 Found 201 hits of ic50 data for polymerid = 5424
Found 201 hits of ic50 data for polymerid = 5424 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50221566
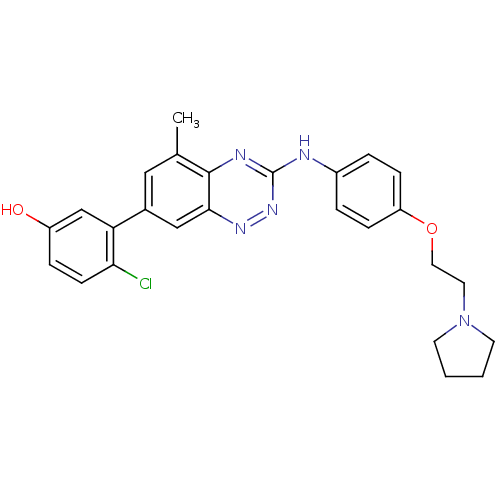
(4-chloro-3-(5-methyl-3-(4-(2-(pyrrolidin-1-yl)etho...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(OCCN4CCCC4)cc3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C26H26ClN5O2/c1-17-14-18(22-16-20(33)6-9-23(22)27)15-24-25(17)29-26(31-30-24)28-19-4-7-21(8-5-19)34-13-12-32-10-2-3-11-32/h4-9,14-16,33H,2-3,10-13H2,1H3,(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of YES |
J Med Chem 51: 1546-59 (2008)
Article DOI: 10.1021/jm7011276
BindingDB Entry DOI: 10.7270/Q2NV9K3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM4552
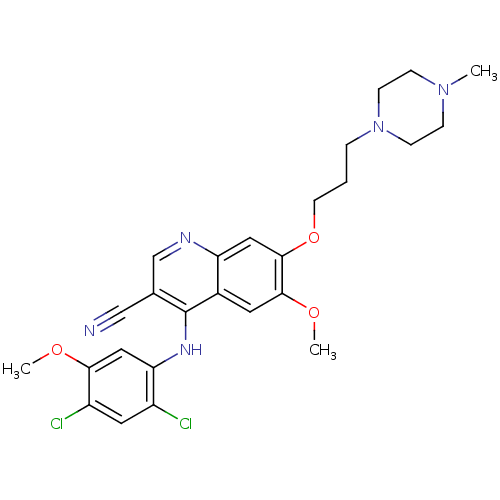
(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Molecular Medicine of the Austrian Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of YES1 |
Leukemia 23: 477-85 (2009)
Article DOI: 10.1038/leu.2008.334
BindingDB Entry DOI: 10.7270/Q22Z15R6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50221566

(4-chloro-3-(5-methyl-3-(4-(2-(pyrrolidin-1-yl)etho...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(OCCN4CCCC4)cc3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C26H26ClN5O2/c1-17-14-18(22-16-20(33)6-9-23(22)27)15-24-25(17)29-26(31-30-24)28-19-4-7-21(8-5-19)34-13-12-32-10-2-3-11-32/h4-9,14-16,33H,2-3,10-13H2,1H3,(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 25 |
TargeGen, Inc.
US Patent
| Assay Description
Testing of inhibition of kinases in vitro using luciferase-based assay from KinaseGlo, Promega Corp. |
US Patent US8481536 (2013)
BindingDB Entry DOI: 10.7270/Q2GB22PZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50184767
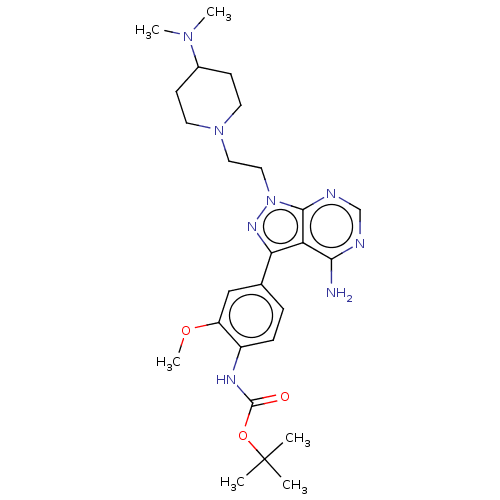
(CHEMBL3824089 | US10294227, Code 506)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C26H38N8O3/c1-26(2,3)37-25(35)30-19-8-7-17(15-20(19)36-6)22-21-23(27)28-16-29-24(21)34(31-22)14-13-33-11-9-18(10-12-33)32(4)5/h7-8,15-16,18H,9-14H2,1-6H3,(H,30,35)(H2,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human YES using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P] ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Yes1 (unknown origin) assessed as kinase-dependent enzymatic production of ADP from ATP using coupled luminescence-based reaction by AD... |
Bioorg Med Chem Lett 23: 4398-403 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.072
BindingDB Entry DOI: 10.7270/Q2BR8W3F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human YES using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P] ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of yes kinase |
J Med Chem 47: 6658-61 (2004)
Article DOI: 10.1021/jm049486a
BindingDB Entry DOI: 10.7270/Q2ZG6RRC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM12255
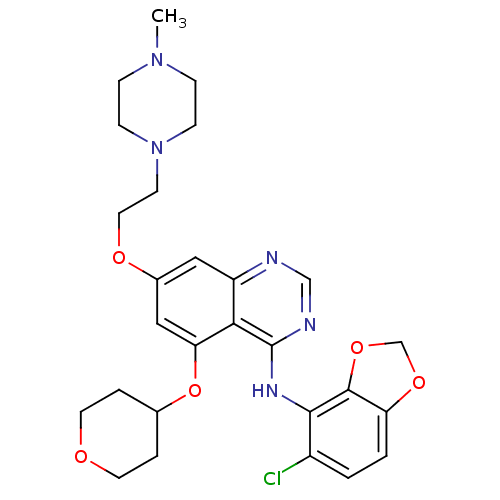
(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Yes1 (unknown origin) by [gamma-33P]-ATP radiolabeled enzyme activity assay |
Bioorg Med Chem Lett 23: 4398-403 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.072
BindingDB Entry DOI: 10.7270/Q2BR8W3F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM97937
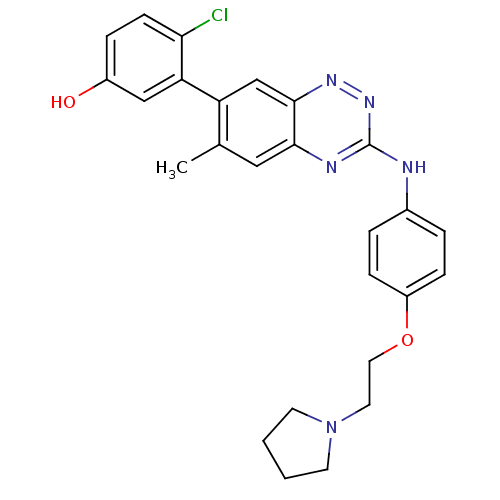
(US8481536, 507 | US8481536, 612)Show SMILES Cc1cc2nc(Nc3ccc(OCCN4CCCC4)cc3)nnc2cc1-c1cc(O)ccc1Cl Show InChI InChI=1S/C26H26ClN5O2/c1-17-14-24-25(16-21(17)22-15-19(33)6-9-23(22)27)30-31-26(29-24)28-18-4-7-20(8-5-18)34-13-12-32-10-2-3-11-32/h4-9,14-16,33H,2-3,10-13H2,1H3,(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 25 |
TargeGen, Inc.
