 Found 98 hits of kd for UniProtKB: O60341
Found 98 hits of kd for UniProtKB: O60341 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50240772

((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant LSD1(unknown origin) |
J Med Chem 58: 1705-16 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00037
BindingDB Entry DOI: 10.7270/Q2CC12CZ |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50075474
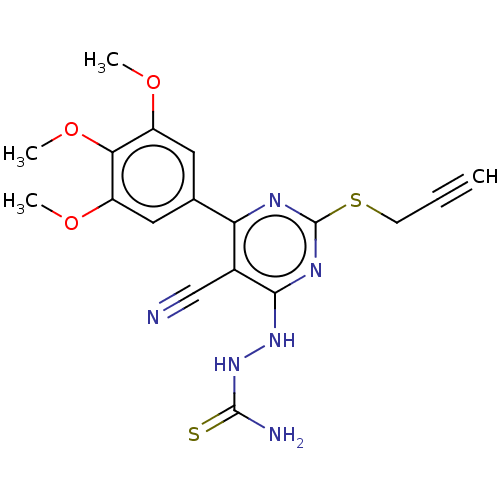
(CHEMBL3415352)Show SMILES COc1cc(cc(OC)c1OC)-c1nc(SCC#C)nc(NNC(N)=S)c1C#N Show InChI InChI=1S/C18H18N6O3S2/c1-5-6-29-18-21-14(11(9-19)16(22-18)23-24-17(20)28)10-7-12(25-2)15(27-4)13(8-10)26-3/h1,7-8H,6H2,2-4H3,(H3,20,24,28)(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant LSD1(unknown origin) |
J Med Chem 58: 1705-16 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00037
BindingDB Entry DOI: 10.7270/Q2CC12CZ |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50240772

((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.63E+4 | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant LSD1 (157 to 852 aa) expressed in Escherichia coli BL21(DE) by microscale thermophoresis assay |
J Med Chem 56: 8543-60 (2013)
Article DOI: 10.1021/jm401002r
BindingDB Entry DOI: 10.7270/Q2XD1333 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50441978
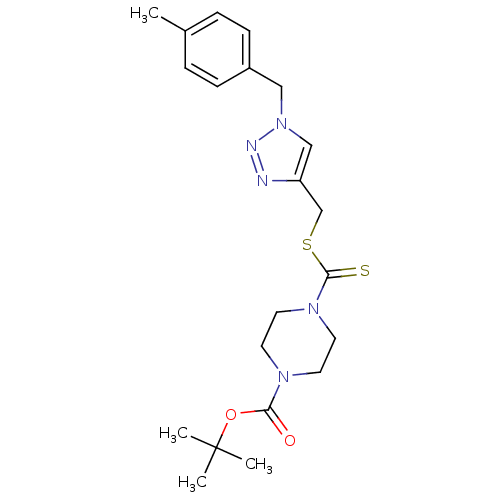
(CHEMBL2334501)Show SMILES Cc1ccc(Cn2cc(CSC(=S)N3CCN(CC3)C(=O)OC(C)(C)C)nn2)cc1 Show InChI InChI=1S/C21H29N5O2S2/c1-16-5-7-17(8-6-16)13-26-14-18(22-23-26)15-30-20(29)25-11-9-24(10-12-25)19(27)28-21(2,3)4/h5-8,14H,9-13,15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant LSD1 (157 to 852 aa) expressed in Escherichia coli BL21(DE) by microscale thermophoresis assay |
J Med Chem 56: 8543-60 (2013)
Article DOI: 10.1021/jm401002r
BindingDB Entry DOI: 10.7270/Q2XD1333 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50242883

(CHEMBL4062785)Show SMILES Cc1cc(COc2cc(nn2-c2ccc(cc2)C#N)C(=O)N2CCC(N)CC2)on1 Show InChI InChI=1S/C21H22N6O3/c1-14-10-18(30-25-14)13-29-20-11-19(21(28)26-8-6-16(23)7-9-26)24-27(20)17-4-2-15(12-22)3-5-17/h2-5,10-11,16H,6-9,13,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli by SPR analysis |
Bioorg Med Chem Lett 27: 3190-3195 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.018
BindingDB Entry DOI: 10.7270/Q2M047W8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50242884

(CHEMBL4090735)Show SMILES Cc1cc(COc2cc(nn2-c2ccc(cc2)C#N)C(=O)N2CC[C@@H](F)[C@@H](N)C2)on1 |r| Show InChI InChI=1S/C21H21FN6O3/c1-13-8-16(31-26-13)12-30-20-9-19(21(29)27-7-6-17(22)18(24)11-27)25-28(20)15-4-2-14(10-23)3-5-15/h2-5,8-9,17-18H,6-7,11-12,24H2,1H3/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli by SPR analysis |
Bioorg Med Chem Lett 27: 3190-3195 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.018
BindingDB Entry DOI: 10.7270/Q2M047W8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50242886
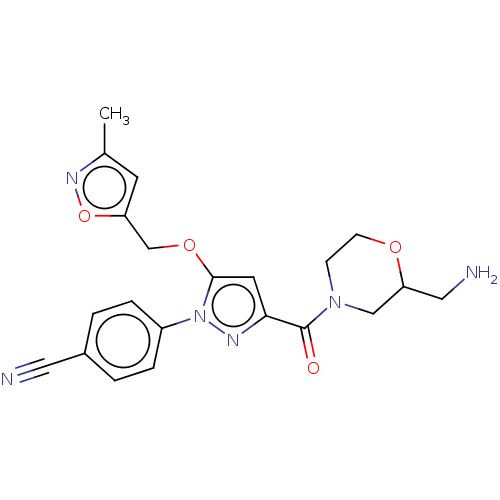
(CHEMBL4104407)Show SMILES Cc1cc(COc2cc(nn2-c2ccc(cc2)C#N)C(=O)N2CCOC(CN)C2)on1 Show InChI InChI=1S/C21H22N6O4/c1-14-8-17(31-25-14)13-30-20-9-19(21(28)26-6-7-29-18(11-23)12-26)24-27(20)16-4-2-15(10-22)3-5-16/h2-5,8-9,18H,6-7,11-13,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli by SPR analysis |
Bioorg Med Chem Lett 27: 3190-3195 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.018
BindingDB Entry DOI: 10.7270/Q2M047W8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50242890
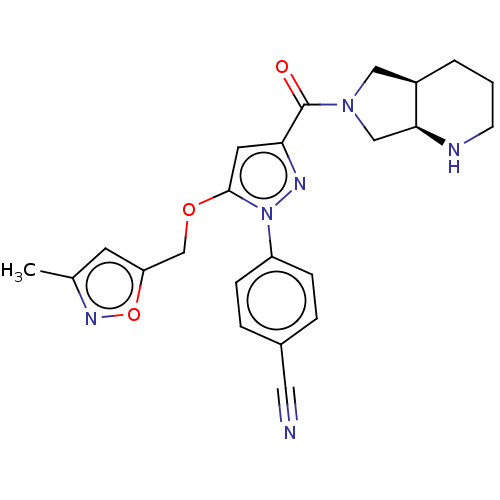
(CHEMBL4077739)Show SMILES [H][C@@]12CN(C[C@]1([H])NCCC2)C(=O)c1cc(OCc2cc(C)no2)n(n1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C23H24N6O3/c1-15-9-19(32-27-15)14-31-22-10-20(26-29(22)18-6-4-16(11-24)5-7-18)23(30)28-12-17-3-2-8-25-21(17)13-28/h4-7,9-10,17,21,25H,2-3,8,12-14H2,1H3/t17-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli by SPR analysis |
Bioorg Med Chem Lett 27: 3190-3195 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.018
BindingDB Entry DOI: 10.7270/Q2M047W8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50242894

