 Found 11836 hits of ic50 for UniProtKB: Q9NWZ3
Found 11836 hits of ic50 for UniProtKB: Q9NWZ3 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50515378

(CHEMBL4443947)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1C[C@H](C[C@H]1C(=O)N[C@@H]1CCCc2ccccc12)NC(=O)CCOCCOCCOCC#Cc1cnc(OC[C@@H]2CCC(=O)N2)c2cc(OC)c(cc12)C(N)=O |r| Show InChI InChI=1S/C53H70N8O11/c1-33(55-2)50(65)60-48(35-12-5-4-6-13-35)53(67)61-31-38(27-44(61)51(66)59-43-17-9-14-34-11-7-8-16-39(34)43)58-47(63)20-22-70-24-26-71-25-23-69-21-10-15-36-30-56-52(72-32-37-18-19-46(62)57-37)41-29-45(68-3)42(49(54)64)28-40(36)41/h7-8,11,16,28-30,33,35,37-38,43-44,48,55H,4-6,9,12-14,17-27,31-32H2,1-3H3,(H2,54,64)(H,57,62)(H,58,63)(H,59,66)(H,60,65)/t33-,37-,38-,43+,44-,48-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity to human IRK4 using myelin basic protein as substrate by [gamma-33P]-ATP assay |
ACS Med Chem Lett 10: 1081-1085 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00219
BindingDB Entry DOI: 10.7270/Q2RF5ZCB |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50511360
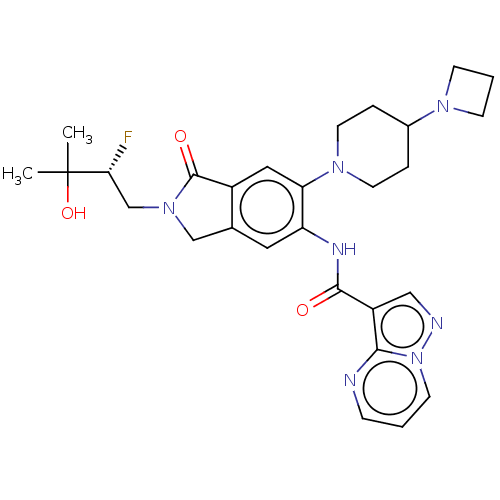
(CHEMBL4556091)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(cc2C1=O)N1CCC(CC1)N1CCC1 |r| Show InChI InChI=1S/C28H34FN7O3/c1-28(2,39)24(29)17-35-16-18-13-22(32-26(37)21-15-31-36-10-3-7-30-25(21)36)23(14-20(18)27(35)38)34-11-5-19(6-12-34)33-8-4-9-33/h3,7,10,13-15,19,24,39H,4-6,8-9,11-12,16-17H2,1-2H3,(H,32,37)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human whole blood assessed as inhibition of R848-induced IL-6 production preincubated for 90 mins followed by R848 stimulation... |
ACS Med Chem Lett 11: 327-333 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00380
BindingDB Entry DOI: 10.7270/Q26W9FDM |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50599226
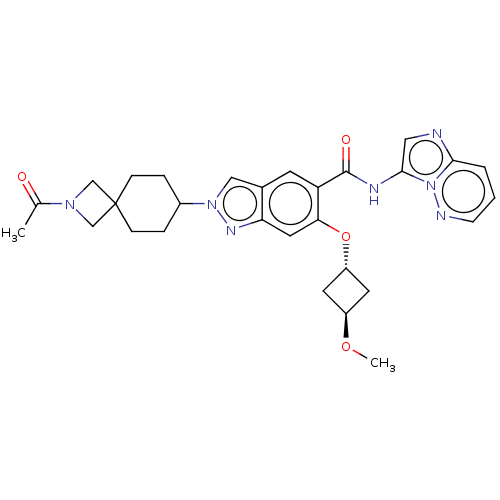
(CHEMBL5201062 | US11866405, Example 100)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc2nn(cc2cc1C(=O)Nc1cnc2cccnn12)C1CCC2(CN(C2)C(C)=O)CC1 |r,wU:4.6,wD:2.1,(5.55,-5.49,;4.22,-4.72,;4.22,-3.18,;5.31,-2.09,;4.2,-.98,;3.11,-2.07,;4.2,.56,;2.87,1.33,;1.54,.57,;.21,1.32,;-1.25,.85,;-2.16,2.09,;-1.25,3.34,;.21,2.86,;1.54,3.63,;2.87,2.87,;4.2,3.64,;4.2,5.18,;5.53,2.87,;6.87,3.64,;7.03,5.17,;8.53,5.49,;9.3,4.15,;10.81,3.83,;11.28,2.37,;10.25,1.22,;8.75,1.54,;8.27,3.01,;-3.7,2.09,;-4.47,.76,;-6.01,.76,;-6.78,2.09,;-7.88,.99,;-8.97,2.08,;-7.87,3.18,;-10.51,2.08,;-11.28,.74,;-11.28,3.41,;-6.01,3.43,;-4.47,3.43,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50599226