US Patent
| Assay Description
Testing of inhibition of kinases in vitro using luciferase-based assay from KinaseGlo, Promega Corp. |
US Patent US8481536 (2013)
BindingDB Entry DOI: 10.7270/Q2GB22PZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM97672
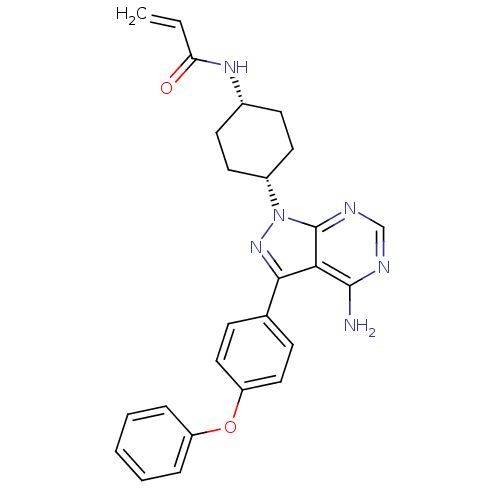
(US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)NC(=O)C=C |r,wU:23.26,26.33,(-3.76,2.88,;-3.76,1.34,;-5.09,.57,;-5.09,-.97,;-3.76,-1.74,;-2.43,-.97,;-.96,-1.45,;-.06,-.2,;-.96,1.05,;-.56,2.53,;.92,2.93,;1.32,4.42,;.23,5.51,;.63,7,;2.12,7.4,;3.21,6.31,;4.7,6.7,;5.09,8.19,;4.01,9.28,;2.52,8.88,;-1.25,5.11,;-1.65,3.62,;-2.43,.57,;-.56,-2.93,;-1.65,-4.02,;-1.25,-5.51,;.23,-5.91,;1.32,-4.82,;.92,-3.33,;.63,-7.4,;2.12,-7.79,;2.52,-9.28,;3.21,-6.7,;4.7,-7.1,)| Show InChI InChI=1S/C26H26N6O2/c1-2-22(33)30-18-10-12-19(13-11-18)32-26-23(25(27)28-16-29-26)24(31-32)17-8-14-21(15-9-17)34-20-6-4-3-5-7-20/h2-9,14-16,18-19H,1,10-13H2,(H,30,33)(H2,27,28,29)/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC
US Patent
| Assay Description
IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... |
US Patent US9181263 (2015)
BindingDB Entry DOI: 10.7270/Q2765D5Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50086441
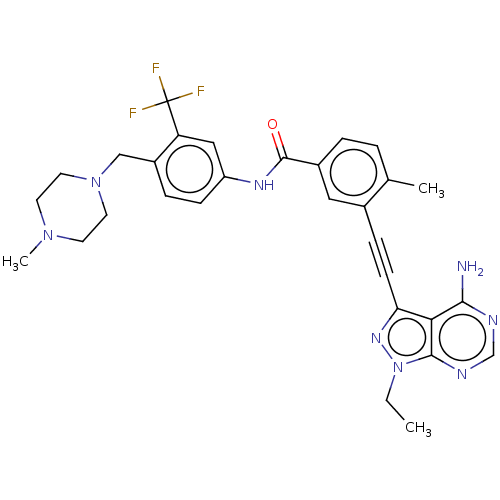
(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Yes |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM97672

(US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)NC(=O)C=C |r,wU:23.26,26.33,(-3.76,2.88,;-3.76,1.34,;-5.09,.57,;-5.09,-.97,;-3.76,-1.74,;-2.43,-.97,;-.96,-1.45,;-.06,-.2,;-.96,1.05,;-.56,2.53,;.92,2.93,;1.32,4.42,;.23,5.51,;.63,7,;2.12,7.4,;3.21,6.31,;4.7,6.7,;5.09,8.19,;4.01,9.28,;2.52,8.88,;-1.25,5.11,;-1.65,3.62,;-2.43,.57,;-.56,-2.93,;-1.65,-4.02,;-1.25,-5.51,;.23,-5.91,;1.32,-4.82,;.92,-3.33,;.63,-7.4,;2.12,-7.79,;2.52,-9.28,;3.21,-6.7,;4.7,-7.1,)| Show InChI InChI=1S/C26H26N6O2/c1-2-22(33)30-18-10-12-19(13-11-18)32-26-23(25(27)28-16-29-26)24(31-32)17-8-14-21(15-9-17)34-20-6-4-3-5-7-20/h2-9,14-16,18-19H,1,10-13H2,(H,30,33)(H2,27,28,29)/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC
US Patent
| Assay Description
IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... |
US Patent US9278100 (2016)
BindingDB Entry DOI: 10.7270/Q20C4TMX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50322535
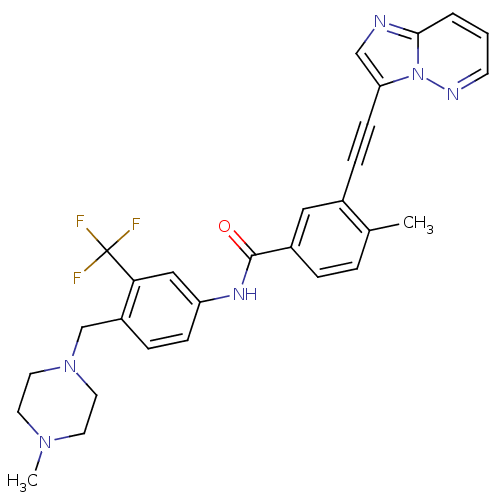
(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human YES using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Yes1 (unknown origin) by [gamma-33P]-ATP radiolabeled enzyme activity assay |
