

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50022815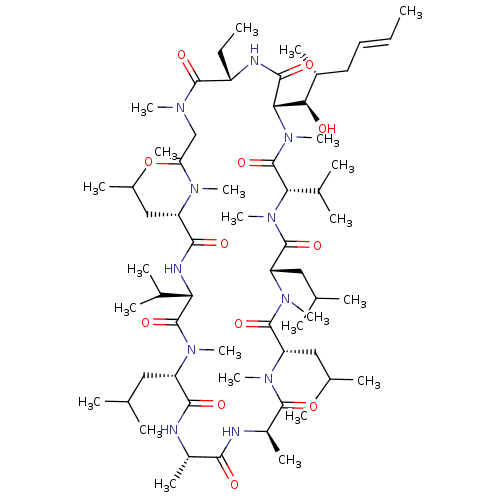 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.78 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330185 (2-((2R,5S,8S,11S,14S,17S,23S,26S,29S,32S)-17-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.45 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50323721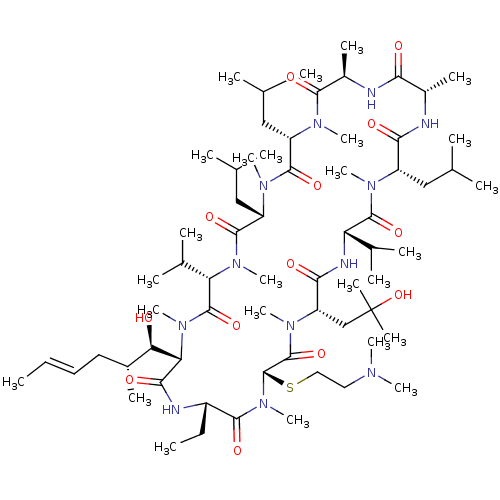 ((3S,6S,9S,12R,15S,18S,21S,24S,27S,30S,33S)-27-(2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330190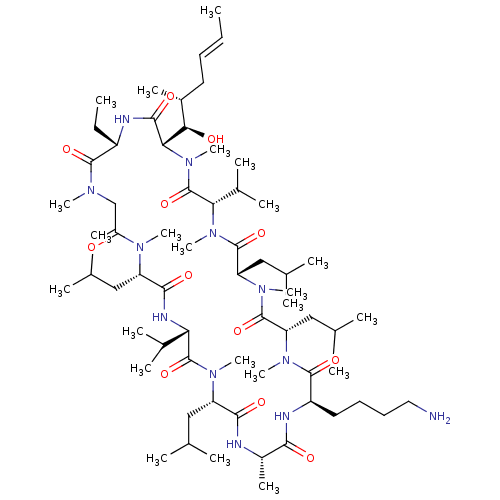 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-12-(4-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330197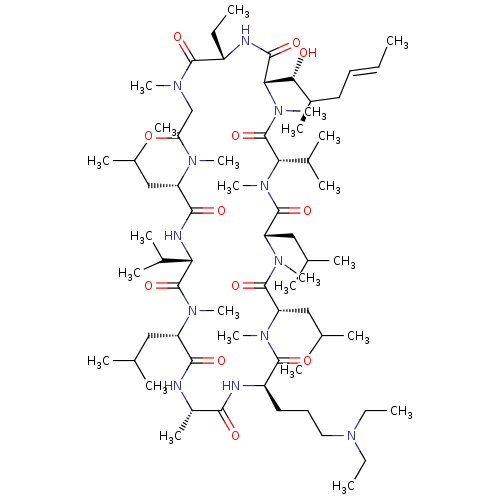 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-12-(3-(diet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330198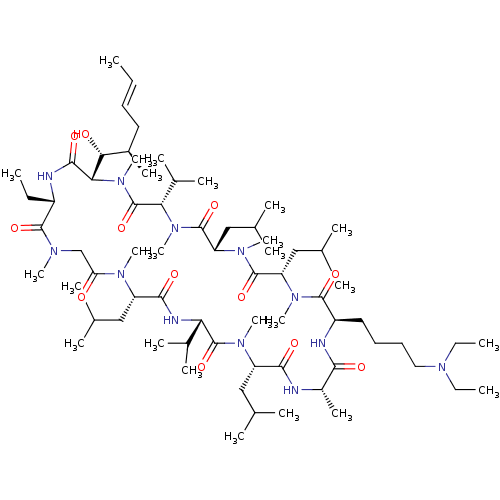 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-12-(4-(diet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.8 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330184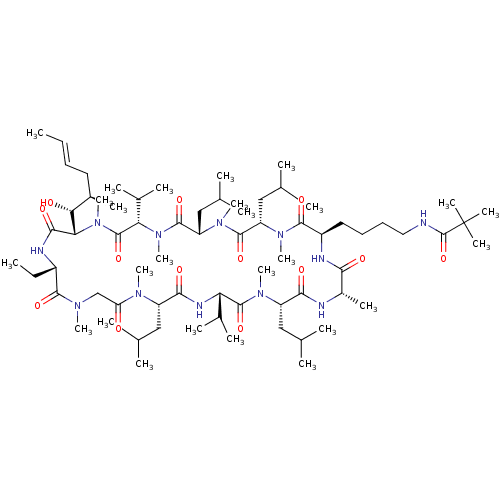 (CHEMBL1269582 | N-(4-((2R,5S,8S,11S,14S,17S,23S,26...