(CHEMBL4074323)Show SMILES CC(C)c1cc(COc2cc(nn2-c2ccc(cc2)C#N)C(=O)N2CCC[C@@H](N)C2)on1 |r| Show InChI InChI=1S/C23H26N6O3/c1-15(2)20-10-19(32-27-20)14-31-22-11-21(23(30)28-9-3-4-17(25)13-28)26-29(22)18-7-5-16(12-24)6-8-18/h5-8,10-11,15,17H,3-4,9,13-14,25H2,1-2H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli by SPR analysis |
Bioorg Med Chem Lett 27: 3190-3195 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.018
BindingDB Entry DOI: 10.7270/Q2M047W8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50242896

(CHEMBL4093330)Show SMILES Cc1nnc(COc2cc(nn2-c2ccc(cc2)C#N)C(=O)N2CCC[C@@H](N)C2)o1 |r| Show InChI InChI=1S/C20H21N7O3/c1-13-23-24-18(30-13)12-29-19-9-17(20(28)26-8-2-3-15(22)11-26)25-27(19)16-6-4-14(10-21)5-7-16/h4-7,9,15H,2-3,8,11-12,22H2,1H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli by SPR analysis |
Bioorg Med Chem Lett 27: 3190-3195 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.018
BindingDB Entry DOI: 10.7270/Q2M047W8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50242906

(CHEMBL4095757)Show SMILES Cn1ncnc1COc1cc(nn1-c1ccc(cc1)C#N)C(=O)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C20H22N8O2/c1-26-18(23-13-24-26)12-30-19-9-17(20(29)27-8-2-3-15(22)11-27)25-28(19)16-6-4-14(10-21)5-7-16/h4-7,9,13,15H,2-3,8,11-12,22H2,1H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli by SPR analysis |
Bioorg Med Chem Lett 27: 3190-3195 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.018
BindingDB Entry DOI: 10.7270/Q2M047W8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50242942
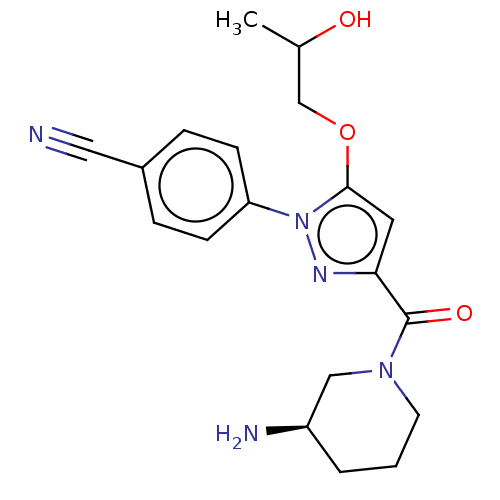
(CHEMBL4092725)Show SMILES CC(O)COc1cc(nn1-c1ccc(cc1)C#N)C(=O)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C19H23N5O3/c1-13(25)12-27-18-9-17(19(26)23-8-2-3-15(21)11-23)22-24(18)16-6-4-14(10-20)5-7-16/h4-7,9,13,15,25H,2-3,8,11-12,21H2,1H3/t13?,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli by SPR analysis |
Bioorg Med Chem Lett 27: 3190-3195 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.018
BindingDB Entry DOI: 10.7270/Q2M047W8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50242943
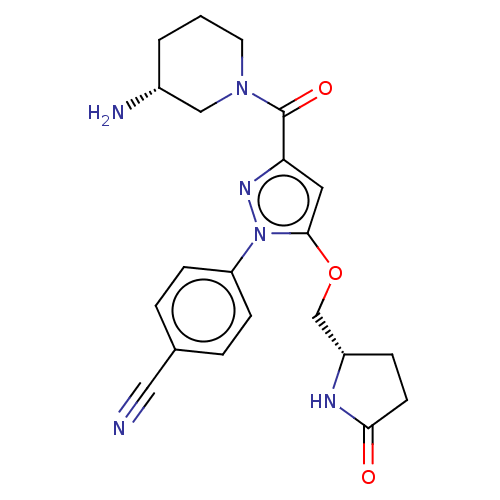
(CHEMBL4065043)Show SMILES N[C@@H]1CCCN(C1)C(=O)c1cc(OC[C@@H]2CCC(=O)N2)n(n1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C21H24N6O3/c22-11-14-3-6-17(7-4-14)27-20(30-13-16-5-8-19(28)24-16)10-18(25-27)21(29)26-9-1-2-15(23)12-26/h3-4,6-7,10,15-16H,1-2,5,8-9,12-13,23H2,(H,24,28)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli by SPR analysis |
Bioorg Med Chem Lett 27: 3190-3195 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.018
BindingDB Entry DOI: 10.7270/Q2M047W8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50242945
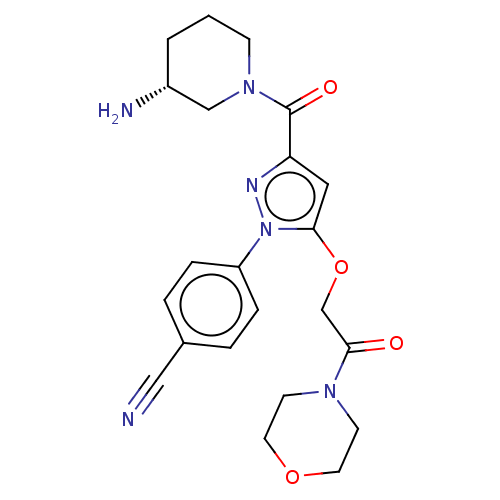
(CHEMBL4073624)Show SMILES N[C@@H]1CCCN(C1)C(=O)c1cc(OCC(=O)N2CCOCC2)n(n1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H26N6O4/c23-13-16-3-5-18(6-4-16)28-21(32-15-20(29)26-8-10-31-11-9-26)12-19(25-28)22(30)27-7-1-2-17(24)14-27/h3-6,12,17H,1-2,7-11,14-15,24H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli by SPR analysis |
Bioorg Med Chem Lett 27: 3190-3195 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.018
BindingDB Entry DOI: 10.7270/Q2M047W8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50242952