Bioorg Med Chem Lett 23: 4398-403 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.072
BindingDB Entry DOI: 10.7270/Q2BR8W3F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM378887
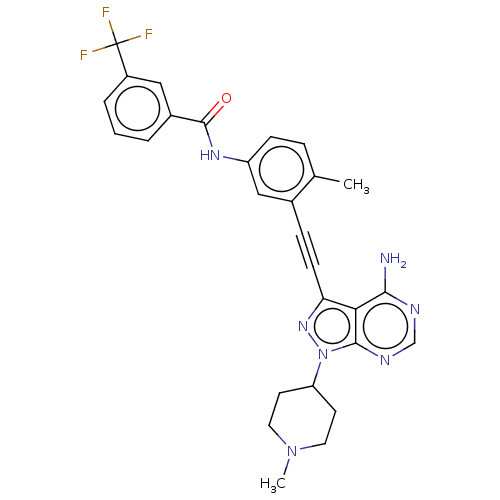
(US10266537, Compound 31)Show SMILES CN1CCC(CC1)n1nc(C#Cc2cc(NC(=O)c3cccc(c3)C(F)(F)F)ccc2C)c2c(N)ncnc12 Show InChI InChI=1S/C28H26F3N7O/c1-17-6-8-21(35-27(39)19-4-3-5-20(14-19)28(29,30)31)15-18(17)7-9-23-24-25(32)33-16-34-26(24)38(36-23)22-10-12-37(2)13-11-22/h3-6,8,14-16,22H,10-13H2,1-2H3,(H,35,39)(H2,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
| Assay Description
The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... |
Bioorg Med Chem Lett 17: 3562-9 (2007)
BindingDB Entry DOI: 10.7270/Q21C2065 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant YES using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
| Assay Description
The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... |
Bioorg Med Chem Lett 17: 3562-9 (2007)
BindingDB Entry DOI: 10.7270/Q21C2065 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
| Assay Description
The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... |
Bioorg Med Chem Lett 17: 3562-9 (2007)
BindingDB Entry DOI: 10.7270/Q21C2065 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM378888
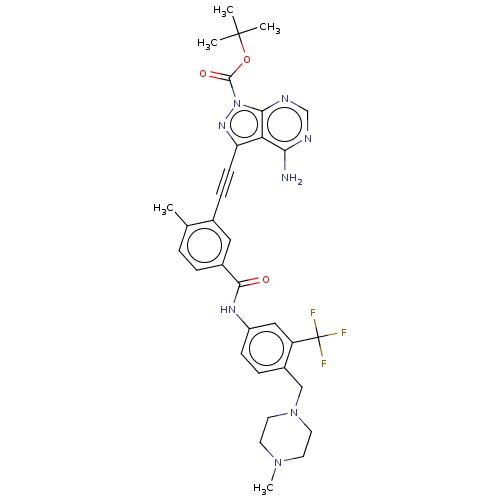
(Preparation of 3-(4-amino-1-(piperidin-4-yl)-1H-py...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C(=O)OC(C)(C)C)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C33H35F3N8O3/c1-20-6-7-22(30(45)40-24-10-8-23(25(17-24)33(34,35)36)18-43-14-12-42(5)13-15-43)16-21(20)9-11-26-27-28(37)38-19-39-29(27)44(41-26)31(46)47-32(2,3)4/h6-8,10,16-17,19H,12-15,18H2,1-5H3,(H,40,45)(H2,37,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
| Assay Description
The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... |
Bioorg Med Chem Lett 17: 3562-9 (2007)
BindingDB Entry DOI: 10.7270/Q21C2065 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50193874
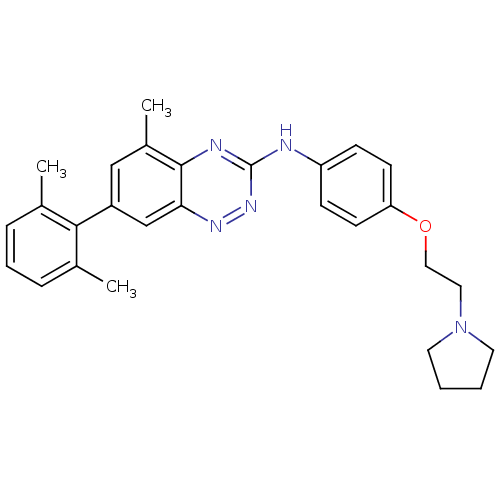
(7-(2,6-dimethylphenyl)-5-methyl-N-(4-(2-(pyrrolidi...)Show SMILES Cc1cccc(C)c1-c1cc(C)c2nc(Nc3ccc(OCCN4CCCC4)cc3)nnc2c1 Show InChI InChI=1S/C28H31N5O/c1-19-7-6-8-20(2)26(19)22-17-21(3)27-25(18-22)31-32-28(30-27)29-23-9-11-24(12-10-23)34-16-15-33-13-4-5-14-33/h6-12,17-18H,4-5,13-16H2,1-3H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Yes |