(CHEMBL5201062 | US11866405, Example 100)Show SMILES CO[C@H]1C[C@@H](C1)Oc1cc2nn(cc2cc1C(=O)Nc1cnc2cccnn12)C1CCC2(CN(C2)C(C)=O)CC1 |r,wU:4.6,wD:2.1,(5.55,-5.49,;4.22,-4.72,;4.22,-3.18,;5.31,-2.09,;4.2,-.98,;3.11,-2.07,;4.2,.56,;2.87,1.33,;1.54,.57,;.21,1.32,;-1.25,.85,;-2.16,2.09,;-1.25,3.34,;.21,2.86,;1.54,3.63,;2.87,2.87,;4.2,3.64,;4.2,5.18,;5.53,2.87,;6.87,3.64,;7.03,5.17,;8.53,5.49,;9.3,4.15,;10.81,3.83,;11.28,2.37,;10.25,1.22,;8.75,1.54,;8.27,3.01,;-3.7,2.09,;-4.47,.76,;-6.01,.76,;-6.78,2.09,;-7.88,.99,;-8.97,2.08,;-7.87,3.18,;-10.51,2.08,;-11.28,.74,;-11.28,3.41,;-6.01,3.43,;-4.47,3.43,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00324
BindingDB Entry DOI: 10.7270/Q2W381C3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50511348

(CHEMBL4566431 | US11034698, Example 105)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(cc2C1=O)N1CCN(CC1)C1COC1 |r| Show InChI InChI=1S/C27H32FN7O4/c1-27(2,38)23(28)14-34-13-17-10-21(31-25(36)20-12-30-35-5-3-4-29-24(20)35)22(11-19(17)26(34)37)33-8-6-32(7-9-33)18-15-39-16-18/h3-5,10-12,18,23,38H,6-9,13-16H2,1-2H3,(H,31,36)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human whole blood assessed as inhibition of R848-induced IL-6 production preincubated for 90 mins followed by R848 stimulation... |
ACS Med Chem Lett 11: 327-333 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00380
BindingDB Entry DOI: 10.7270/Q26W9FDM |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50511359
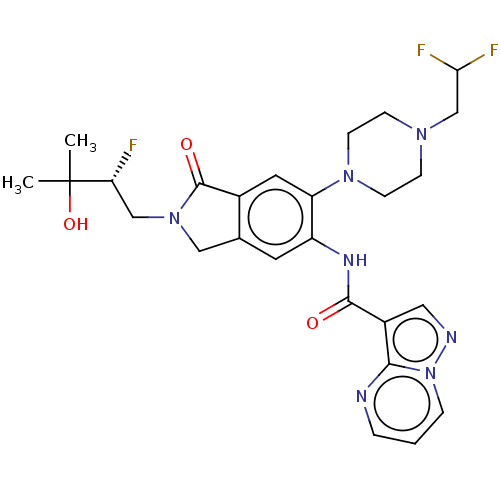
(CHEMBL4545898 | US10988478, Example 258)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(cc2C1=O)N1CCN(CC(F)F)CC1 |r| Show InChI InChI=1S/C26H30F3N7O3/c1-26(2,39)21(27)14-35-13-16-10-19(32-24(37)18-12-31-36-5-3-4-30-23(18)36)20(11-17(16)25(35)38)34-8-6-33(7-9-34)15-22(28)29/h3-5,10-12,21-22,39H,6-9,13-15H2,1-2H3,(H,32,37)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human whole blood assessed as inhibition of R848-induced IL-6 production preincubated for 90 mins followed by R848 stimulation... |
ACS Med Chem Lett 11: 327-333 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00380
BindingDB Entry DOI: 10.7270/Q26W9FDM |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50515375