(CHEMBL4088035)Show SMILES CC(C)(O)CCOc1cc(nn1-c1ccc(cc1)C#N)C(=O)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C21H27N5O3/c1-21(2,28)9-11-29-19-12-18(20(27)25-10-3-4-16(23)14-25)24-26(19)17-7-5-15(13-22)6-8-17/h5-8,12,16,28H,3-4,9-11,14,23H2,1-2H3/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli by SPR analysis |
Bioorg Med Chem Lett 27: 3190-3195 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.018
BindingDB Entry DOI: 10.7270/Q2M047W8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50242957
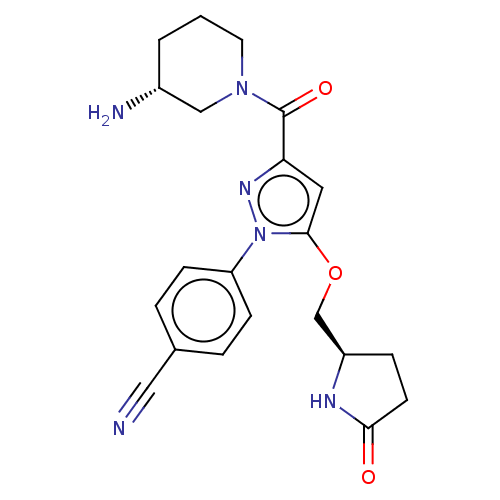
(CHEMBL4100451)Show SMILES N[C@@H]1CCCN(C1)C(=O)c1cc(OC[C@H]2CCC(=O)N2)n(n1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C21H24N6O3/c22-11-14-3-6-17(7-4-14)27-20(30-13-16-5-8-19(28)24-16)10-18(25-27)21(29)26-9-1-2-15(23)12-26/h3-4,6-7,10,15-16H,1-2,5,8-9,12-13,23H2,(H,24,28)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli by SPR analysis |
Bioorg Med Chem Lett 27: 3190-3195 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.018
BindingDB Entry DOI: 10.7270/Q2M047W8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50242948
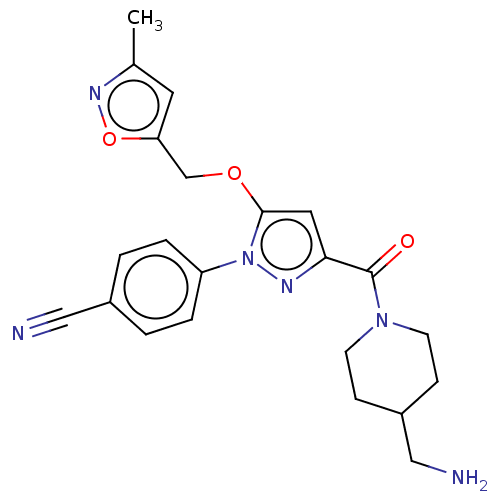
(CHEMBL4082935)Show SMILES Cc1cc(COc2cc(nn2-c2ccc(cc2)C#N)C(=O)N2CCC(CN)CC2)on1 Show InChI InChI=1S/C22H24N6O3/c1-15-10-19(31-26-15)14-30-21-11-20(22(29)27-8-6-17(13-24)7-9-27)25-28(21)18-4-2-16(12-23)3-5-18/h2-5,10-11,17H,6-9,13-14,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli by SPR analysis |
Bioorg Med Chem Lett 27: 3190-3195 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.018
BindingDB Entry DOI: 10.7270/Q2M047W8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50242951
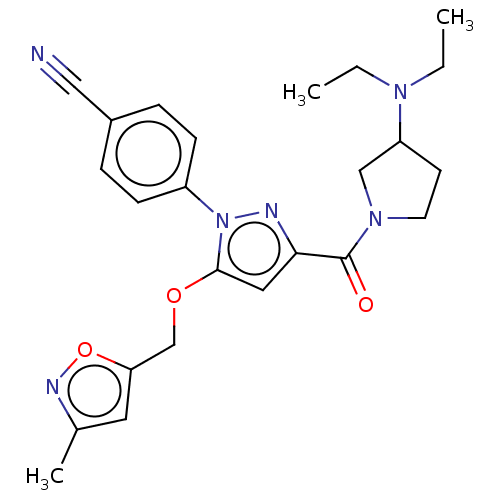
(CHEMBL4096682)Show SMILES CCN(CC)C1CCN(C1)C(=O)c1cc(OCc2cc(C)no2)n(n1)-c1ccc(cc1)C#N Show InChI InChI=1S/C24H28N6O3/c1-4-28(5-2)20-10-11-29(15-20)24(31)22-13-23(32-16-21-12-17(3)27-33-21)30(26-22)19-8-6-18(14-25)7-9-19/h6-9,12-13,20H,4-5,10-11,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli by SPR analysis |
Bioorg Med Chem Lett 27: 3190-3195 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.018
BindingDB Entry DOI: 10.7270/Q2M047W8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50262026

(CHEMBL4063365)Show SMILES COc1ccc(cn1)[C@@H]1CN(C[C@H]1c1ccc(cc1)C#N)C(=O)C[C@@H]1CC[C@H](N)CC1 |r,wU:12.14,24.26,27.30,wD:8.8,(41.88,-24.7,;40.35,-24.52,;39.42,-25.77,;37.88,-25.59,;36.97,-26.82,;37.58,-28.24,;39.11,-28.42,;40.03,-27.19,;36.65,-29.47,;37.11,-30.95,;35.85,-31.85,;34.62,-30.91,;35.1,-29.45,;34.22,-28.19,;34.86,-26.79,;33.97,-25.53,;32.43,-25.68,;31.78,-27.08,;32.68,-28.34,;31.55,-24.42,;30.65,-23.14,;35.83,-33.39,;34.49,-34.14,;37.16,-34.17,;37.14,-35.71,;35.8,-36.46,;35.78,-37.99,;37.1,-38.78,;37.08,-40.31,;38.45,-38.02,;38.47,-36.48,)| Show InChI InChI=1S/C25H30N4O2/c1-31-24-11-8-20(14-28-24)23-16-29(25(30)12-17-4-9-21(27)10-5-17)15-22(23)19-6-2-18(13-26)3-7-19/h2-3,6-8,11,14,17,21-23H,4-5,9-10,12,15-16,27H2,1H3/t17-,21+,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Unit, Cancer Research UK Manchester Institute, University of Manchester, Wilmslow Road, Manchester M20 4BX, UK. Electronic address: Daniel.mould@cruk.manchester.ac.uk.
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 4755-4759 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.052
BindingDB Entry DOI: 10.7270/Q28918CT |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50262050