Bioorg Med Chem Lett 16: 5546-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.035
BindingDB Entry DOI: 10.7270/Q2SN08MP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50193874

(7-(2,6-dimethylphenyl)-5-methyl-N-(4-(2-(pyrrolidi...)Show SMILES Cc1cccc(C)c1-c1cc(C)c2nc(Nc3ccc(OCCN4CCCC4)cc3)nnc2c1 Show InChI InChI=1S/C28H31N5O/c1-19-7-6-8-20(2)26(19)22-17-21(3)27-25(18-22)31-32-28(30-27)29-23-9-11-24(12-10-23)34-16-15-33-13-4-5-14-33/h6-12,17-18H,4-5,13-16H2,1-3H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of YES |
J Med Chem 51: 1546-59 (2008)
Article DOI: 10.1021/jm7011276
BindingDB Entry DOI: 10.7270/Q2NV9K3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM97982
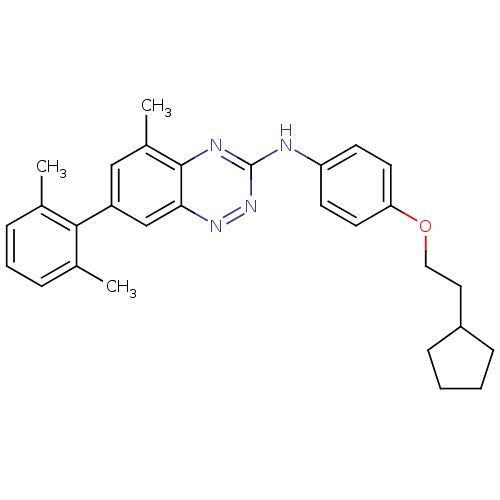
(US8481536, 574)Show SMILES Cc1cccc(C)c1-c1cc(C)c2nc(Nc3ccc(OCCC4CCCC4)cc3)nnc2c1 Show InChI InChI=1S/C29H32N4O/c1-19-7-6-8-20(2)27(19)23-17-21(3)28-26(18-23)32-33-29(31-28)30-24-11-13-25(14-12-24)34-16-15-22-9-4-5-10-22/h6-8,11-14,17-18,22H,4-5,9-10,15-16H2,1-3H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | 25 |
TargeGen, Inc.
US Patent
| Assay Description
Testing of inhibition of kinases in vitro using luciferase-based assay from KinaseGlo, Promega Corp. |
US Patent US8481536 (2013)
BindingDB Entry DOI: 10.7270/Q2GB22PZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM97907
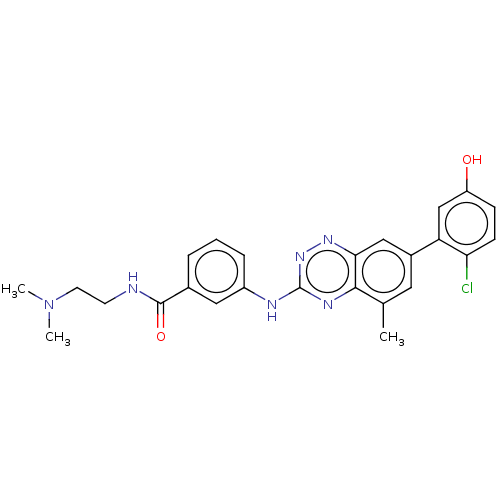
(US8481536, 471 | US8481536, 606)Show SMILES CN(C)CCNC(=O)c1cccc(Nc2nnc3cc(cc(C)c3n2)-c2cc(O)ccc2Cl)c1 Show InChI InChI=1S/C25H25ClN6O2/c1-15-11-17(20-14-19(33)7-8-21(20)26)13-22-23(15)29-25(31-30-22)28-18-6-4-5-16(12-18)24(34)27-9-10-32(2)3/h4-8,11-14,33H,9-10H2,1-3H3,(H,27,34)(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.24 | n/a | n/a | n/a | n/a | n/a | 25 |
TargeGen, Inc.
US Patent
| Assay Description
Testing of inhibition of kinases in vitro using luciferase-based assay from KinaseGlo, Promega Corp. |
US Patent US8481536 (2013)
BindingDB Entry DOI: 10.7270/Q2GB22PZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50221553
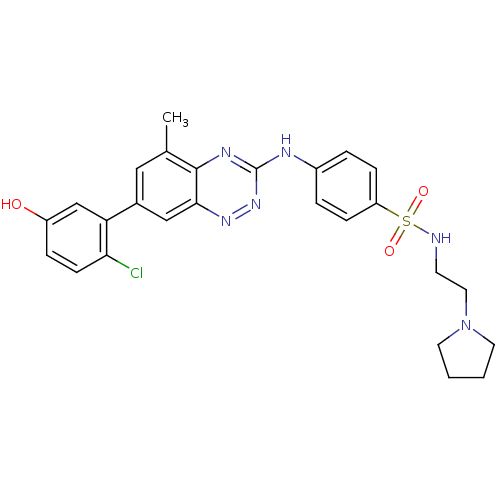
(4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][1...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(cc3)S(=O)(=O)NCCN3CCCC3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C26H27ClN6O3S/c1-17-14-18(22-16-20(34)6-9-23(22)27)15-24-25(17)30-26(32-31-24)29-19-4-7-21(8-5-19)37(35,36)28-10-13-33-11-2-3-12-33/h4-9,14-16,28,34H,2-3,10-13H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 1.28 | n/a | n/a | n/a | n/a | n/a | 25 |
TargeGen, Inc.