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.6 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330186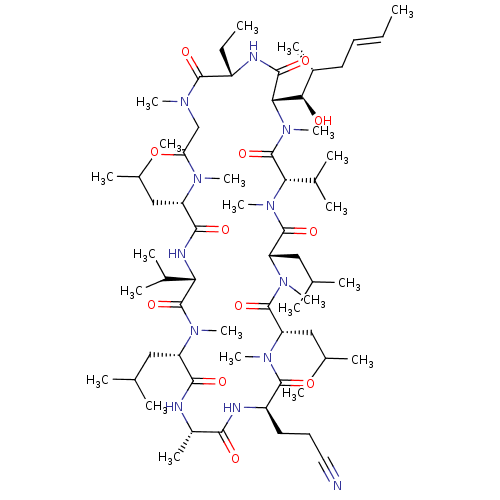 (3-((2R,5S,8S,11S,14S,17S,23S,26S,29S,32S)-17-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.2 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330191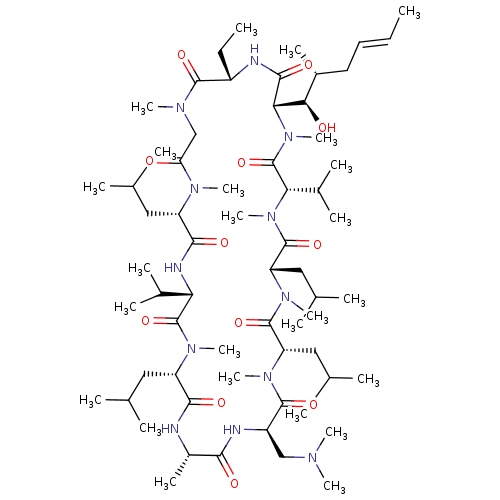 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-12-((dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25.2 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330182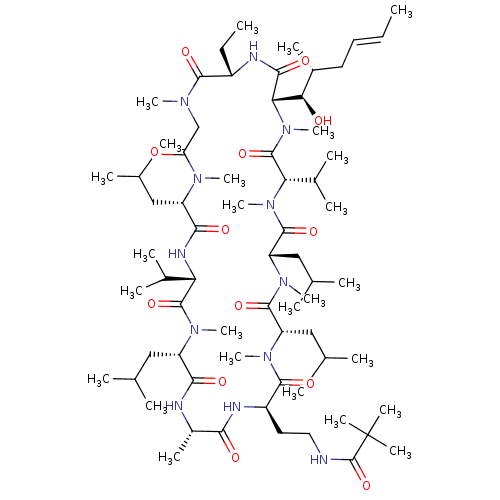 (CHEMBL1269580 | N-(2-((2R,5S,8S,11S,14S,17S,23S,26...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26.1 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330193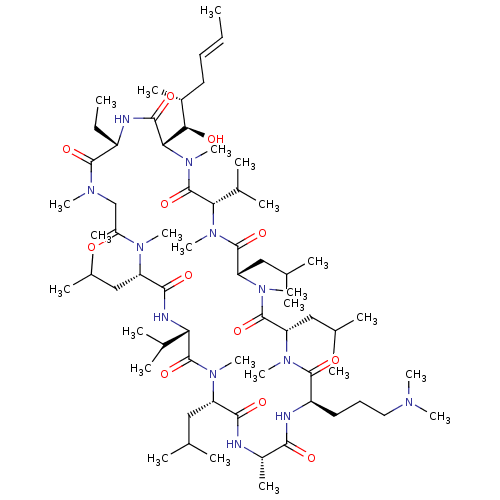 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-12-(3-(dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28.9 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330183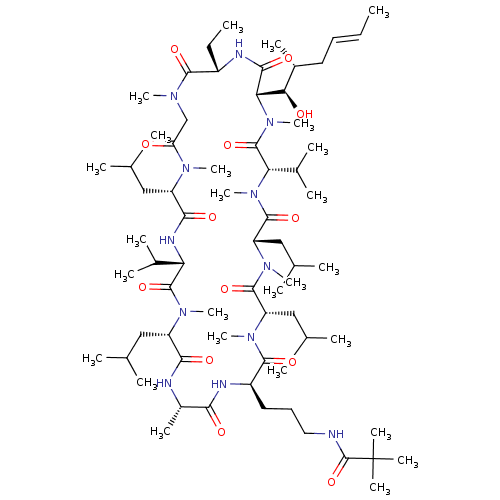 (CHEMBL1269581 | N-(3-((2R,5S,8S,11S,14S,17S,23S,26...