(CHEMBL4066293)Show SMILES COc1ccc(cc1)[C@H]1[C@@H](N([C@H]2CC[C@H](N)CC2)C1=O)c1ccc(cc1)C#N |r,wU:9.22,11.11,wD:8.8,14.15,(24.78,-17.37,;24.38,-18.86,;25.47,-19.95,;26.96,-19.55,;28.05,-20.64,;27.65,-22.12,;26.17,-22.53,;25.08,-21.45,;28.74,-23.21,;28.74,-24.75,;30.28,-24.75,;31.37,-25.84,;32.85,-25.43,;33.93,-26.52,;33.53,-28.01,;34.62,-29.1,;32.04,-28.4,;30.96,-27.32,;30.28,-23.21,;31.37,-22.12,;27.65,-25.84,;26.16,-25.43,;25.07,-26.52,;25.47,-28.01,;26.97,-28.41,;28.05,-27.32,;24.38,-29.1,;23.3,-30.19,)| Show InChI InChI=1S/C23H25N3O2/c1-28-20-12-6-16(7-13-20)21-22(17-4-2-15(14-24)3-5-17)26(23(21)27)19-10-8-18(25)9-11-19/h2-7,12-13,18-19,21-22H,8-11,25H2,1H3/t18-,19-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Unit, Cancer Research UK Manchester Institute, University of Manchester, Wilmslow Road, Manchester M20 4BX, UK. Electronic address: Daniel.mould@cruk.manchester.ac.uk.
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 4755-4759 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.052
BindingDB Entry DOI: 10.7270/Q28918CT |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50262027

(CHEMBL4087649)Show SMILES Cc1ccc(cc1)[C@@H]1[C@@H](N([C@H]2CC[C@H](N)CC2)C1=O)c1ccc(cc1)C#N |r,wU:7.7,8.21,10.10,wD:13.14,(25.54,-20.09,;26.63,-21.18,;28.12,-20.78,;29.21,-21.87,;28.81,-23.35,;27.33,-23.76,;26.24,-22.67,;29.9,-24.44,;29.9,-25.98,;31.44,-25.98,;32.53,-27.07,;34,-26.65,;35.09,-27.75,;34.69,-29.24,;35.77,-30.33,;33.2,-29.63,;32.12,-28.54,;31.44,-24.44,;32.53,-23.35,;28.81,-27.07,;27.32,-26.66,;26.23,-27.75,;26.63,-29.24,;28.13,-29.64,;29.21,-28.54,;25.54,-30.33,;24.46,-31.42,)| Show InChI InChI=1S/C23H25N3O/c1-15-2-6-17(7-3-15)21-22(18-8-4-16(14-24)5-9-18)26(23(21)27)20-12-10-19(25)11-13-20/h2-9,19-22H,10-13,25H2,1H3/t19-,20-,21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Unit, Cancer Research UK Manchester Institute, University of Manchester, Wilmslow Road, Manchester M20 4BX, UK. Electronic address: Daniel.mould@cruk.manchester.ac.uk.
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 4755-4759 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.052
BindingDB Entry DOI: 10.7270/Q28918CT |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50262051
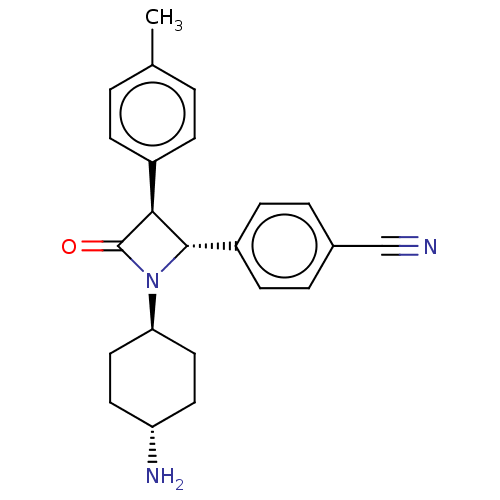
(CHEMBL4104027)Show SMILES Cc1ccc(cc1)[C@H]1[C@@H](N([C@H]2CC[C@H](N)CC2)C1=O)c1ccc(cc1)C#N |r,wU:8.21,10.10,wD:7.7,13.14,(25.54,-20.09,;26.63,-21.18,;28.12,-20.78,;29.21,-21.87,;28.81,-23.35,;27.33,-23.76,;26.24,-22.67,;29.9,-24.44,;29.9,-25.98,;31.44,-25.98,;32.53,-27.07,;34,-26.65,;35.09,-27.75,;34.69,-29.24,;35.77,-30.33,;33.2,-29.63,;32.12,-28.54,;31.44,-24.44,;32.53,-23.35,;28.81,-27.07,;27.32,-26.66,;26.23,-27.75,;26.63,-29.24,;28.13,-29.64,;29.21,-28.54,;25.54,-30.33,;24.46,-31.42,)| Show InChI InChI=1S/C23H25N3O/c1-15-2-6-17(7-3-15)21-22(18-8-4-16(14-24)5-9-18)26(23(21)27)20-12-10-19(25)11-13-20/h2-9,19-22H,10-13,25H2,1H3/t19-,20-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Unit, Cancer Research UK Manchester Institute, University of Manchester, Wilmslow Road, Manchester M20 4BX, UK. Electronic address: Daniel.mould@cruk.manchester.ac.uk.
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 4755-4759 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.052
BindingDB Entry DOI: 10.7270/Q28918CT |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50262052

(CHEMBL4066130)Show SMILES Cn1ncc2cc(ccc12)[C@@H]1CN(C[C@H]1c1ccc(cc1)C#N)C(=O)[C@H]1CC[C@H](CN)CC1 |r,wU:25.28,14.17,wD:10.11,28.32,(69.23,-20.88,;69.38,-22.41,;70.71,-23.2,;70.37,-24.71,;68.83,-24.85,;67.91,-26.09,;66.38,-25.9,;65.77,-24.49,;66.68,-23.26,;68.22,-23.44,;65.45,-27.14,;65.91,-28.61,;64.65,-29.51,;63.42,-28.58,;63.91,-27.12,;63.02,-25.86,;63.66,-24.46,;62.78,-23.2,;61.24,-23.35,;60.59,-24.74,;61.49,-26,;60.36,-22.09,;59.46,-20.82,;64.63,-31.05,;63.29,-31.8,;65.96,-31.83,;67.29,-31.07,;68.61,-31.86,;68.6,-33.4,;69.92,-34.18,;69.9,-35.72,;67.25,-34.15,;65.94,-33.36,)| Show InChI InChI=1S/C27H31N5O/c1-31-26-11-10-22(12-23(26)15-30-31)25-17-32(27(33)21-8-4-19(14-29)5-9-21)16-24(25)20-6-2-18(13-28)3-7-20/h2-3,6-7,10-12,15,19,21,24-25H,4-5,8-9,14,16-17,29H2,1H3/t19-,21-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Unit, Cancer Research UK Manchester Institute, University of Manchester, Wilmslow Road, Manchester M20 4BX, UK. Electronic address: Daniel.mould@cruk.manchester.ac.uk.
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 4755-4759 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.052
BindingDB Entry DOI: 10.7270/Q28918CT |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50262068