US Patent
| Assay Description
Testing of inhibition of kinases in vitro using luciferase-based assay from KinaseGlo, Promega Corp. |
US Patent US8481536 (2013)
BindingDB Entry DOI: 10.7270/Q2GB22PZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50221553

(4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][1...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(cc3)S(=O)(=O)NCCN3CCCC3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C26H27ClN6O3S/c1-17-14-18(22-16-20(34)6-9-23(22)27)15-24-25(17)30-26(32-31-24)29-19-4-7-21(8-5-19)37(35,36)28-10-13-33-11-2-3-12-33/h4-9,14-16,28,34H,2-3,10-13H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of YES |
J Med Chem 51: 1546-59 (2008)
Article DOI: 10.1021/jm7011276
BindingDB Entry DOI: 10.7270/Q2NV9K3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM25118
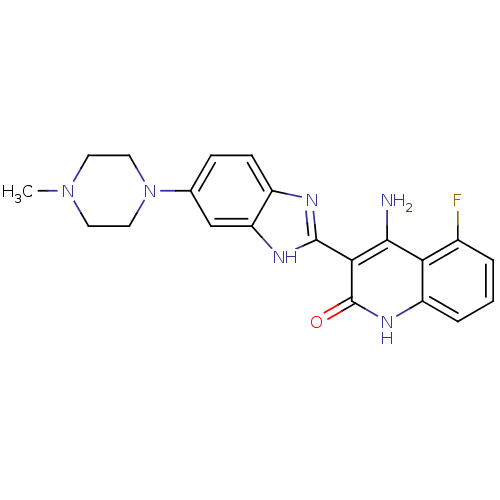
((3Z)-4-amino-5-fluoro-3-[5-(4-methylpiperazino)-1,...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2c(F)cccc2[nH]c1=O Show InChI InChI=1S/C21H21FN6O/c1-27-7-9-28(10-8-27)12-5-6-14-16(11-12)25-20(24-14)18-19(23)17-13(22)3-2-4-15(17)26-21(18)29/h2-6,11H,7-10H2,1H3,(H,24,25)(H3,23,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Yes1 (unknown origin) by [gamma-33P]-ATP radiolabeled enzyme activity assay |
Bioorg Med Chem Lett 23: 4398-403 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.072
BindingDB Entry DOI: 10.7270/Q2BR8W3F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM185674
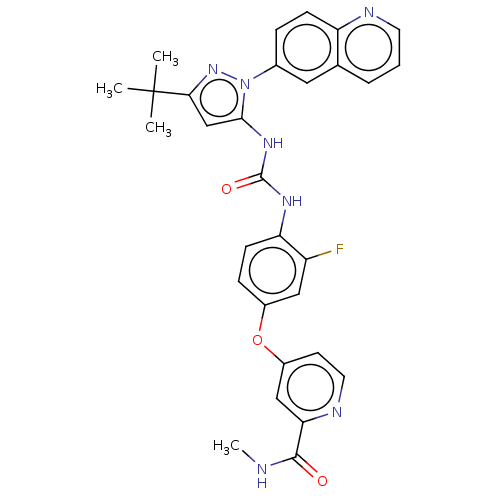
(4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)ca...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3cc(nn3-c3ccc4ncccc4c3)C(C)(C)C)c(F)c2)ccn1 Show InChI InChI=1S/C30H28FN7O3/c1-30(2,3)26-17-27(38(37-26)19-7-9-23-18(14-19)6-5-12-33-23)36-29(40)35-24-10-8-20(15-22(24)31)41-21-11-13-34-25(16-21)28(39)32-4/h5-17H,1-4H3,(H,32,39)(H2,35,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Yes1 (unknown origin) by [gamma-33P]-ATP radiolabeled enzyme activity assay |
Bioorg Med Chem Lett 23: 4398-403 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.072
BindingDB Entry DOI: 10.7270/Q2BR8W3F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
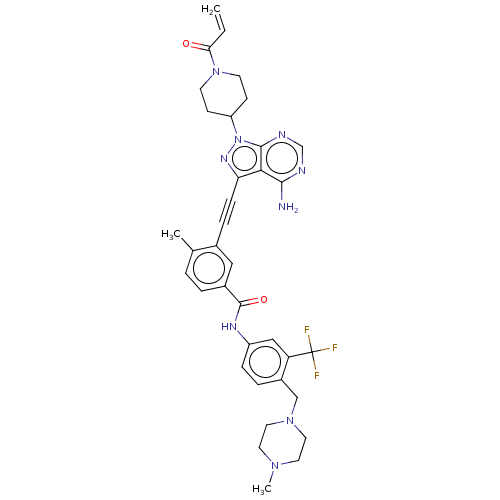
(Homo sapiens (Human)) | BDBM50086442
(CHEMBL3426233 | US10266537, Compound 121)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C4CCN(CC4)C(=O)C=C)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C36H38F3N9O2/c1-4-31(49)47-13-11-28(12-14-47)48-34-32(33(40)41-22-42-34)30(44-48)10-8-24-19-25(6-5-23(24)2)35(50)43-27-9-7-26(29(20-27)36(37,38)39)21-46-17-15-45(3)16-18-46/h4-7,9,19-20,22,28H,1,11-18,21H2,2-3H3,(H,43,50)(H2,40,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
| Assay Description
The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... |
Bioorg Med Chem Lett 17: 3562-9 (2007)
BindingDB Entry DOI: 10.7270/Q21C2065 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50430875

(CHEMBL2336005 | US9062066, LCB 03-0110)Show SMILES Oc1cccc(Nc2ccnc3cc(sc23)-c2cccc(CN3CCOCC3)c2)c1 Show InChI InChI=1S/C24H23N3O2S/c28-20-6-2-5-19(14-20)26-21-7-8-25-22-15-23(30-24(21)22)18-4-1-3-17(13-18)16-27-9-11-29-12-10-27/h1-8,13-15,28H,9-12,16H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 8.0 | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
| Assay Description
The inhibitory activity measurement against the kinases mentioned above was measured in the kinase inhibition reaction mixture containing 2 ul purifi... |
US Patent US9062066 (2015)
BindingDB Entry DOI: 10.7270/Q21G0K17 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.35 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of human YES/YES1 using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-32P]ATP |
Bioorg Med Chem 25: 2156-2166 (2017)
Article DOI: 10.1016/j.bmc.2017.02.030
BindingDB Entry DOI: 10.7270/Q2571FGM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human YES using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM185674

(4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)ca...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3cc(nn3-c3ccc4ncccc4c3)C(C)(C)C)c(F)c2)ccn1 Show InChI InChI=1S/C30H28FN7O3/c1-30(2,3)26-17-27(38(37-26)19-7-9-23-18(14-19)6-5-12-33-23)36-29(40)35-24-10-8-20(15-22(24)31)41-21-11-13-34-25(16-21)28(39)32-4/h5-17H,1-4H3,(H,32,39)(H2,35,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Yes1 (unknown origin) assessed as kinase-dependent enzymatic production of ADP from ATP using coupled luminescence-based reaction by AD... |
Bioorg Med Chem Lett 23: 4398-403 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.072
BindingDB Entry DOI: 10.7270/Q2BR8W3F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
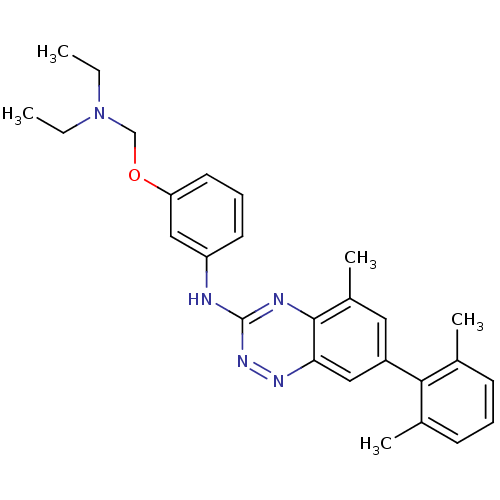
(Homo sapiens (Human)) | BDBM97983
(US8481536, 578)Show SMILES CCN(CC)COc1cccc(Nc2nnc3cc(cc(C)c3n2)-c2c(C)cccc2C)c1 Show InChI InChI=1S/C27H31N5O/c1-6-32(7-2)17-33-23-13-9-12-22(16-23)28-27-29-26-20(5)14-21(15-24(26)30-31-27)25-18(3)10-8-11-19(25)4/h8-16H,6-7,17H2,1-5H3,(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | 25 |
TargeGen, Inc.
