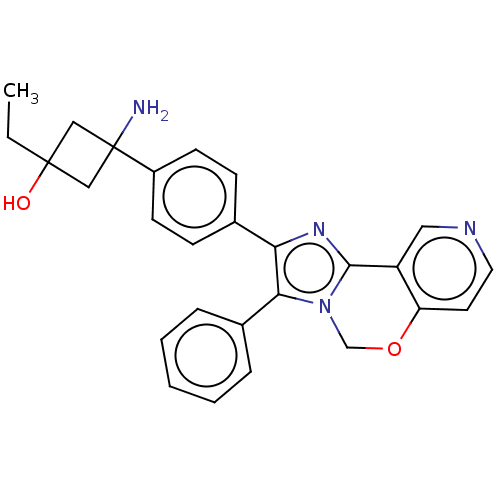 (US8772283, 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | VEVORISERTIB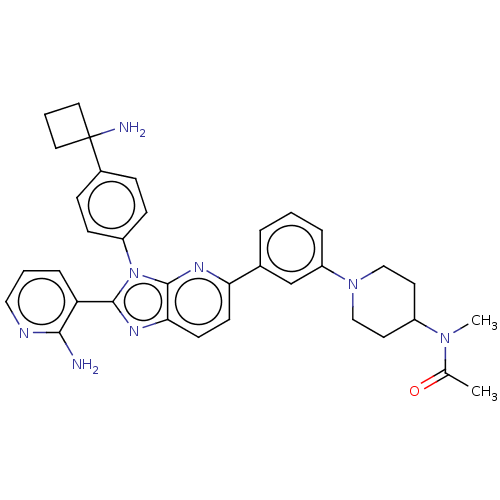 (Vevorisertib) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of acetylcholinesterase (AChE) activity | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126606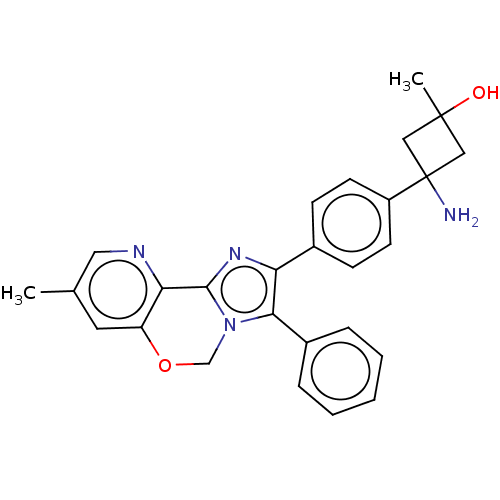 (US8772283, 50) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126619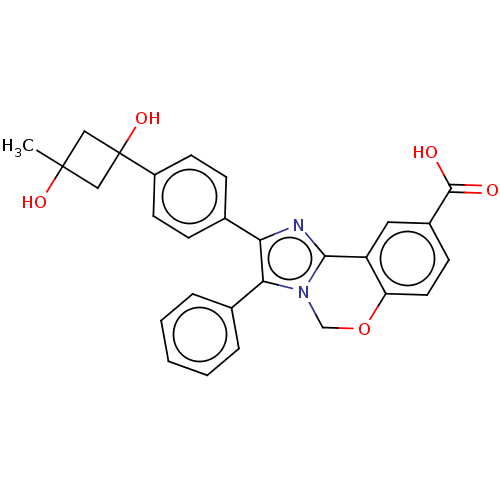 (US8772283, 63) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126577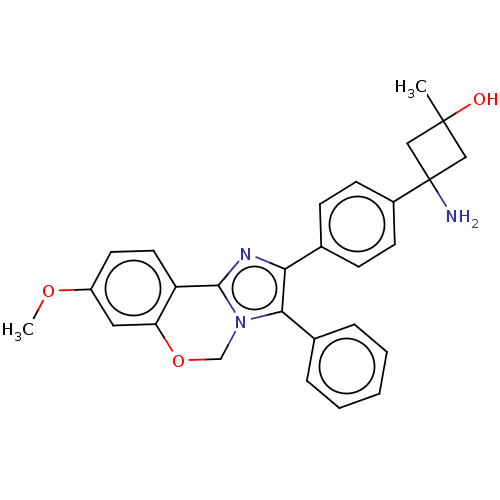 (US8772283, 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126594 (US8772283, 38) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126561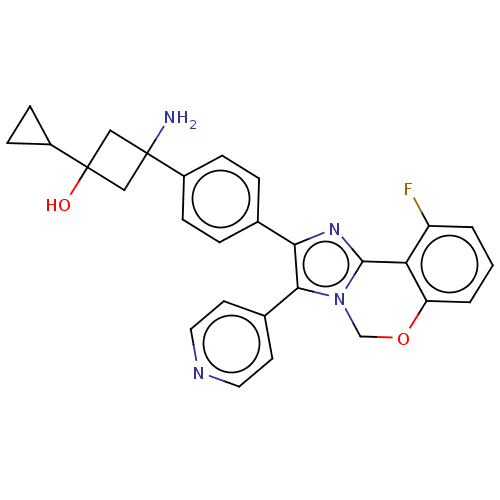 (US8772283, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126562 (US8772283, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126620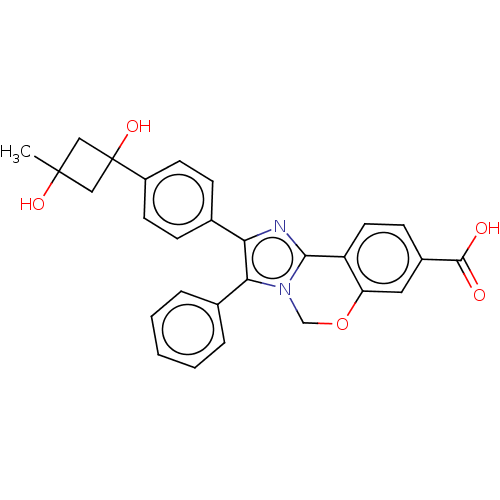 (US8772283, 64) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126603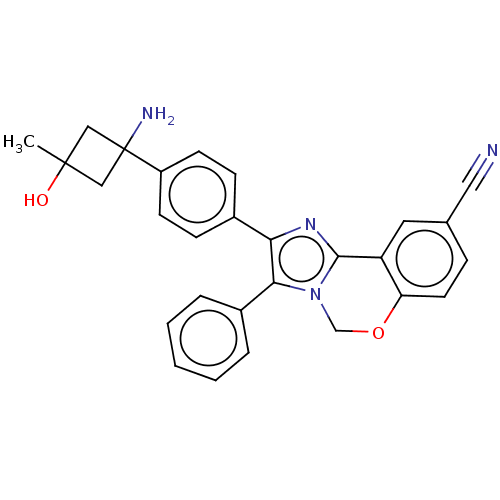 (US8772283, 46) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126595 (US8772283, 39) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha/RAC-beta/RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM182517 (US9145392, 218) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Akt (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128352 BindingDB Entry DOI: 10.7270/Q2SB49H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126567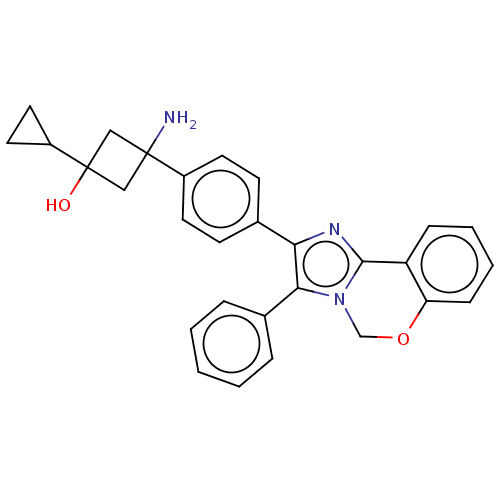 (US8772283, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126564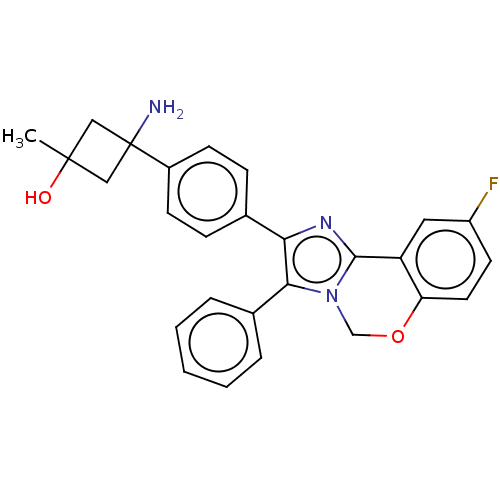 (US8772283, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50170284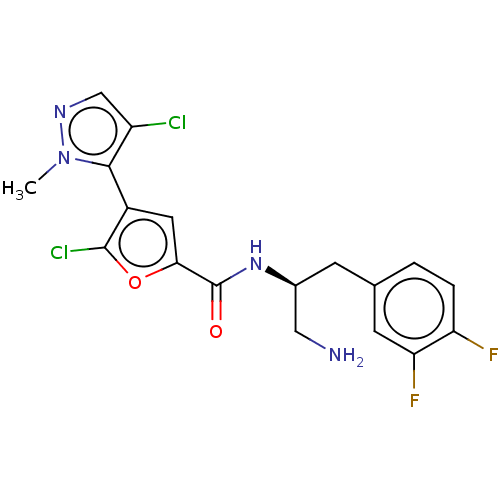 (GSK2141795 | GSK2141795C | Uprosertib) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human Akt2 by mobile shift assay | J Med Chem 62: 7264-7288 (2019) Article DOI: 10.1021/acs.jmedchem.9b00891 BindingDB Entry DOI: 10.7270/Q2XW4P7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126593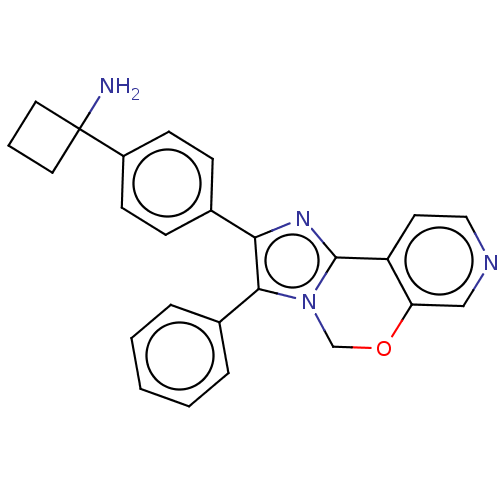 (US8772283, 37) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126607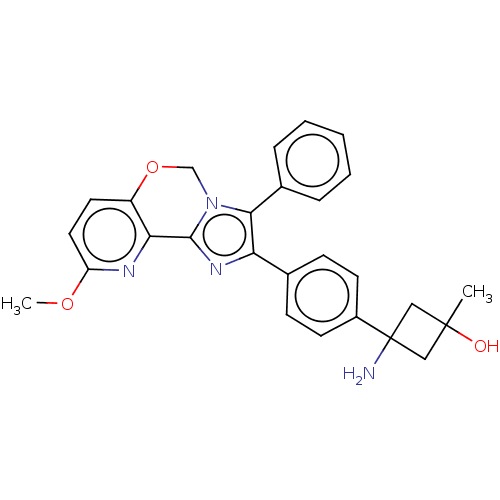 (US8772283, 51) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126563 (US8772283, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126588 (US8772283, 32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50528416 (CHEMBL4440965) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human Akt2 by mobile shift assay | J Med Chem 62: 7264-7288 (2019) Article DOI: 10.1021/acs.jmedchem.9b00891 BindingDB Entry DOI: 10.7270/Q2XW4P7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50606346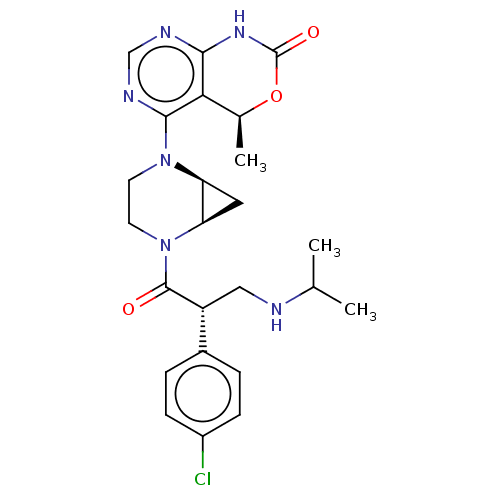 (CHEMBL5197007 | US20230321108, Isomer 4 of Example...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00527 BindingDB Entry DOI: 10.7270/Q2TX3KGT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50606346 (CHEMBL5197007 | US20230321108, Isomer 4 of Example...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126602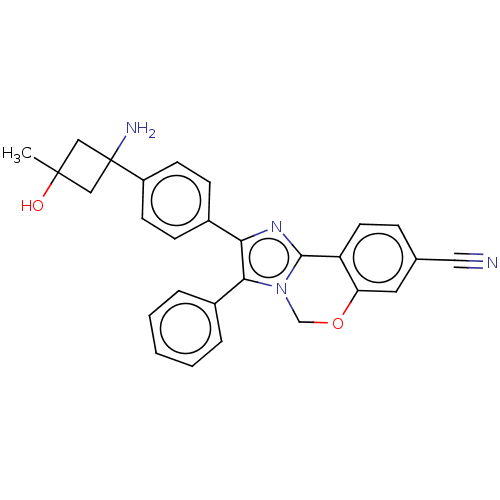 (US8772283, 47) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1251 total ) | Next | Last >> |