(CHEMBL4448950)Show SMILES COc1cc2c(OC[C@@H]3CCC(=O)N3)ncc(C#CCCCCCCCCCCC(=O)N[C@H](C(=O)N3C[C@H](O)C[C@H]3C(=O)NCc3ccc(cc3)-c3scnc3C)C(C)(C)C)c2cc1C(N)=O |r| Show InChI InChI=1S/C51H65N7O8S/c1-32-45(67-31-55-32)34-20-18-33(19-21-34)27-53-48(63)41-24-37(59)29-58(41)50(64)46(51(2,3)4)57-43(60)17-15-13-11-9-7-6-8-10-12-14-16-35-28-54-49(66-30-36-22-23-44(61)56-36)39-26-42(65-5)40(47(52)62)25-38(35)39/h18-21,25-26,28,31,36-37,41,46,59H,6-13,15,17,22-24,27,29-30H2,1-5H3,(H2,52,62)(H,53,63)(H,56,61)(H,57,60)/t36-,37+,41-,46+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0990 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity to human IRK4 using myelin basic protein as substrate by [gamma-33P]-ATP assay |
ACS Med Chem Lett 10: 1081-1085 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00219
BindingDB Entry DOI: 10.7270/Q2RF5ZCB |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239500
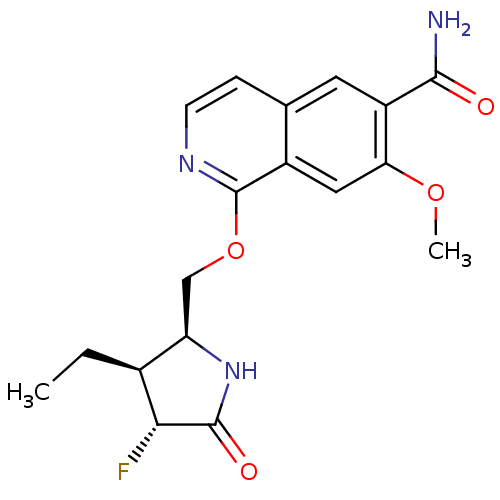
(CHEMBL4066705 | US10329302, Example 337 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27D308N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital
| Assay Description
This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... |
ACS Chem Biol 4: 834-43 (2009)
BindingDB Entry DOI: 10.7270/Q2FT8PC5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM403214

(US10329302, Example 370 | US10793579, Example 370 ...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@@H](F)[C@H]3CCF)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C18H19F2N3O4/c1-26-14-7-11-9(6-12(14)16(21)24)3-5-22-18(11)27-8-13-10(2-4-19)15(20)17(25)23-13/h3,5-7,10,13,15H,2,4,8H2,1H3,(H2,21,24)(H,23,25)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27D308N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27D308N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50599222

(CHEMBL5190644 | US11866405, Example 12)Show SMILES COc1cc2nn(cc2cc1C(=O)Nc1cnc2cccnn12)[C@H]1CC[C@@]2(CCC(=O)N2C)CC1 |r,wU:23.26,26.30,(3.8,-3.23,;3.8,-1.69,;2.46,-.92,;1.14,-1.69,;-.19,-.93,;-1.65,-1.41,;-2.56,-.16,;-1.65,1.09,;-.19,.61,;1.14,1.38,;2.46,.61,;3.8,1.38,;3.8,2.92,;5.13,.61,;6.47,1.38,;6.63,2.91,;8.13,3.23,;8.9,1.9,;10.41,1.58,;10.89,.12,;9.86,-1.03,;8.35,-.71,;7.87,.76,;-4.1,-.16,;-4.87,-1.49,;-6.41,-1.49,;-7.18,-.16,;-8.09,-1.4,;-9.55,-.93,;-9.55,.61,;-10.89,1.38,;-8.09,1.09,;-7.69,2.57,;-6.41,1.18,;-4.87,1.18,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00324
BindingDB Entry DOI: 10.7270/Q2W381C3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50599222

(CHEMBL5190644 | US11866405, Example 12)Show SMILES COc1cc2nn(cc2cc1C(=O)Nc1cnc2cccnn12)[C@H]1CC[C@@]2(CCC(=O)N2C)CC1 |r,wU:23.26,26.30,(3.8,-3.23,;3.8,-1.69,;2.46,-.92,;1.14,-1.69,;-.19,-.93,;-1.65,-1.41,;-2.56,-.16,;-1.65,1.09,;-.19,.61,;1.14,1.38,;2.46,.61,;3.8,1.38,;3.8,2.92,;5.13,.61,;6.47,1.38,;6.63,2.91,;8.13,3.23,;8.9,1.9,;10.41,1.58,;10.89,.12,;9.86,-1.03,;8.35,-.71,;7.87,.76,;-4.1,-.16,;-4.87,-1.49,;-6.41,-1.49,;-7.18,-.16,;-8.09,-1.4,;-9.55,-.93,;-9.55,.61,;-10.89,1.38,;-8.09,1.09,;-7.69,2.57,;-6.41,1.18,;-4.87,1.18,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50511361
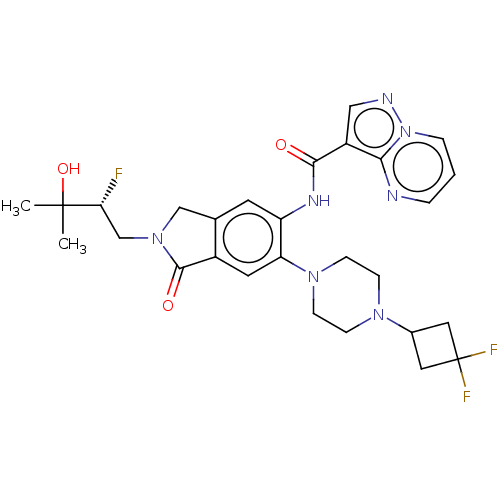
(CHEMBL4445098)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(cc2C1=O)N1CCN(CC1)C1CC(F)(F)C1 |r| Show InChI InChI=1S/C28H32F3N7O3/c1-27(2,41)23(29)16-37-15-17-10-21(34-25(39)20-14-33-38-5-3-4-32-24(20)38)22(11-19(17)26(37)40)36-8-6-35(7-9-36)18-12-28(30,31)13-18/h3-5,10-11,14,18,23,41H,6-9,12-13,15-16H2,1-2H3,(H,34,39)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human whole blood assessed as inhibition of R848-induced IL-6 production preincubated for 90 mins followed by R848 stimulation... |
ACS Med Chem Lett 11: 327-333 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00380
BindingDB Entry DOI: 10.7270/Q26W9FDM |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239500

