

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasminogen (Rattus norvegicus) | BDBM50074920 (((R)-3-Phenyl-1-{(S)-2-phenyl-1-[3,3,3-trifluoro-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Procter& Gamble Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against Plasmin (HP) | Bioorg Med Chem Lett 9: 301-6 (1999) BindingDB Entry DOI: 10.7270/Q2C24WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM50074921 ([(R)-1-({(S)-Naphthalen-2-yl-[3,3,3-trifluoro-1-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Procter& Gamble Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against Plasmin (HP) | Bioorg Med Chem Lett 9: 301-6 (1999) BindingDB Entry DOI: 10.7270/Q2C24WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM50074915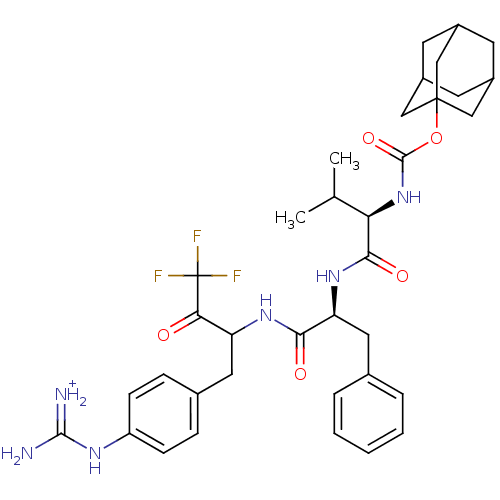 (((R)-2-Methyl-1-{(S)-2-phenyl-1-[3,3,3-trifluoro-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Procter& Gamble Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against Plasmin (HP) | Bioorg Med Chem Lett 9: 301-6 (1999) BindingDB Entry DOI: 10.7270/Q2C24WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM50074918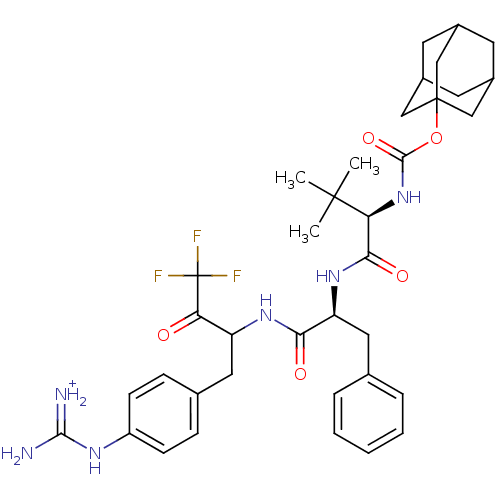 (((R)-2,2-Dimethyl-1-{(S)-2-phenyl-1-[3,3,3-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Procter& Gamble Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against