US Patent
| Assay Description
Testing of inhibition of kinases in vitro using luciferase-based assay from KinaseGlo, Promega Corp. |
US Patent US8481536 (2013)
BindingDB Entry DOI: 10.7270/Q2GB22PZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human YES using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50392788
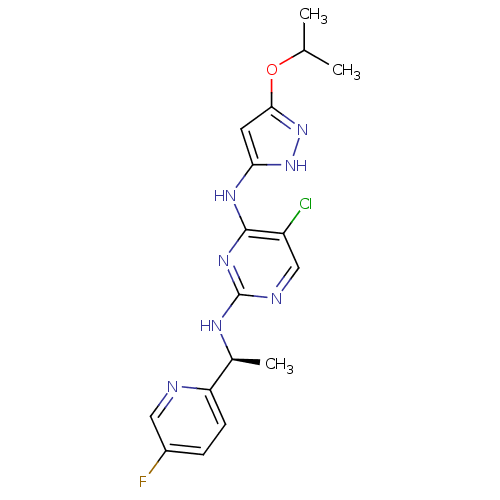
(CHEMBL457614)Show SMILES CC(C)Oc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cn3)ncc2Cl)[nH]n1 |r| Show InChI InChI=1S/C17H19ClFN7O/c1-9(2)27-15-6-14(25-26-15)23-16-12(18)8-21-17(24-16)22-10(3)13-5-4-11(19)7-20-13/h4-10H,1-3H3,(H3,21,22,23,24,25,26)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Yes1 (unknown origin) by [gamma-33P]-ATP radiolabeled enzyme activity assay |
Bioorg Med Chem Lett 23: 4398-403 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.072
BindingDB Entry DOI: 10.7270/Q2BR8W3F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
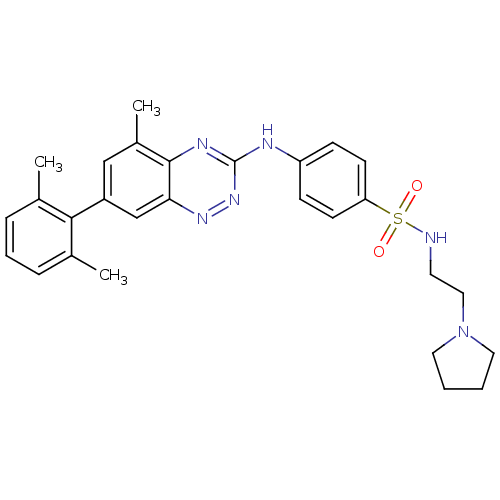
(Homo sapiens (Human)) | BDBM50198817
(4-(7-(2,6-dimethylphenyl)-5-methylbenzo[e][1,2,4]t...)Show SMILES Cc1cccc(C)c1-c1cc(C)c2nc(Nc3ccc(cc3)S(=O)(=O)NCCN3CCCC3)nnc2c1 Show InChI InChI=1S/C28H32N6O2S/c1-19-7-6-8-20(2)26(19)22-17-21(3)27-25(18-22)32-33-28(31-27)30-23-9-11-24(12-10-23)37(35,36)29-13-16-34-14-4-5-15-34/h6-12,17-18,29H,4-5,13-16H2,1-3H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | 25 |
TargeGen, Inc.
US Patent
| Assay Description
Testing of inhibition of kinases in vitro using luciferase-based assay from KinaseGlo, Promega Corp. |
US Patent US8481536 (2013)
BindingDB Entry DOI: 10.7270/Q2GB22PZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50193891
(7-(2,6-dimethylphenyl)-5-methyl-N-(3-(2-(pyrrolidi...)Show SMILES Cc1cccc(C)c1-c1cc(C)c2nc(Nc3cccc(OCCN4CCCC4)c3)nnc2c1 Show InChI InChI=1S/C28H31N5O/c1-19-8-6-9-20(2)26(19)22-16-21(3)27-25(17-22)31-32-28(30-27)29-23-10-7-11-24(18-23)34-15-14-33-12-4-5-13-33/h6-11,16-18H,4-5,12-15H2,1-3H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | 25 |
TargeGen, Inc.