(CHEMBL4074738)Show SMILES Cn1ncc2cc(ccc12)[C@@H]1CN(C[C@H]1c1ccc(cc1)C#N)C(=O)[C@@H]1CC[C@H](N)CC1 |r,wU:14.17,wD:10.11,25.28,28.32,(40.71,-22.41,;40.86,-23.94,;42.18,-24.72,;41.85,-26.23,;40.31,-26.37,;39.39,-27.61,;37.86,-27.42,;37.25,-26.01,;38.16,-24.78,;39.7,-24.96,;36.93,-28.65,;37.39,-30.12,;36.14,-31.02,;34.91,-30.09,;35.39,-28.63,;34.51,-27.38,;35.14,-25.98,;34.26,-24.72,;32.73,-24.87,;32.08,-26.26,;32.97,-27.52,;31.84,-23.61,;30.95,-22.34,;36.12,-32.56,;34.77,-33.31,;37.44,-33.34,;38.77,-32.58,;40.09,-33.37,;40.08,-34.91,;41.39,-35.69,;38.73,-35.66,;37.42,-34.88,)| Show InChI InChI=1S/C26H29N5O/c1-30-25-11-8-20(12-21(25)14-29-30)24-16-31(26(32)19-6-9-22(28)10-7-19)15-23(24)18-4-2-17(13-27)3-5-18/h2-5,8,11-12,14,19,22-24H,6-7,9-10,15-16,28H2,1H3/t19-,22+,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Unit, Cancer Research UK Manchester Institute, University of Manchester, Wilmslow Road, Manchester M20 4BX, UK. Electronic address: Daniel.mould@cruk.manchester.ac.uk.
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 4755-4759 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.052
BindingDB Entry DOI: 10.7270/Q28918CT |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50262071

(CHEMBL4100487)Show SMILES Cc1ccc(cc1)[C@@H]1CN(C[C@H]1c1ccc(cc1)C#N)C(=O)C[C@@H]1CC[C@H](N)CC1 |r,wU:23.25,11.13,26.29,wD:7.7,(42.56,-23.35,;41.63,-24.6,;40.1,-24.42,;39.18,-25.65,;39.8,-27.06,;41.32,-27.25,;42.24,-26.01,;38.86,-28.29,;39.33,-29.76,;38.07,-30.66,;36.84,-29.73,;37.33,-28.27,;36.44,-27.02,;37.08,-25.62,;36.2,-24.37,;34.66,-24.51,;34.01,-25.91,;34.91,-27.16,;33.78,-23.25,;32.88,-21.98,;38.05,-32.2,;36.71,-32.94,;39.37,-32.98,;39.35,-34.52,;38.01,-35.26,;37.98,-36.8,;39.3,-37.59,;39.28,-39.12,;40.65,-36.84,;40.68,-35.3,)| Show InChI InChI=1S/C26H31N3O/c1-18-2-8-21(9-3-18)24-16-29(26(30)14-19-6-12-23(28)13-7-19)17-25(24)22-10-4-20(15-27)5-11-22/h2-5,8-11,19,23-25H,6-7,12-14,16-17,28H2,1H3/t19-,23+,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Unit, Cancer Research UK Manchester Institute, University of Manchester, Wilmslow Road, Manchester M20 4BX, UK. Electronic address: Daniel.mould@cruk.manchester.ac.uk.
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 4755-4759 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.052
BindingDB Entry DOI: 10.7270/Q28918CT |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50262072

(CHEMBL4079770)Show SMILES CNC[C@H]1CC[C@@H](CC1)C(=O)N1C[C@H]([C@@H](C1)c1ccc2n(C)ncc2c1)c1ccc(cc1)C#N |r,wU:6.9,13.29,wD:14.17,3.2,(68.45,-37.02,;69.8,-36.27,;69.82,-34.74,;68.49,-33.95,;68.51,-32.41,;67.19,-31.62,;65.85,-32.38,;65.83,-33.92,;67.15,-34.7,;64.53,-31.6,;63.18,-32.35,;64.55,-30.06,;63.32,-29.12,;63.8,-27.66,;65.35,-27.68,;65.81,-29.16,;66.28,-26.45,;65.66,-25.03,;66.58,-23.8,;68.12,-23.98,;69.28,-22.96,;69.13,-21.43,;70.61,-23.74,;70.27,-25.25,;68.73,-25.4,;67.81,-26.63,;62.92,-26.41,;63.55,-25,;62.67,-23.75,;61.13,-23.89,;60.48,-25.29,;61.38,-26.55,;60.25,-22.63,;59.35,-21.36,)| Show InChI InChI=1S/C28H33N5O/c1-30-15-20-5-9-22(10-6-20)28(34)33-17-25(21-7-3-19(14-29)4-8-21)26(18-33)23-11-12-27-24(13-23)16-31-32(27)2/h3-4,7-8,11-13,16,20,22,25-26,30H,5-6,9-10,15,17-18H2,1-2H3/t20-,22-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Unit, Cancer Research UK Manchester Institute, University of Manchester, Wilmslow Road, Manchester M20 4BX, UK. Electronic address: Daniel.mould@cruk.manchester.ac.uk.
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 4755-4759 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.052
BindingDB Entry DOI: 10.7270/Q28918CT |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50262073