Plasmin (HP) | Bioorg Med Chem Lett 9: 301-6 (1999) BindingDB Entry DOI: 10.7270/Q2C24WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98600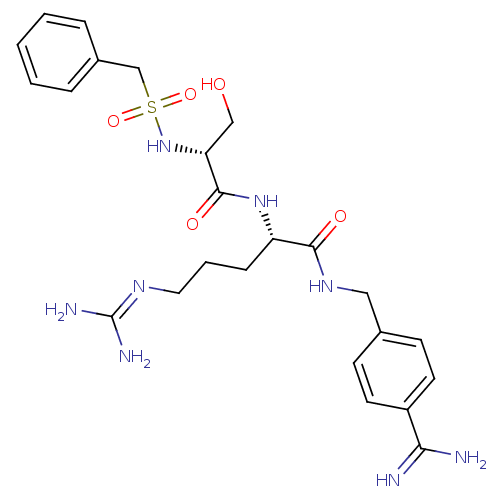 ((2S)-2-[[(2R)-2-(benzylsulfonylamino)-3-hydroxy-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 200 | -9.10 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98587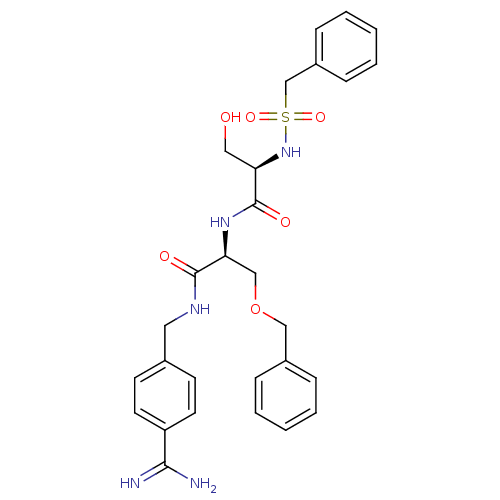 ((2R)-N-[(1S)-1-(benzyloxymethyl)-2-[(4-carbamimido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | US Patent | 270 | -8.92 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98599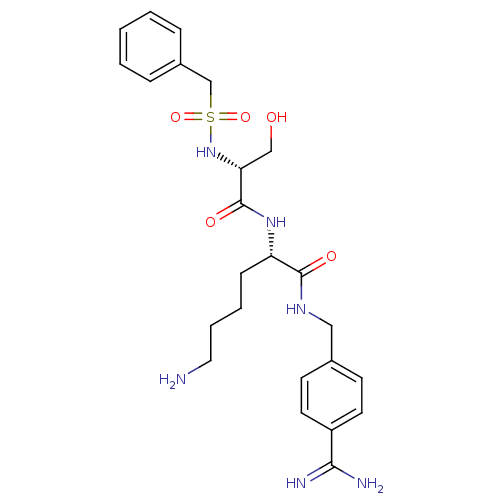 ((2S)-6-amino-2-[[(2R)-2-(benzylsulfonylamino)-3-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 360 | -8.75 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98603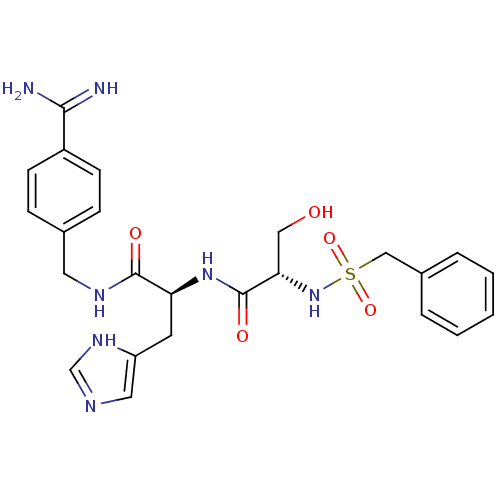 (3-amino-N-[4-[[(2S)-2-[[(2R)-2-(benzylsulfonylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 400 | -8.69 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM50074916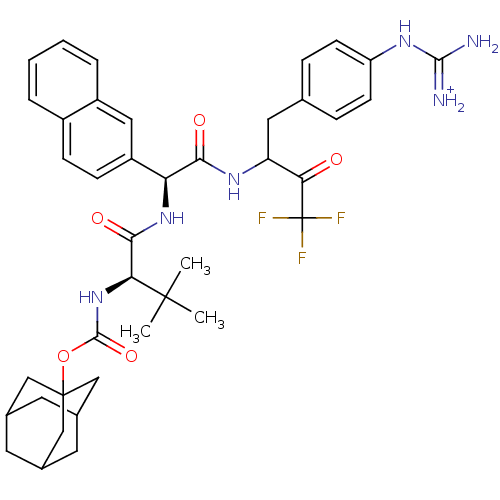 ([(R)-2,2-Dimethyl-1-({(S)-naphthalen-2-yl-[3,3,3-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Procter& Gamble Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against Plasmin (HP) | Bioorg Med