(CHEMBL4066705 | US10329302, Example 337 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... |
US Patent US10793579 (2020)
BindingDB Entry DOI: 10.7270/Q2H9988P |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM403204

(US10329302, Example 360 | US10793579, Example 360 ...)Show SMILES CC[C@H]1[C@@H](COc2ncc(F)c3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H19F2N3O4/c1-3-8-13(23-17(25)15(8)20)7-27-18-10-5-14(26-2)11(16(21)24)4-9(10)12(19)6-22-18/h4-6,8,13,15H,3,7H2,1-2H3,(H2,21,24)(H,23,25)/t8-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... |
US Patent US10793579 (2020)
BindingDB Entry DOI: 10.7270/Q2H9988P |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM403214

(US10329302, Example 370 | US10793579, Example 370 ...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@@H](F)[C@H]3CCF)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C18H19F2N3O4/c1-26-14-7-11-9(6-12(14)16(21)24)3-5-22-18(11)27-8-13-10(2-4-19)15(20)17(25)23-13/h3,5-7,10,13,15H,2,4,8H2,1H3,(H2,21,24)(H,23,25)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... |
US Patent US10793579 (2020)
BindingDB Entry DOI: 10.7270/Q2H9988P |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... |
US Patent US10793579 (2020)
BindingDB Entry DOI: 10.7270/Q2H9988P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM319575

(5-(((2S,3S,4S)-3-ethyl-4-fluoro-5-oxopyrrolidin-2-...)Show SMILES CC[C@H]1[C@@H](COc2cccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C19H21FN2O4/c1-3-11-14(22-19(24)17(11)20)9-26-15-6-4-5-10-7-13(18(21)23)16(25-2)8-12(10)15/h4-8,11,14,17H,3,9H2,1-2H3,(H2,21,23)(H,22,24)/t11-,14+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... |
US Patent US10174000 (2019)
BindingDB Entry DOI: 10.7270/Q27S7QWQ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239500

(CHEMBL4066705 | US10329302, Example 337 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239500

(CHEMBL4066705 | US10329302, Example 337 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital
| Assay Description
This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... |
ACS Chem Biol 4: 834-43 (2009)
BindingDB Entry DOI: 10.7270/Q2FT8PC5 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM403204

(US10329302, Example 360 | US10793579, Example 360 ...)Show SMILES CC[C@H]1[C@@H](COc2ncc(F)c3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H19F2N3O4/c1-3-8-13(23-17(25)15(8)20)7-27-18-10-5-14(26-2)11(16(21)24)4-9(10)12(19)6-22-18/h4-6,8,13,15H,3,7H2,1-2H3,(H2,21,24)(H,23,25)/t8-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital
| Assay Description
This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... |
ACS Chem Biol 4: 834-43 (2009)
BindingDB Entry DOI: 10.7270/Q2FT8PC5 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM403214

(US10329302, Example 370 | US10793579, Example 370 ...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@@H](F)[C@H]3CCF)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C18H19F2N3O4/c1-26-14-7-11-9(6-12(14)16(21)24)3-5-22-18(11)27-8-13-10(2-4-19)15(20)17(25)23-13/h3,5-7,10,13,15H,2,4,8H2,1H3,(H2,21,24)(H,23,25)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital
| Assay Description
This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... |
ACS Chem Biol 4: 834-43 (2009)
BindingDB Entry DOI: 10.7270/Q2FT8PC5 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM403204