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330196 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-12-(2-(diet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55.9 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330192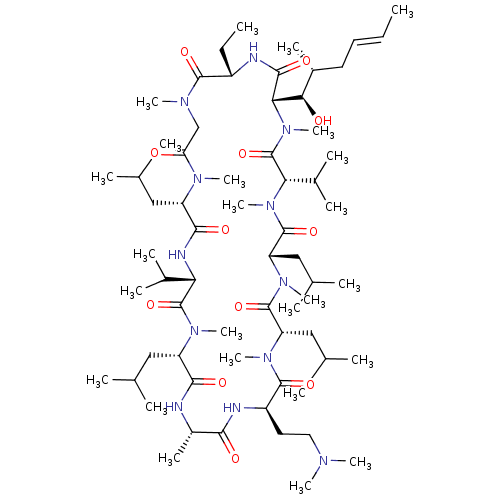 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-12-(2-(dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330181 (CHEMBL1269579 | tert-butyl((2R,5S,8S,11S,14S,17S,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 66.8 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330187 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-12-(aminome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79.7 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50546259 (CHEMBL4092230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal His6-tagged CypB (unknown origin) expressed in Escherichia coli C41(DE3) using Suc-Ala-Ala-Cis-Pro-Phe-pNA as substrate by s... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00242 BindingDB Entry DOI: 10.7270/Q2KD22HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330194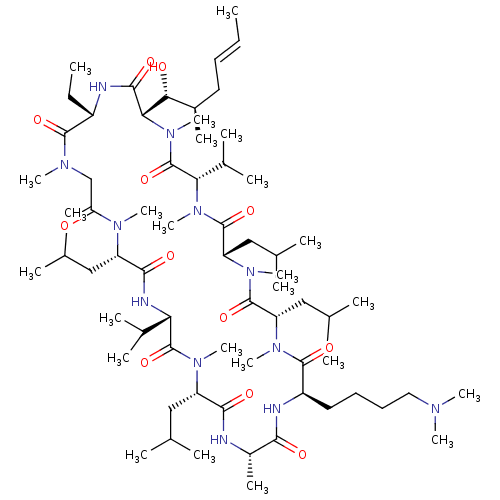 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-12-(4-(dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97.1 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330188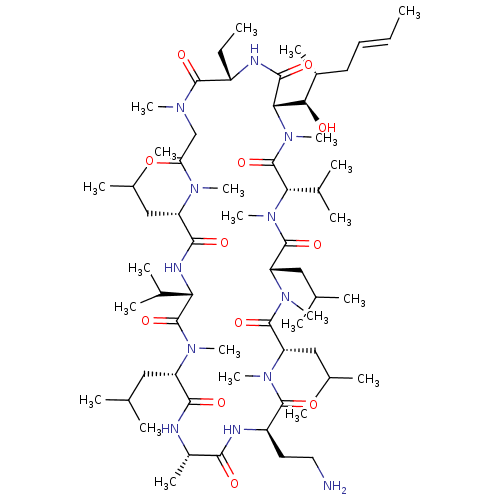 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-12-(2-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330195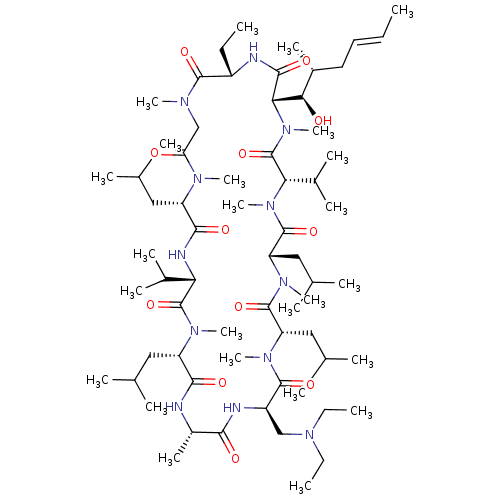 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-12-((diethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM140005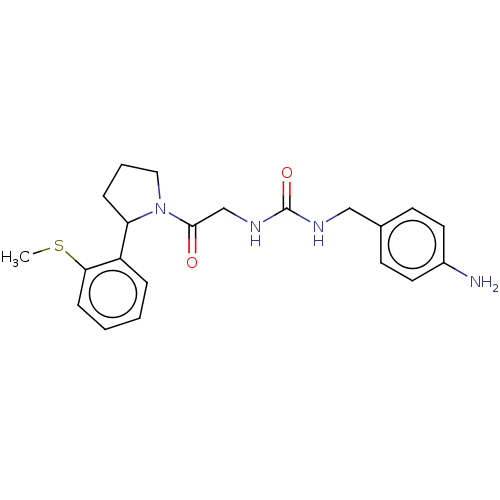 (US8901295, F684) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM140004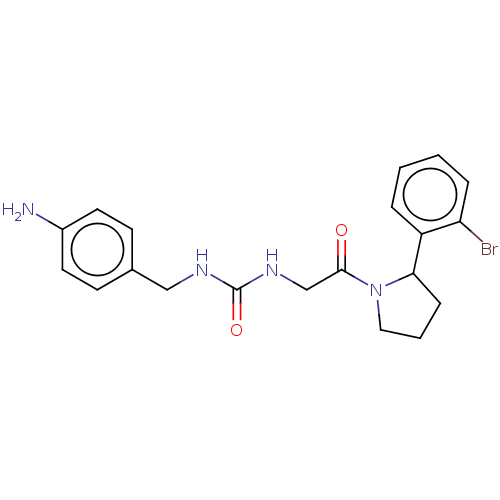 (US8901295, F680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 760 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM140001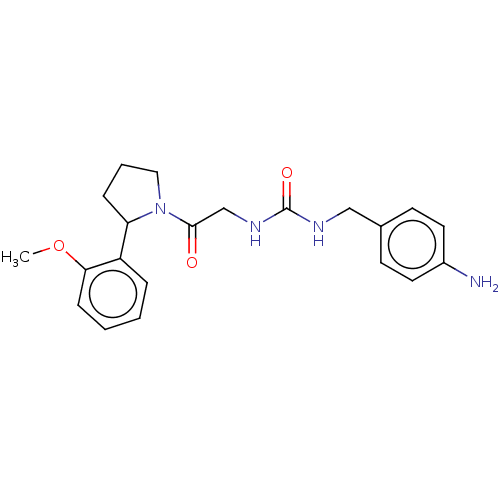 (US8901295, F609) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50330189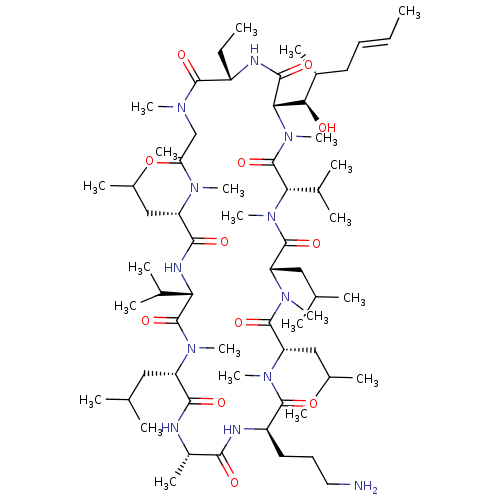 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-12-(3-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SCYNEXIS, Inc. Curated by ChEMBL | Assay Description Binding affinity to cyclophilin B by ELISA | Bioorg Med Chem Lett 20: 6542-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.036 BindingDB Entry DOI: 10.7270/Q2M909NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM46034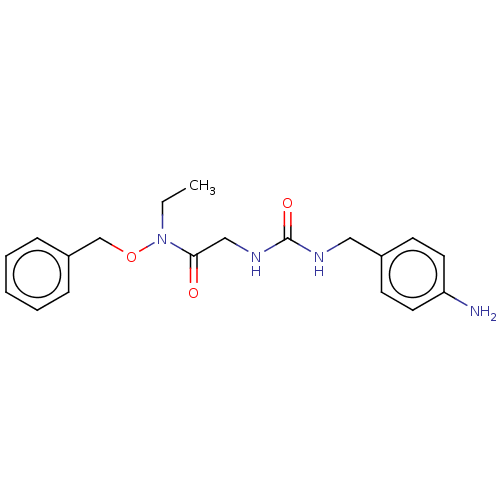 (US8901295, F607) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM140000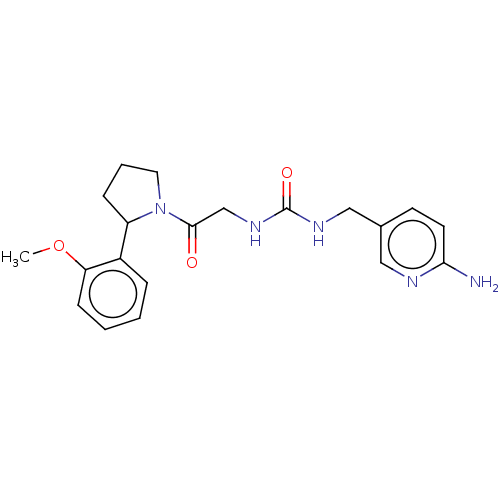 (US8901295, F587) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM139995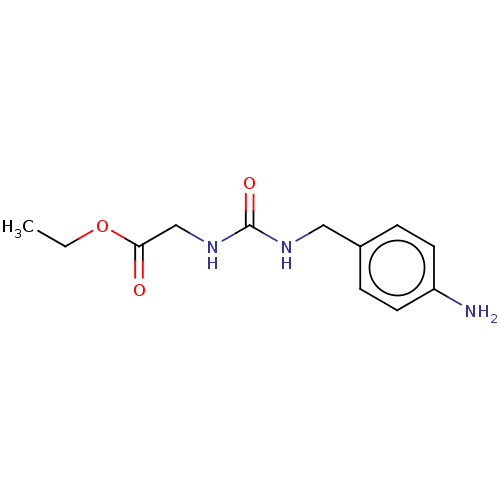 (US8901295, F428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM139995 (US8901295, F428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal His6-tagged CypB (unknown origin) expressed in Escherichia coli C41(DE3) using Suc-Ala-Ala-Cis-Pro-Phe-pNA as substrate by s... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00242 BindingDB Entry DOI: 10.7270/Q2KD22HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM139997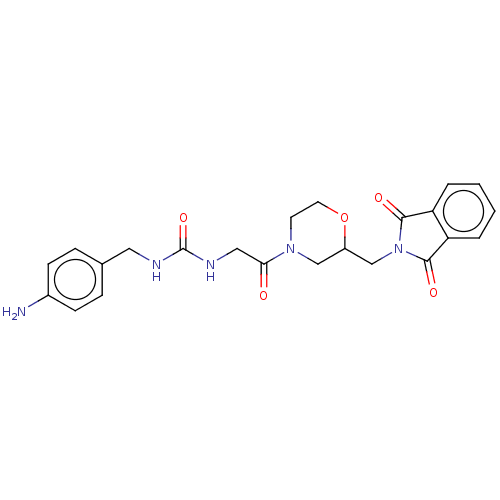 (US8901295, F554) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.46E+4 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM139998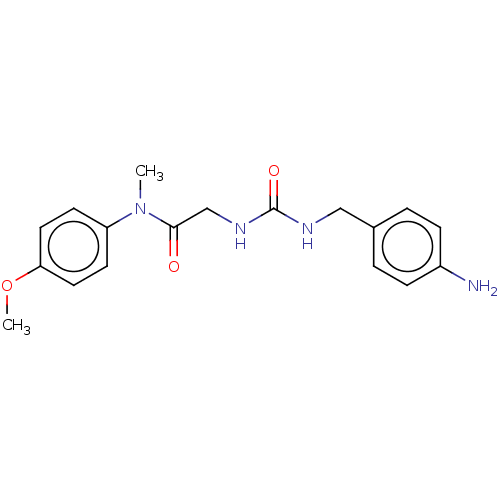 (US8901295, F555) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.78E+4 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM46031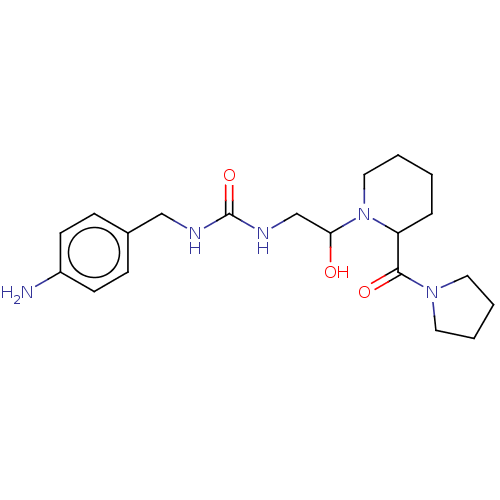 (US8901295, F542) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.66E+4 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM139999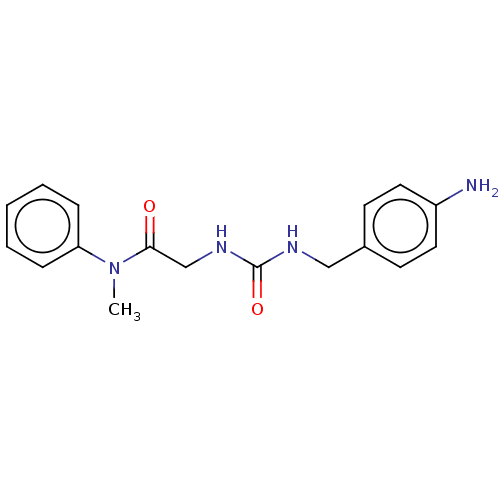 (US8901295, F557) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.45E+4 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM139996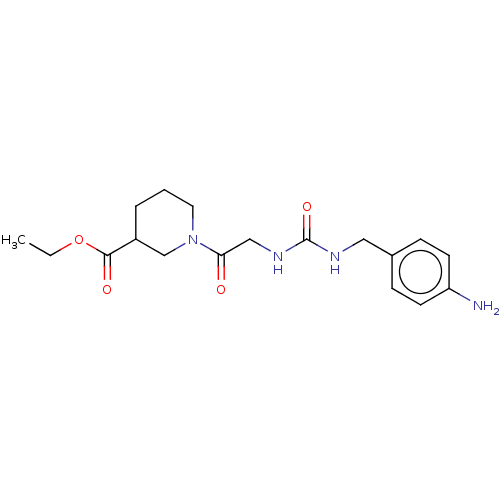 (US8901295, F545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.51E+4 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50556732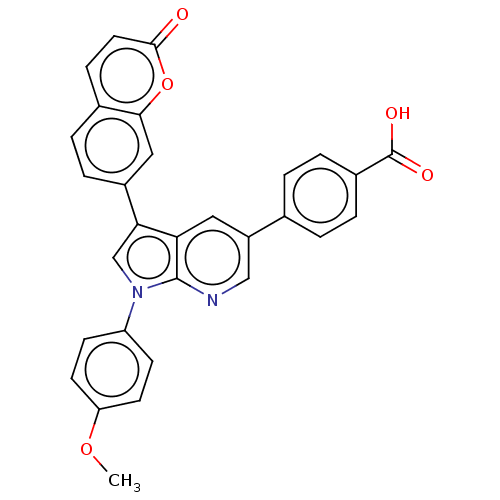 (CHEMBL4750435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PPIB (unknown origin) pre-incubated for 20 mins followed by fluorescence substrate addition and measured after 120 mins by DiFMUP assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01265 BindingDB Entry DOI: 10.7270/Q24171QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50546257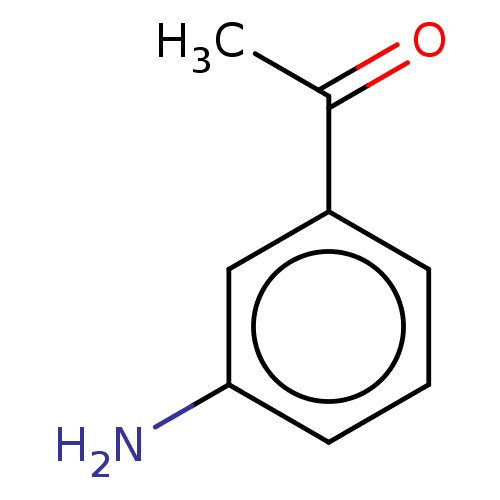 (CHEMBL3408644) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal His6-tagged CypB (unknown origin) expressed in Escherichia coli C41(DE3) using Suc-Ala-Ala-Cis-Pro-Phe-pNA as substrate by s... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00242 BindingDB Entry DOI: 10.7270/Q2KD22HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50546258 (CHEMBL4745604) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal His6-tagged CypB (unknown origin) expressed in Escherichia coli C41(DE3) using Suc-Ala-Ala-Cis-Pro-Phe-pNA as substrate by s... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00242 BindingDB Entry DOI: 10.7270/Q2KD22HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||