Chem Lett 9: 301-6 (1999) BindingDB Entry DOI: 10.7270/Q2C24WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98601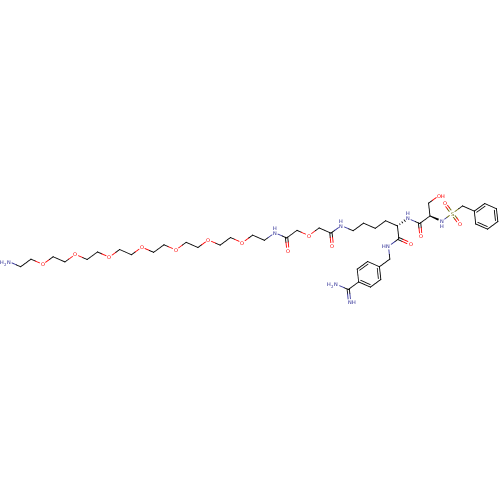 ((2S)-6-[[2-[2-[2-[2-[2-[2-[2-[2-[2-(2-aminoethoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 530 | -8.53 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM50074917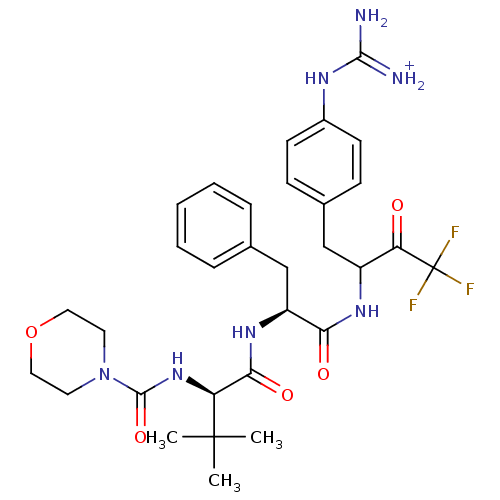 (Morpholine-4-carboxylic acid ((R)-2,2-dimethyl-1-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Procter& Gamble Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against Plasmin (HP) | Bioorg Med Chem Lett 9: 301-6 (1999) BindingDB Entry DOI: 10.7270/Q2C24WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM50076221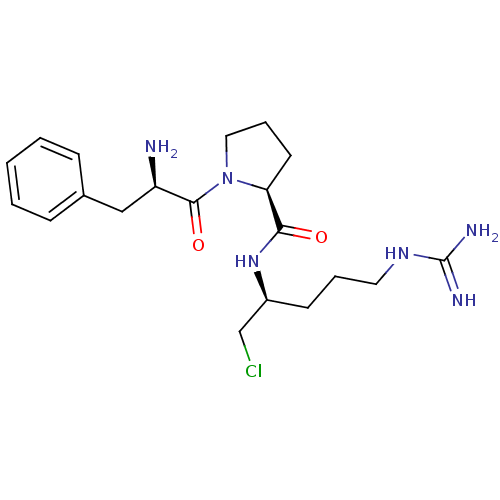 ((S)-1-((R)-2-Amino-3-phenyl-propionyl)-pyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 699 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against plasmin | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM50231520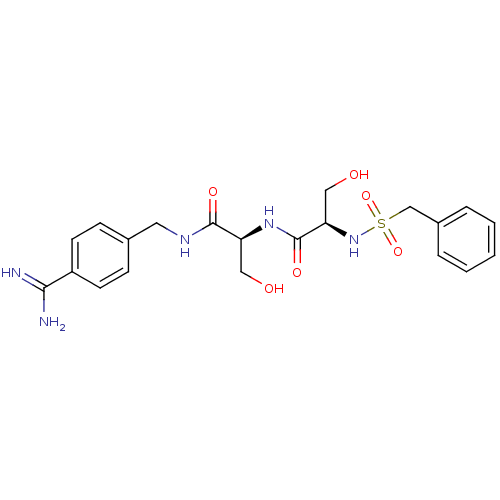 ((R)-N-[(S)-1-(4-carbamimidoyl-benzylcarbamoyl)-2-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | 750 | -8.32 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98588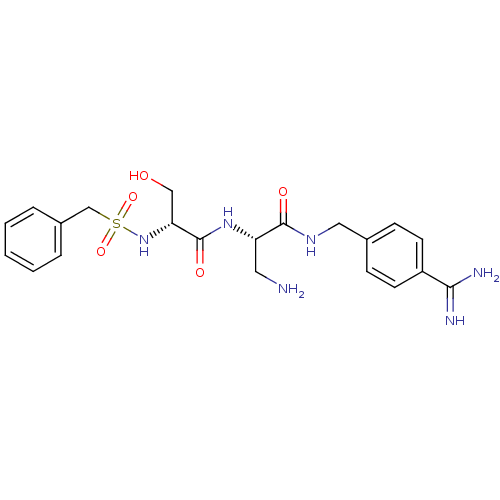 ((2S)-3-amino-2-[[(2R)-2-(benzylsulfonylamino)-3-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 