(CHEMBL4101423)Show SMILES Cn1ncc2cc(ccc12)[C@@H]1CN(C[C@H]1c1ccc(cc1)C#N)C(=O)[C@H]1CC[C@H](N)CC1 |r,wU:25.28,14.17,wD:10.11,28.32,(38.91,-22.24,;39.06,-23.76,;40.39,-24.55,;40.05,-26.06,;38.51,-26.2,;37.6,-27.43,;36.07,-27.25,;35.45,-25.84,;36.37,-24.61,;37.9,-24.79,;35.13,-28.48,;35.6,-29.95,;34.34,-30.85,;33.11,-29.92,;33.59,-28.46,;32.71,-27.21,;33.35,-25.81,;32.46,-24.55,;30.93,-24.7,;30.28,-26.09,;31.18,-27.35,;30.05,-23.44,;29.15,-22.17,;34.32,-32.39,;32.98,-33.14,;35.64,-33.17,;36.97,-32.41,;38.3,-33.2,;38.28,-34.74,;39.6,-35.52,;36.94,-35.49,;35.62,-34.71,)| Show InChI InChI=1S/C26H29N5O/c1-30-25-11-8-20(12-21(25)14-29-30)24-16-31(26(32)19-6-9-22(28)10-7-19)15-23(24)18-4-2-17(13-27)3-5-18/h2-5,8,11-12,14,19,22-24H,6-7,9-10,15-16,28H2,1H3/t19-,22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Unit, Cancer Research UK Manchester Institute, University of Manchester, Wilmslow Road, Manchester M20 4BX, UK. Electronic address: Daniel.mould@cruk.manchester.ac.uk.
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 4755-4759 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.052
BindingDB Entry DOI: 10.7270/Q28918CT |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50262074
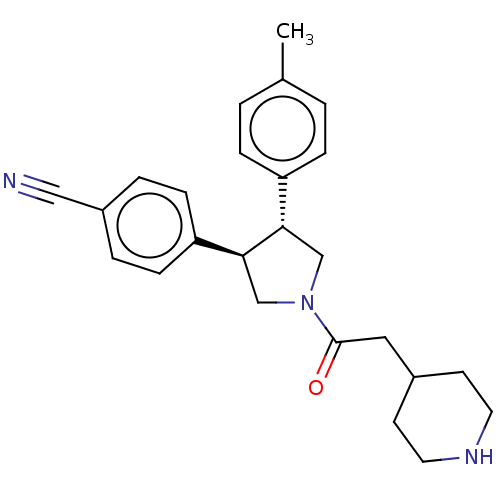
(CHEMBL4105603)Show SMILES Cc1ccc(cc1)[C@@H]1CN(C[C@H]1c1ccc(cc1)C#N)C(=O)CC1CCNCC1 |r| Show InChI InChI=1S/C25H29N3O/c1-18-2-6-21(7-3-18)23-16-28(25(29)14-19-10-12-27-13-11-19)17-24(23)22-8-4-20(15-26)5-9-22/h2-9,19,23-24,27H,10-14,16-17H2,1H3/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Unit, Cancer Research UK Manchester Institute, University of Manchester, Wilmslow Road, Manchester M20 4BX, UK. Electronic address: Daniel.mould@cruk.manchester.ac.uk.
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
Bioorg Med Chem Lett 27: 4755-4759 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.052
BindingDB Entry DOI: 10.7270/Q28918CT |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241486
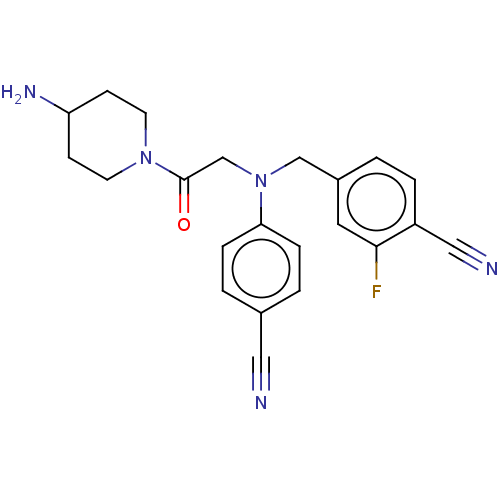
(CHEMBL4097953)Show SMILES NC1CCN(CC1)C(=O)CN(Cc1ccc(C#N)c(F)c1)c1ccc(cc1)C#N Show InChI InChI=1S/C22H22FN5O/c23-21-11-17(1-4-18(21)13-25)14-28(20-5-2-16(12-24)3-6-20)15-22(29)27-9-7-19(26)8-10-27/h1-6,11,19H,7-10,14-15,26H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241492
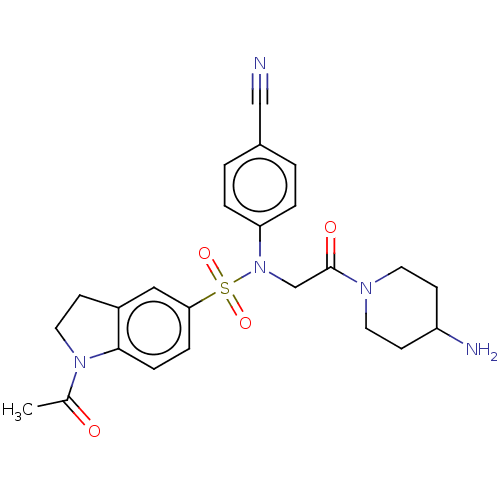
(CHEMBL4063384)Show SMILES CC(=O)N1CCc2cc(ccc12)S(=O)(=O)N(CC(=O)N1CCC(N)CC1)c1ccc(cc1)C#N Show InChI InChI=1S/C24H27N5O4S/c1-17(30)28-13-8-19-14-22(6-7-23(19)28)34(32,33)29(21-4-2-18(15-25)3-5-21)16-24(31)27-11-9-20(26)10-12-27/h2-7,14,20H,8-13,16,26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241505
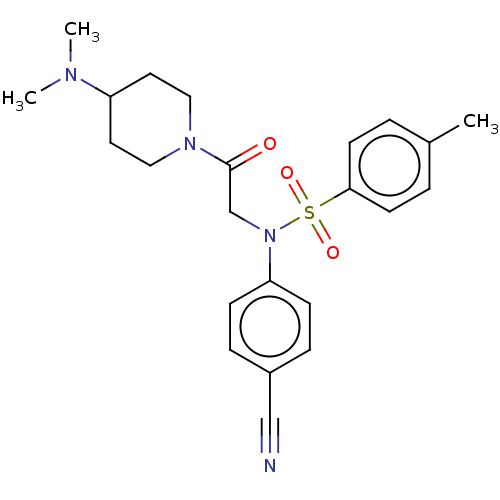
(CHEMBL4073334)Show SMILES CN(C)C1CCN(CC1)C(=O)CN(c1ccc(cc1)C#N)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C23H28N4O3S/c1-18-4-10-22(11-5-18)31(29,30)27(21-8-6-19(16-24)7-9-21)17-23(28)26-14-12-20(13-15-26)25(2)3/h4-11,20H,12-15,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241506
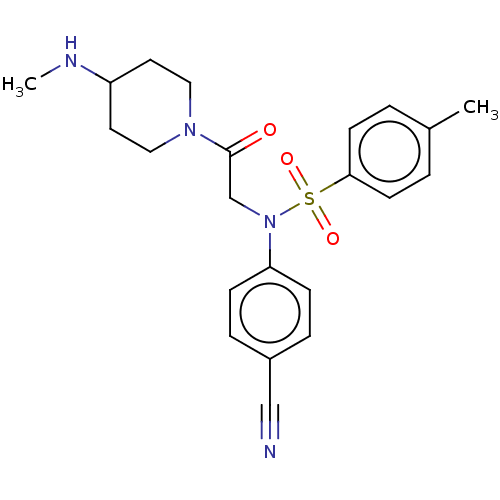
(CHEMBL4101729)Show SMILES CNC1CCN(CC1)C(=O)CN(c1ccc(cc1)C#N)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C22H26N4O3S/c1-17-3-9-21(10-4-17)30(28,29)26(20-7-5-18(15-23)6-8-20)16-22(27)25-13-11-19(24-2)12-14-25/h3-10,19,24H,11-14,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241507