US Patent
| Assay Description
Testing of inhibition of kinases in vitro using luciferase-based assay from KinaseGlo, Promega Corp. |
US Patent US8481536 (2013)
BindingDB Entry DOI: 10.7270/Q2GB22PZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50201641
(CHEMBL3923175)Show SMILES CCS(=O)(=O)c1cc(Nc2ncc(F)c(n2)N(Cc2ccccc2C#N)c2cc(CO)ccc2C)ccc1N1CCN(C)CC1 Show InChI InChI=1S/C33H36FN7O3S/c1-4-45(43,44)31-18-27(11-12-29(31)40-15-13-39(3)14-16-40)37-33-36-20-28(34)32(38-33)41(21-26-8-6-5-7-25(26)19-35)30-17-24(22-42)10-9-23(30)2/h5-12,17-18,20,42H,4,13-16,21-22H2,1-3H3,(H,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6His-tagged full length recombinant human YES expressed in baculovirus expression system |
ACS Med Chem Lett 7: 1118-1123 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00322
BindingDB Entry DOI: 10.7270/Q2PV6NCH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of YES |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of c-Yes |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50221556
(4-chloro-3-(5-methyl-3-(4-(piperidin-4-ylsulfonyl)...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(cc3)S(=O)(=O)C3CCNCC3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C25H24ClN5O3S/c1-15-12-16(21-14-18(32)4-7-22(21)26)13-23-24(15)29-25(31-30-23)28-17-2-5-19(6-3-17)35(33,34)20-8-10-27-11-9-20/h2-7,12-14,20,27,32H,8-11H2,1H3,(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of YES |
J Med Chem 51: 1546-59 (2008)
Article DOI: 10.1021/jm7011276
BindingDB Entry DOI: 10.7270/Q2NV9K3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University
Curated by ChEMBL
| Assay Description
Inhibition of YES (unknown origin) incubated for 1 hr by spectrophotometric analysis |
Bioorg Med Chem 24: 3483-93 (2016)
Article DOI: 10.1016/j.bmc.2016.05.057
BindingDB Entry DOI: 10.7270/Q29G5PQT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50151366
((2-Chloro-6-methyl-phenyl)-[8-((S)-3-methyl-pipera...)Show SMILES C[C@H]1CN(CCN1)c1ccc2nc(Nc3c(C)cccc3Cl)c3cncn3c2n1 Show InChI InChI=1S/C21H22ClN7/c1-13-4-3-5-15(22)19(13)27-20-17-10-23-12-29(17)21-16(25-20)6-7-18(26-21)28-9-8-24-14(2)11-28/h3-7,10,12,14,24H,8-9,11H2,1-2H3,(H,25,27)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Yes kinase |
J Med Chem 47: 4517-29 (2004)
Article DOI: 10.1021/jm030217e
BindingDB Entry DOI: 10.7270/Q2R210VQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50221547
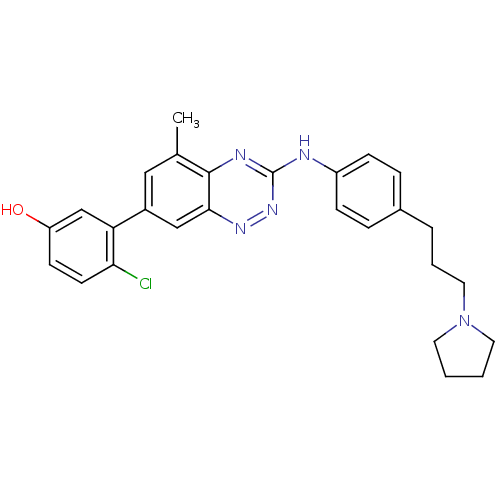
(4-chloro-3-(5-methyl-3-(4-(3-(pyrrolidin-1-yl)prop...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(CCCN4CCCC4)cc3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C27H28ClN5O/c1-18-15-20(23-17-22(34)10-11-24(23)28)16-25-26(18)30-27(32-31-25)29-21-8-6-19(7-9-21)5-4-14-33-12-2-3-13-33/h6-11,15-17,34H,2-5,12-14H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of YES |
J Med Chem 51: 1546-59 (2008)
Article DOI: 10.1021/jm7011276
BindingDB Entry DOI: 10.7270/Q2NV9K3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM473174
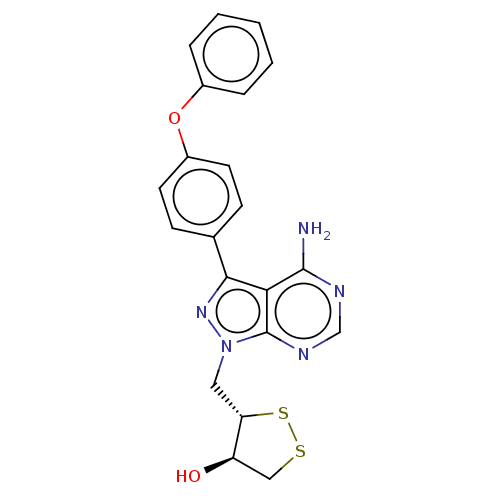
((3S,4R)-3-((4-amino-3-(4-phenoxyphenyl)-1H-pyrazol...)Show SMILES Nc1ncnc2n(C[C@@H]3SSC[C@H]3O)nc(-c3ccc(Oc4ccccc4)cc3)c12 |r| Show InChI InChI=1S/C21H19N5O2S2/c22-20-18-19(13-6-8-15(9-7-13)28-14-4-2-1-3-5-14)25-26(21(18)24-12-23-20)10-17-16(27)11-29-30-17/h1-9,12,16-17,27H,10-11H2,(H2,22,23,24)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SABILA BIOSCIENCES LLC
US Patent
| Assay Description
IC50: Methods for In Vitro Evaluation Assays known in the art for testing compounds are used to test compounds of this invention and to assess the bi... |
US Patent US10844038 (2020)