810 | -8.28 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98593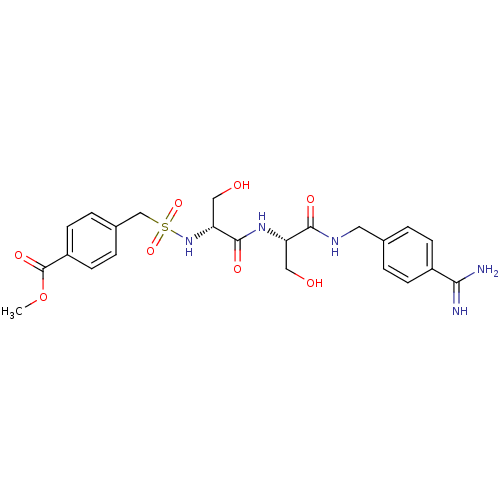 (US8476306, 6.19 | methyl 4-[[(1R)-2-[[(1S)-2-[(4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 960 | -8.18 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM50076220 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 978 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against plasmin | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98590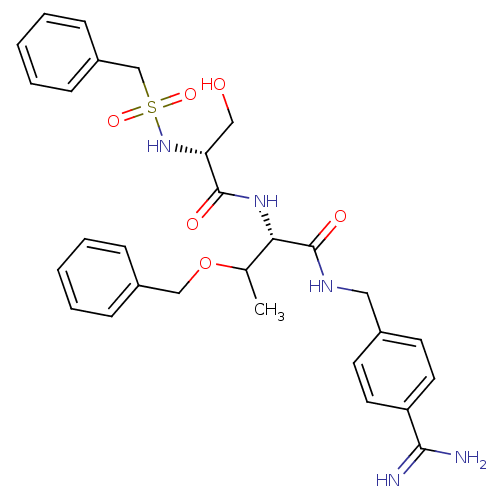 ((2S)-3-benzyloxy-2-[[(2R)-2-(benzylsulfonylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10E+3 | -8.10 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98589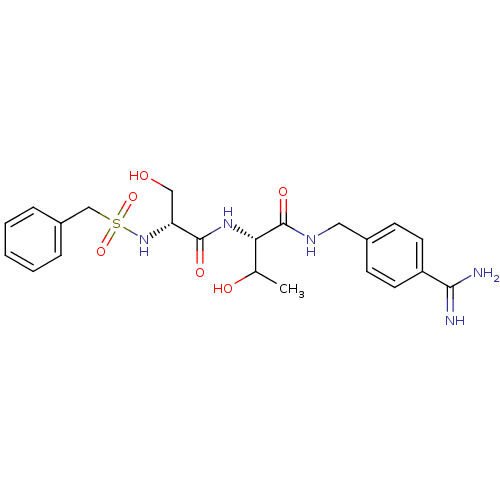 ((2S)-2-[[(2R)-2-(benzylsulfonylamino)-3-hydroxy-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40E+3 | -7.95 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98583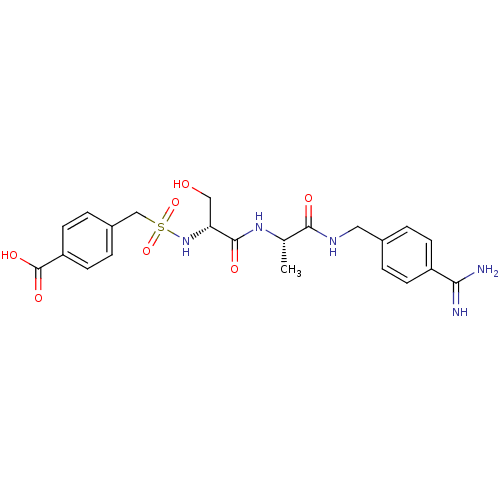 (4-[[(1R)-2-[[(1S)-2-[(4-carbamimidoylphenyl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.00E+3 | -7.50 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98591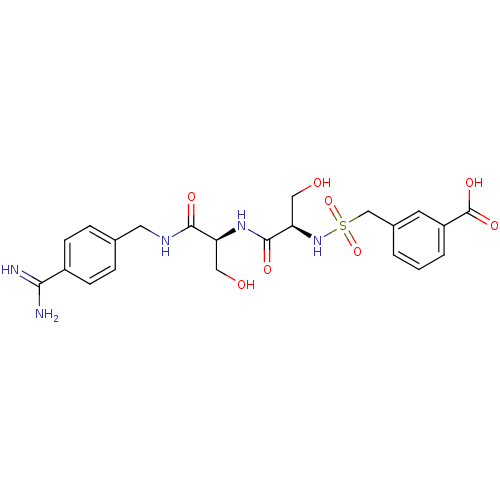 (3-[[(1R)-2-[[(1S)-2-[(4-carbamimidoylphenyl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.20E+3 | -7.31 