(CHEMBL4083732)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N1CCC(C)(N)CC1)c1ccc(cc1)C#N Show InChI InChI=1S/C22H26N4O3S/c1-17-3-9-20(10-4-17)30(28,29)26(19-7-5-18(15-23)6-8-19)16-21(27)25-13-11-22(2,24)12-14-25/h3-10H,11-14,16,24H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241508

(CHEMBL4102632)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N1CC[C@H](N)C1)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C20H22N4O3S/c1-15-2-8-19(9-3-15)28(26,27)24(18-6-4-16(12-21)5-7-18)14-20(25)23-11-10-17(22)13-23/h2-9,17H,10-11,13-14,22H2,1H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241509

(CHEMBL4086227)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)NC1CCNCC1)c1ccc(cc1)C#N Show InChI InChI=1S/C21H24N4O3S/c1-16-2-8-20(9-3-16)29(27,28)25(19-6-4-17(14-22)5-7-19)15-21(26)24-18-10-12-23-13-11-18/h2-9,18,23H,10-13,15H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241510
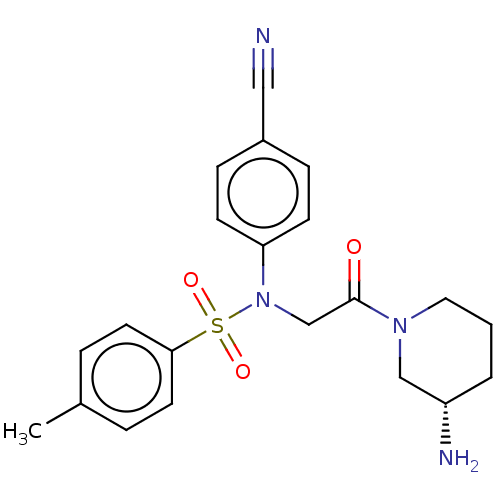
(CHEMBL4079604)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N1CCC[C@H](N)C1)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C21H24N4O3S/c1-16-4-10-20(11-5-16)29(27,28)25(19-8-6-17(13-22)7-9-19)15-21(26)24-12-2-3-18(23)14-24/h4-11,18H,2-3,12,14-15,23H2,1H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241511
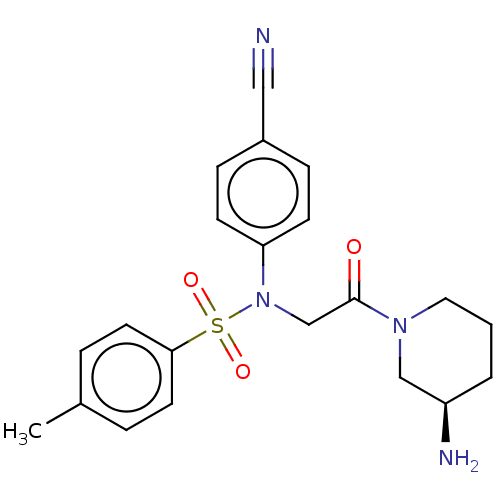
(CHEMBL4097636)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N1CCC[C@@H](N)C1)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C21H24N4O3S/c1-16-4-10-20(11-5-16)29(27,28)25(19-8-6-17(13-22)7-9-19)15-21(26)24-12-2-3-18(23)14-24/h4-11,18H,2-3,12,14-15,23H2,1H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241516

(CHEMBL4083030)Show SMILES [H][C@]12CN(C(=O)CN(c3ccc(cc3)C#N)S(=O)(=O)c3ccc(C)cc3)[C@]([H])(CN1)C2 |r| Show InChI InChI=1S/C21H22N4O3S/c1-15-2-8-20(9-3-15)29(27,28)25(18-6-4-16(11-22)5-7-18)14-21(26)24-13-17-10-19(24)12-23-17/h2-9,17,19,23H,10,12-14H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241517
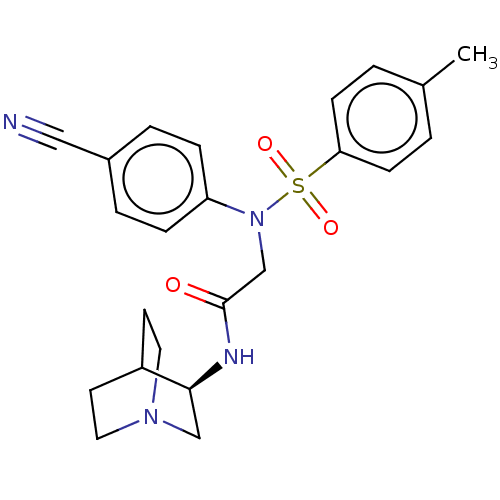
(CHEMBL4072551)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N[C@H]1CN2CCC1CC2)c1ccc(cc1)C#N |r,wD:15.15,(17.25,-37.07,;15.92,-37.85,;15.92,-39.39,;14.6,-40.16,;13.27,-39.39,;13.25,-37.86,;14.58,-37.08,;11.93,-40.16,;12.69,-41.49,;11.16,-41.48,;10.6,-39.4,;10.59,-37.86,;9.25,-37.09,;7.92,-37.87,;9.25,-35.55,;10.58,-34.78,;11.92,-35.54,;13.25,-34.77,;13.25,-33.23,;11.91,-32.46,;10.57,-33.24,;12.1,-33.23,;11.7,-34.72,;9.26,-40.17,;7.93,-39.41,;6.6,-40.18,;6.6,-41.72,;7.93,-42.5,;9.27,-41.72,;5.27,-42.5,;3.93,-43.27,)| Show InChI InChI=1S/C23H26N4O3S/c1-17-2-8-21(9-3-17)31(29,30)27(20-6-4-18(14-24)5-7-20)16-23(28)25-22-15-26-12-10-19(22)11-13-26/h2-9,19,22H,10-13,15-16H2,1H3,(H,25,28)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241518
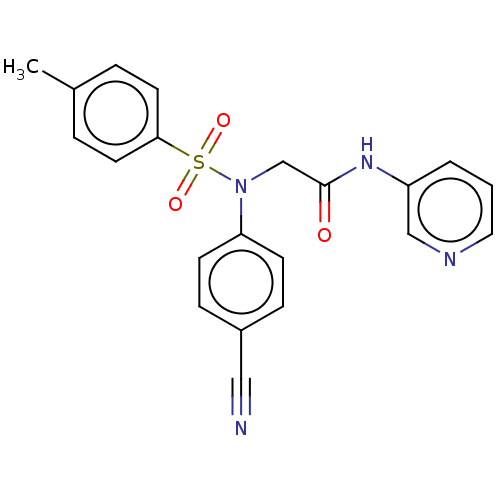
(CHEMBL4093280)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)Nc1cccnc1)c1ccc(cc1)C#N Show InChI InChI=1S/C21H18N4O3S/c1-16-4-10-20(11-5-16)29(27,28)25(19-8-6-17(13-22)7-9-19)15-21(26)24-18-3-2-12-23-14-18/h2-12,14H,15H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241519
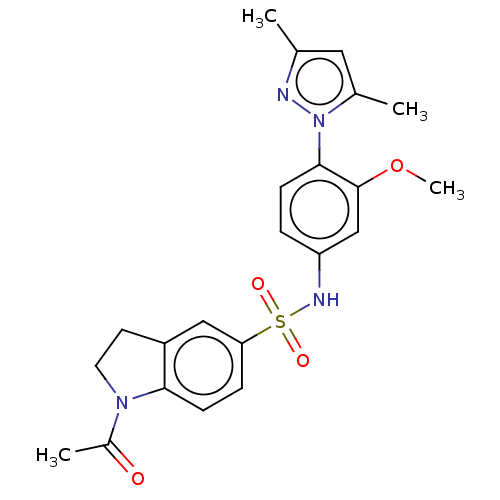
(CHEMBL4073481)Show SMILES COc1cc(NS(=O)(=O)c2ccc3N(CCc3c2)C(C)=O)ccc1-n1nc(C)cc1C Show InChI InChI=1S/C22H24N4O4S/c1-14-11-15(2)26(23-14)21-7-5-18(13-22(21)30-4)24-31(28,29)19-6-8-20-17(12-19)9-10-25(20)16(3)27/h5-8,11-13,24H,9-10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241533