BindingDB Entry DOI: 10.7270/Q25D8VZ7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM473172
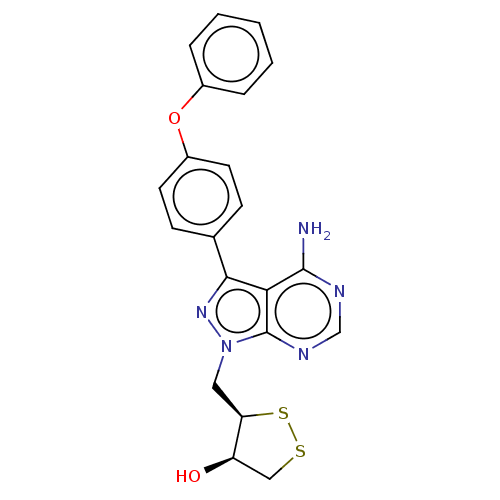
((3R,4R)-3-((4-amino-3-(4-phenoxyphenyl)-1H-pyrazol...)Show SMILES Nc1ncnc2n(C[C@H]3SSC[C@H]3O)nc(-c3ccc(Oc4ccccc4)cc3)c12 |r| Show InChI InChI=1S/C21H19N5O2S2/c22-20-18-19(13-6-8-15(9-7-13)28-14-4-2-1-3-5-14)25-26(21(18)24-12-23-20)10-17-16(27)11-29-30-17/h1-9,12,16-17,27H,10-11H2,(H2,22,23,24)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SABILA BIOSCIENCES LLC
US Patent
| Assay Description
IC50: Methods for In Vitro Evaluation Assays known in the art for testing compounds are used to test compounds of this invention and to assess the bi... |
US Patent US10844038 (2020)
BindingDB Entry DOI: 10.7270/Q25D8VZ7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM473171
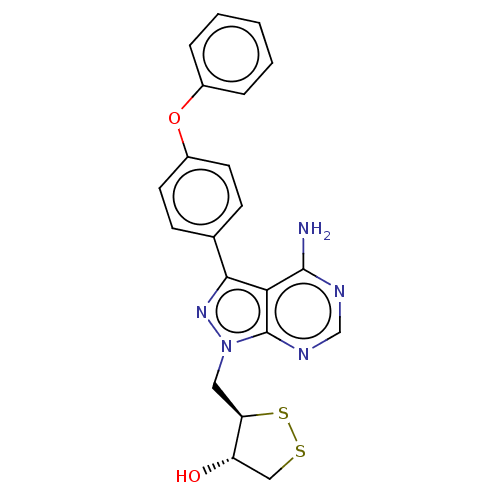
((3R,4S)-3-((4-amino-3-(4-phenoxyphenyl)-1H-pyrazol...)Show SMILES Nc1ncnc2n(C[C@H]3SSC[C@@H]3O)nc(-c3ccc(Oc4ccccc4)cc3)c12 |r| Show InChI InChI=1S/C21H19N5O2S2/c22-20-18-19(13-6-8-15(9-7-13)28-14-4-2-1-3-5-14)25-26(21(18)24-12-23-20)10-17-16(27)11-29-30-17/h1-9,12,16-17,27H,10-11H2,(H2,22,23,24)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SABILA BIOSCIENCES LLC
US Patent
| Assay Description
IC50: Methods for In Vitro Evaluation Assays known in the art for testing compounds are used to test compounds of this invention and to assess the bi... |
US Patent US10844038 (2020)
BindingDB Entry DOI: 10.7270/Q25D8VZ7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM473174

((3S,4R)-3-((4-amino-3-(4-phenoxyphenyl)-1H-pyrazol...)Show SMILES Nc1ncnc2n(C[C@@H]3SSC[C@H]3O)nc(-c3ccc(Oc4ccccc4)cc3)c12 |r| Show InChI InChI=1S/C21H19N5O2S2/c22-20-18-19(13-6-8-15(9-7-13)28-14-4-2-1-3-5-14)25-26(21(18)24-12-23-20)10-17-16(27)11-29-30-17/h1-9,12,16-17,27H,10-11H2,(H2,22,23,24)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SABILA BIOSCIENCES LLC
US Patent
| Assay Description
IC50: Methods for In Vitro Evaluation Assays known in the art for testing compounds are used to test compounds of this invention and to assess the bi... |
US Patent US10844038 (2020)
BindingDB Entry DOI: 10.7270/Q25D8VZ7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM473172

((3R,4R)-3-((4-amino-3-(4-phenoxyphenyl)-1H-pyrazol...)Show SMILES Nc1ncnc2n(C[C@H]3SSC[C@H]3O)nc(-c3ccc(Oc4ccccc4)cc3)c12 |r| Show InChI InChI=1S/C21H19N5O2S2/c22-20-18-19(13-6-8-15(9-7-13)28-14-4-2-1-3-5-14)25-26(21(18)24-12-23-20)10-17-16(27)11-29-30-17/h1-9,12,16-17,27H,10-11H2,(H2,22,23,24)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SABILA BIOSCIENCES LLC
US Patent
| Assay Description
IC50: Methods for In Vitro Evaluation Assays known in the art for testing compounds are used to test compounds of this invention and to assess the bi... |
US Patent US10844038 (2020)
BindingDB Entry DOI: 10.7270/Q25D8VZ7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM473175
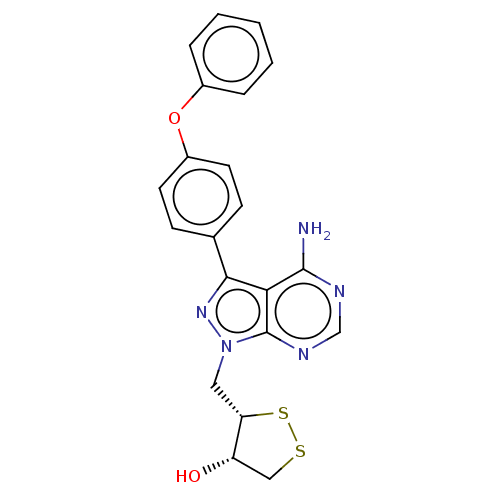
((3S,4S)-3-((4-amino-3-(4-phenoxyphenyl)-1H-pyrazol...)Show SMILES Nc1ncnc2n(C[C@@H]3SSC[C@@H]3O)nc(-c3ccc(Oc4ccccc4)cc3)c12 |r| Show InChI InChI=1S/C21H19N5O2S2/c22-20-18-19(13-6-8-15(9-7-13)28-14-4-2-1-3-5-14)25-26(21(18)24-12-23-20)10-17-16(27)11-29-30-17/h1-9,12,16-17,27H,10-11H2,(H2,22,23,24)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SABILA BIOSCIENCES LLC
US Patent
| Assay Description
IC50: Methods for In Vitro Evaluation Assays known in the art for testing compounds are used to test compounds of this invention and to assess the bi... |
US Patent US10844038 (2020)
BindingDB Entry DOI: 10.7270/Q25D8VZ7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data