(US10329302, Example 360 | US10793579, Example 360 ...)Show SMILES CC[C@H]1[C@@H](COc2ncc(F)c3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H19F2N3O4/c1-3-8-13(23-17(25)15(8)20)7-27-18-10-5-14(26-2)11(16(21)24)4-9(10)12(19)6-22-18/h4-6,8,13,15H,3,7H2,1-2H3,(H2,21,24)(H,23,25)/t8-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27D308N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50511366
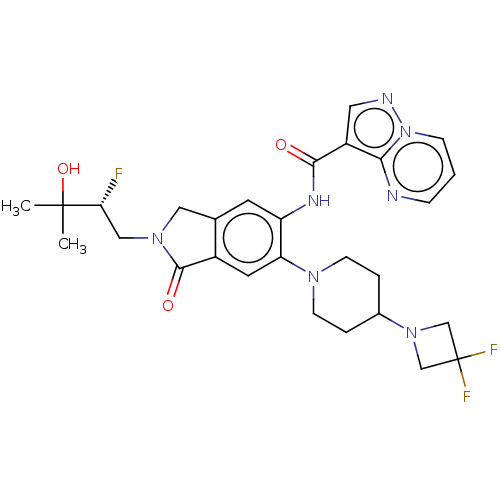
(CHEMBL4568894)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(cc2C1=O)N1CCC(CC1)N1CC(F)(F)C1 |r| Show InChI InChI=1S/C28H32F3N7O3/c1-27(2,41)23(29)14-36-13-17-10-21(34-25(39)20-12-33-38-7-3-6-32-24(20)38)22(11-19(17)26(36)40)35-8-4-18(5-9-35)37-15-28(30,31)16-37/h3,6-7,10-12,18,23,41H,4-5,8-9,13-16H2,1-2H3,(H,34,39)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human whole blood assessed as inhibition of R848-induced IL-6 production preincubated for 90 mins followed by R848 stimulation... |
ACS Med Chem Lett 11: 327-333 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00380
BindingDB Entry DOI: 10.7270/Q26W9FDM |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50511358
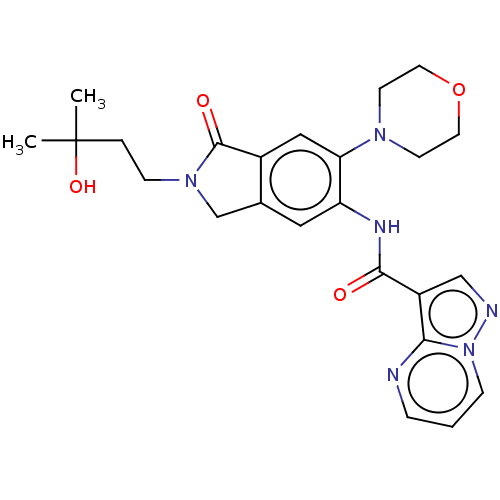
(CHEMBL4472406 | US10988478, Example 175)Show SMILES CC(C)(O)CCN1Cc2cc(NC(=O)c3cnn4cccnc34)c(cc2C1=O)N1CCOCC1 Show InChI InChI=1S/C24H28N6O4/c1-24(2,33)4-7-29-15-16-12-19(20(13-17(16)23(29)32)28-8-10-34-11-9-28)27-22(31)18-14-26-30-6-3-5-25-21(18)30/h3,5-6,12-14,33H,4,7-11,15H2,1-2H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human whole blood assessed as inhibition of R848-induced IL-6 production preincubated for 90 mins followed by R848 stimulation... |
ACS Med Chem Lett 11: 327-333 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00380
BindingDB Entry DOI: 10.7270/Q26W9FDM |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50511349
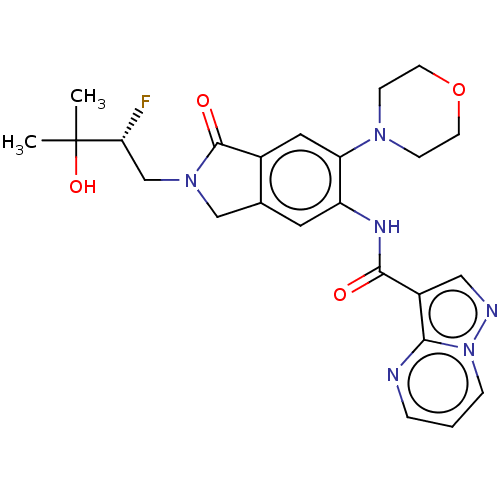
(CHEMBL4475494 | US10988478, Example 139)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(cc2C1=O)N1CCOCC1 |r| Show InChI InChI=1S/C24H27FN6O4/c1-24(2,34)20(25)14-30-13-15-10-18(19(11-16(15)23(30)33)29-6-8-35-9-7-29)28-22(32)17-12-27-31-5-3-4-26-21(17)31/h3-5,10-12,20,34H,6-9,13-14H2,1-2H3,(H,28,32)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 in human whole blood assessed as inhibition of R848-induced IL-6 production preincubated for 90 mins followed by R848 stimulation... |
ACS Med Chem Lett 11: 327-333 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00380
BindingDB Entry DOI: 10.7270/Q26W9FDM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27D308N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM302996
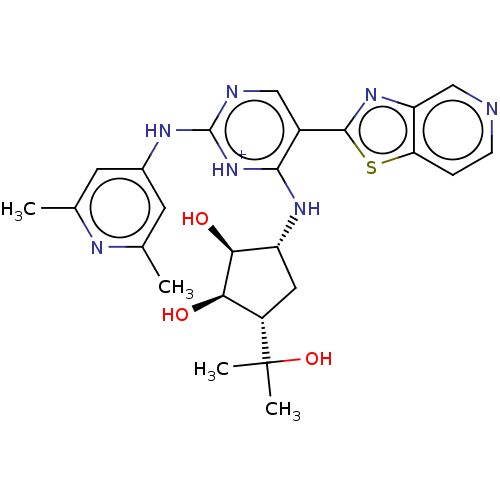
(US9598440, 137)Show SMILES Cc1cc(Nc2ncc(-c3nc4cnccc4s3)c(N[C@@H]3C[C@@H]([C@@H](O)[C@H]3O)C(C)(C)O)[nH+]2)cc(C)n1 |r| Show InChI InChI=1S/C25H29N7O3S/c1-12-7-14(8-13(2)28-12)29-24-27-10-15(23-31-18-11-26-6-5-19(18)36-23)22(32-24)30-17-9-16(25(3,4)35)20(33)21(17)34/h5-8,10-11,16-17,20-21,33-35H,9H2,1-4H3,(H2,27,28,29,30,32)/p+1/t16-,17+,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
Procedure: A 20 μl reaction mixture contains 10 mM TriHCl, pH 7.2, 0.5 nM GST tagged IRAK4 (SignalChem), 100 nM fluorescent peptide substrate (R... |
US Patent US9598440 (2017)
BindingDB Entry DOI: 10.7270/Q2XG9T6V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27D308N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27D308N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM403189