(CHEMBL4102775)Show SMILES COc1ccc(CN(CC(=O)N2CCC3(CNC3)CC2)c2ccc(cc2)C#N)cc1F Show InChI InChI=1S/C24H27FN4O2/c1-31-22-7-4-19(12-21(22)25)14-29(20-5-2-18(13-26)3-6-20)15-23(30)28-10-8-24(9-11-28)16-27-17-24/h2-7,12,27H,8-11,14-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241523

(CHEMBL4084823)Show SMILES COc1ccc(CN(CC(=O)N2CCC(F)(CN)CC2)c2ccc(cc2)C#N)cc1F Show InChI InChI=1S/C23H26F2N4O2/c1-31-21-7-4-18(12-20(21)24)14-29(19-5-2-17(13-26)3-6-19)15-22(30)28-10-8-23(25,16-27)9-11-28/h2-7,12H,8-11,14-16,27H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241534
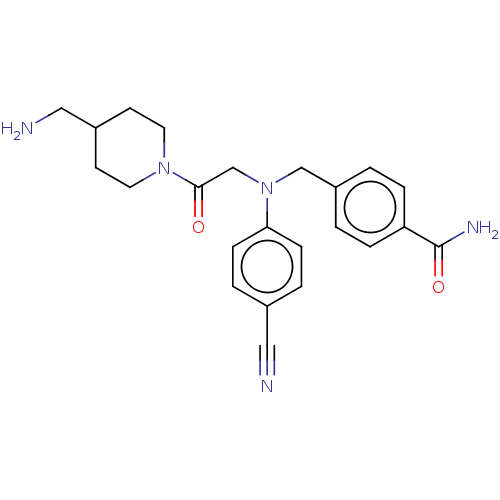
(CHEMBL4074550)Show SMILES NCC1CCN(CC1)C(=O)CN(Cc1ccc(cc1)C(N)=O)c1ccc(cc1)C#N Show InChI InChI=1S/C23H27N5O2/c24-13-17-3-7-21(8-4-17)28(15-19-1-5-20(6-2-19)23(26)30)16-22(29)27-11-9-18(14-25)10-12-27/h1-8,18H,9-12,14-16,25H2,(H2,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241527

(CHEMBL4098894)Show SMILES NCC1CCN(CC1)C(=O)CN(Cc1cccc(c1)C(N)=O)c1ccc(cc1)C#N Show InChI InChI=1S/C23H27N5O2/c24-13-17-4-6-21(7-5-17)28(15-19-2-1-3-20(12-19)23(26)30)16-22(29)27-10-8-18(14-25)9-11-27/h1-7,12,18H,8-11,14-16,25H2,(H2,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241528
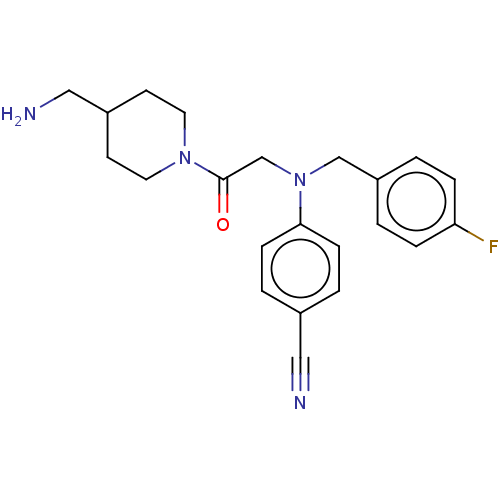
(CHEMBL4071901)Show SMILES NCC1CCN(CC1)C(=O)CN(Cc1ccc(F)cc1)c1ccc(cc1)C#N Show InChI InChI=1S/C22H25FN4O/c23-20-5-1-19(2-6-20)15-27(21-7-3-17(13-24)4-8-21)16-22(28)26-11-9-18(14-25)10-12-26/h1-8,18H,9-12,14-16,25H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241535

(CHEMBL4068129)Show SMILES Cc1ccc(CN(CC(=O)N2CCC(CN)CC2)c2ccc(cc2)C#N)cc1 Show InChI InChI=1S/C23H28N4O/c1-18-2-4-21(5-3-18)16-27(22-8-6-19(14-24)7-9-22)17-23(28)26-12-10-20(15-25)11-13-26/h2-9,20H,10-13,15-17,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241493

(CHEMBL4103264)Show SMILES Cn1ncc2cc(CN(CC(=O)N3CCC(N)CC3)c3ccc(cc3)C#N)ccc12 Show InChI InChI=1S/C23H26N6O/c1-27-22-7-4-18(12-19(22)14-26-27)15-29(21-5-2-17(13-24)3-6-21)16-23(30)28-10-8-20(25)9-11-28/h2-7,12,14,20H,8-11,15-16,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241494

(CHEMBL4079000)Show InChI InChI=1S/C21H24N4O/c22-14-17-6-8-20(9-7-17)25(15-18-4-2-1-3-5-18)16-21(26)24-12-10-19(23)11-13-24/h1-9,19H,10-13,15-16,23H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241497

(CHEMBL4087810)Show SMILES Cc1ccc(CN(CC(=O)N2CCC(N)CC2)c2ccc(cc2)C#N)cc1 Show InChI InChI=1S/C22H26N4O/c1-17-2-4-19(5-3-17)15-26(21-8-6-18(14-23)7-9-21)16-22(27)25-12-10-20(24)11-13-25/h2-9,20H,10-13,15-16,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data