(US10329302, Example 345 | US10793579, Example 345 ...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)C1(F)F |r| Show InChI InChI=1S/C18H19F2N3O4/c1-3-12-13(23-17(25)18(12,19)20)8-27-16-10-7-14(26-2)11(15(21)24)6-9(10)4-5-22-16/h4-7,12-13H,3,8H2,1-2H3,(H2,21,24)(H,23,25)/t12-,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27D308N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50599223

(CHEMBL5202941 | US11866405, Example 23)Show SMILES COc1cc2nn(cc2cc1C(=O)Nc1cnc2cccnn12)[C@H]1CC[C@@H](CC1)N(C)C(C)=O |r,wU:26.33,wD:23.26,(3.87,-3.23,;3.87,-1.69,;2.54,-.92,;1.22,-1.69,;-.12,-.93,;-1.58,-1.41,;-2.49,-.16,;-1.58,1.09,;-.12,.61,;1.21,1.38,;2.54,.61,;3.87,1.38,;3.87,2.92,;5.2,.61,;6.54,1.38,;6.7,2.91,;8.21,3.23,;8.97,1.9,;10.48,1.58,;10.96,.12,;9.93,-1.03,;8.42,-.71,;7.94,.76,;-4.03,-.16,;-4.8,1.17,;-6.34,1.17,;-7.11,-.16,;-6.34,-1.49,;-4.8,-1.49,;-8.65,-.16,;-9.42,1.17,;-9.42,-1.49,;-10.96,-1.49,;-8.65,-2.83,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00324
BindingDB Entry DOI: 10.7270/Q2W381C3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50599224

(CHEMBL5176061 | US11866405, Example 68)Show SMILES COc1cc2nn(cc2cc1C(=O)Nc1cnc2cccnn12)[C@H]1CC[C@@]2(CN(C)C(=O)C2)CC1 |r,wU:23.26,wD:26.36,(3.83,-3.23,;3.83,-1.69,;2.5,-.92,;1.18,-1.69,;-.15,-.93,;-1.62,-1.41,;-2.53,-.16,;-1.62,1.09,;-.15,.61,;1.17,1.38,;2.5,.61,;3.83,1.38,;3.83,2.92,;5.17,.61,;6.5,1.38,;6.66,2.91,;8.17,3.23,;8.94,1.9,;10.44,1.58,;10.92,.12,;9.89,-1.03,;8.38,-.71,;7.91,.76,;-4.07,-.16,;-4.84,-1.49,;-6.38,-1.49,;-7.15,-.16,;-7.92,1.17,;-9.42,.85,;-10.51,1.94,;-9.58,-.68,;-10.92,-1.45,;-8.18,-1.3,;-6.38,1.17,;-4.84,1.17,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00324
BindingDB Entry DOI: 10.7270/Q2W381C3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50599225

(CHEMBL5196239)Show SMILES COc1cc2nn(cc2cc1C(=O)Nc1cnc2cccnn12)[C@H]1CC[C@@H](CC1)N(C)C(=O)[C@@H](C)O |r,wU:26.33,wD:23.26,33.38,(4.26,-3.23,;4.26,-1.69,;2.92,-.92,;1.6,-1.69,;.27,-.93,;-1.2,-1.41,;-2.1,-.16,;-1.2,1.09,;.27,.61,;1.6,1.38,;2.92,.61,;4.26,1.38,;4.26,2.92,;5.59,.61,;6.92,1.38,;7.08,2.91,;8.59,3.23,;9.36,1.9,;10.87,1.58,;11.34,.12,;10.31,-1.03,;8.81,-.71,;8.33,.76,;-3.64,-.16,;-4.41,1.17,;-5.95,1.17,;-6.72,-.16,;-5.95,-1.49,;-4.41,-1.49,;-8.26,-.16,;-9.03,1.17,;-9.03,-1.49,;-8.26,-2.83,;-10.57,-1.49,;-11.34,-.16,;-11.34,-2.83,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00324
BindingDB Entry DOI: 10.7270/Q2W381C3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM402999

(US10329302, Example 146 | US10793579, Example 146 ...)Show SMILES COc1cc2c(OC[C@@H]3CC(F)(F)C(=O)N3)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C16H15F2N3O4/c1-24-12-5-10-8(4-11(12)13(19)22)2-3-20-14(10)25-7-9-6-16(17,18)15(23)21-9/h2-5,9H,6-7H2,1H3,(H2,19,22)(H,21,23)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27D308N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM403035

(US10329302, Example 183 | US10793579, Example 183 ...)Show SMILES CC(C)Oc1cc2c(OC[C@H]3NC(=O)C(F)(F)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C19H21F2N3O4/c1-9(2)28-15-7-12-11(6-13(15)16(22)25)4-5-23-17(12)27-8-14-10(3)19(20,21)18(26)24-14/h4-7,9-10,14H,8H2,1-3H3,(H2,22,25)(H,24,26)/t10-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27D308N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50599223

(CHEMBL5202941 | US11866405, Example 23)Show SMILES COc1cc2nn(cc2cc1C(=O)Nc1cnc2cccnn12)[C@H]1CC[C@@H](CC1)N(C)C(C)=O |r,wU:26.33,wD:23.26,(3.87,-3.23,;3.87,-1.69,;2.54,-.92,;1.22,-1.69,;-.12,-.93,;-1.58,-1.41,;-2.49,-.16,;-1.58,1.09,;-.12,.61,;1.21,1.38,;2.54,.61,;3.87,1.38,;3.87,2.92,;5.2,.61,;6.54,1.38,;6.7,2.91,;8.21,3.23,;8.97,1.9,;10.48,1.58,;10.96,.12,;9.93,-1.03,;8.42,-.71,;7.94,.76,;-4.03,-.16,;-4.8,1.17,;-6.34,1.17,;-7.11,-.16,;-6.34,-1.49,;-4.8,-1.49,;-8.65,-.16,;-9.42,1.17,;-9.42,-1.49,;-10.96,-1.49,;-8.65,-2.83,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM643809

(US11866405, Example 87)Show SMILES COc1cc2nn(cc2cc1C(=O)Nc1cnn2cccnc12)[C@H]1CC[C@@]2(CN(C)C(=O)C2)CC1 |r,wU:23.26,26.30,(3.8,-3.24,;3.8,-1.7,;2.47,-.93,;1.13,-1.7,;-.2,-.93,;-1.66,-1.4,;-2.57,-.16,;-1.66,1.09,;-.2,.61,;1.13,1.38,;2.47,.61,;3.8,1.38,;3.9,2.93,;5.14,.61,;6.47,1.38,;6.63,2.92,;8.14,3.24,;8.91,1.9,;10.41,1.58,;10.89,.12,;9.86,-1.03,;8.35,-.71,;7.88,.76,;-4.11,-.16,;-4.88,-1.49,;-6.42,-1.49,;-7.19,-.16,;-8.09,1.09,;-9.56,.61,;-10.89,1.58,;-9.56,-.93,;-10.89,-1.89,;-8.09,-1.4,;-6.42,1.18,;-4.88,1.18,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM643825

(US11866405, Example 95)Show SMILES COc1cc2nn(cc2cc1C(=O)Nc1cnc2cccnn12)[C@H]1CC[C@@H](CC1)N(C)C(=O)[C@@H](C)OC(C)=O |r,wU:26.33,wD:33.38,23.26,(5.42,-2.78,;5.42,-1.24,;4.08,-.47,;2.75,-1.24,;1.42,-.47,;-.05,-.94,;-.95,.3,;-.05,1.55,;1.42,1.07,;2.75,1.84,;4.08,1.07,;5.42,1.84,;5.42,3.38,;6.75,1.07,;8.08,1.84,;8.25,3.38,;9.75,3.7,;10.52,2.36,;12.03,2.04,;12.5,.58,;11.47,-.57,;9.97,-.25,;9.49,1.22,;-2.49,.3,;-3.26,1.64,;-4.8,1.64,;-5.57,.3,;-4.8,-1.03,;-3.26,-1.03,;-7.11,.3,;-7.88,1.64,;-7.88,-1.03,;-7.11,-2.36,;-9.42,-1.03,;-10.19,.3,;-10.19,-2.36,;-11.73,-2.36,;-12.5,-3.7,;-12.5,-1.03,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM402999

(US10329302, Example 146 | US10793579, Example 146 ...)Show SMILES COc1cc2c(OC[C@@H]3CC(F)(F)C(=O)N3)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C16H15F2N3O4/c1-24-12-5-10-8(4-11(12)13(19)22)2-3-20-14(10)25-7-9-6-16(17,18)15(23)21-9/h2-5,9H,6-7H2,1H3,(H2,19,22)(H,21,23)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... |
US Patent US10793579 (2020)
BindingDB Entry DOI: 10.7270/Q2H9988P |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM403035

(US10329302, Example 183 | US10793579, Example 183 ...)Show SMILES CC(C)Oc1cc2c(OC[C@H]3NC(=O)C(F)(F)[C@H]3C)nccc2cc1C(N)=O |r| Show InChI InChI=1S/C19H21F2N3O4/c1-9(2)28-15-7-12-11(6-13(15)16(22)25)4-5-23-17(12)27-8-14-10(3)19(20,21)18(26)24-14/h4-7,9-10,14H,8H2,1-3H3,(H2,22,25)(H,24,26)/t10-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... |
US Patent US10793579 (2020)
BindingDB Entry DOI: 10.7270/Q2H9988P |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... |
US Patent US10793579 (2020)
BindingDB Entry DOI: 10.7270/Q2H9988P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM403169

(US10329302, Example 323 | US10793579, Example 323 ...)Show SMILES COc1cc2c(OC[C@H]3NC(=O)[C@]4(F)[C@@H](C)[C@H]34)ccnc2cc1C(N)=O |r| Show InChI InChI=1S/C18H18FN3O4/c1-8-15-12(22-17(24)18(8,15)19)7-26-13-3-4-21-11-5-10(16(20)23)14(25-2)6-9(11)13/h3-6,8,12,15H,7H2,1-2H3,(H2,20,23)(H,22,24)/t8-,12+,15+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... |
US Patent US10793579 (2020)
BindingDB Entry DOI: 10.7270/Q2H9988P |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... |
US Patent US10793579 (2020)
BindingDB Entry DOI: 10.7270/Q2H9988P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... |
US Patent US10793579 (2020)
BindingDB Entry DOI: 10.7270/Q2H9988P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM403189

(US10329302, Example 345 | US10793579, Example 345 ...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)C1(F)F |r| Show InChI InChI=1S/C18H19F2N3O4/c1-3-12-13(23-17(25)18(12,19)20)8-27-16-10-7-14(26-2)11(15(21)24)6-9(10)4-5-22-16/h4-7,12-13H,3,8H2,1-2H3,(H2,21,24)(H,23,25)/t12-,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... |
US Patent US10793579 (2020)
BindingDB Entry DOI: 10.7270/Q2H9988P |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50130661
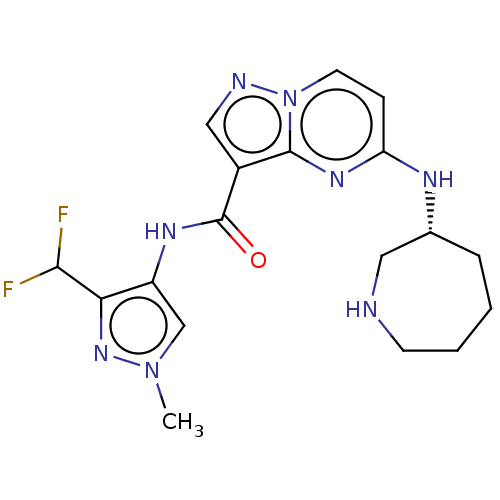
(CHEMBL3634510 | US10329294, Example 162)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCCNC4)nc23)c(n1)C(F)F |r| Show InChI InChI=1S/C18H22F2N8O/c1-27-10-13(15(26-27)16(19)20)24-18(29)12-9-22-28-7-5-14(25-17(12)28)23-11-4-2-3-6-21-8-11/h5,7,9-11,16,21H,2-4,6,8H2,1H3,(H,23,25)(H,24,29)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
| Assay Description
The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... |
Bioorg Med Chem 17: 6590-605 (2009)
BindingDB Entry DOI: 10.7270/Q2V40XJ3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM319575

(5-(((2S,3S,4S)-3-ethyl-4-fluoro-5-oxopyrrolidin-2-...)Show SMILES CC[C@H]1[C@@H](COc2cccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C19H21FN2O4/c1-3-11-14(22-19(24)17(11)20)9-26-15-6-4-5-10-7-13(18(21)23)16(25-2)8-12(10)15/h4-8,11,14,17H,3,9H2,1-2H3,(H2,21,23)(H,22,24)/t11-,14+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 (unknown origin) |
Eur J Med Chem 163: 413-427 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.072
BindingDB Entry DOI: 10.7270/Q2736V7K |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50239499

(CHEMBL4081711 | US10329302, Example 344 | US107935...)Show SMILES CC[C@H]1[C@@H](COc2nccc3cc(C(N)=O)c(OC)cc23)NC(=O)[C@H]1F |r| Show InChI InChI=1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... |
J Med Chem 60: 5521-5542 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00231
BindingDB Entry DOI: 10.7270/Q26D5W42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data