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98584 (3-[[(1R)-2-[[(1S)-2-[(4-carbamimidoylphenyl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.70E+3 | -7.24 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM50147046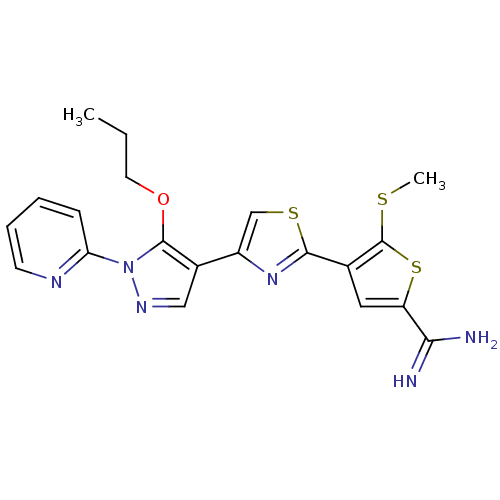 (5-Methylsulfanyl-4-[4-(5-propoxy-1-pyridin-2-yl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc Curated by ChEMBL | Assay Description In vitro binding affinity towards plasmin was determined | Bioorg Med Chem Lett 14: 3043-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.034 BindingDB Entry DOI: 10.7270/Q2K35T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98592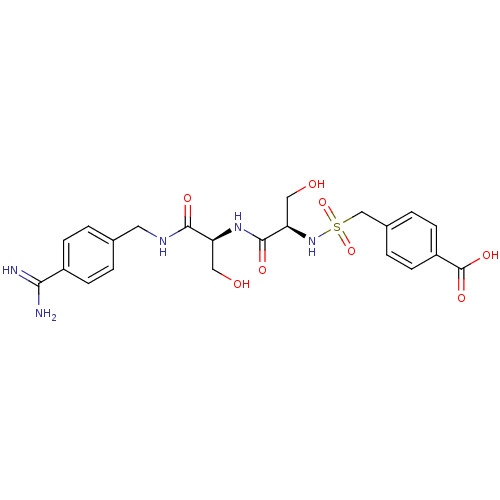 (4-[[(1R)-2-[[(1S)-2-[(4-carbamimidoylphenyl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.20E+3 | -7.08 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98586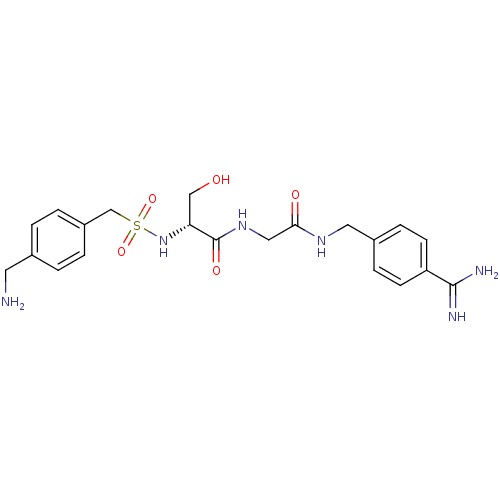 ((2R)-2-[[4-(aminomethyl)phenyl]methylsulfonylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.40E+3 | -6.97 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98602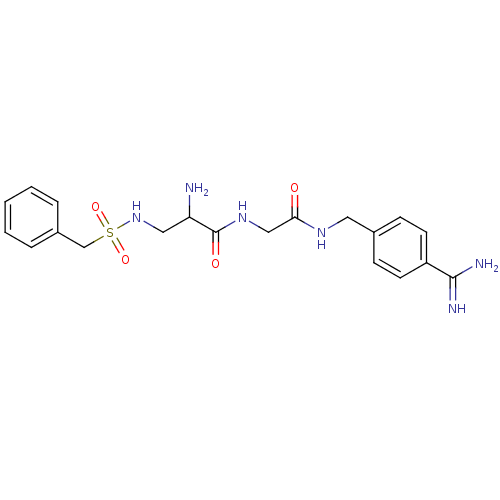 (3-amino-N-[4-[[2-[[(2R)-3-amino-2-(benzylsulfonyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.60E+3 | -6.82 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM50110015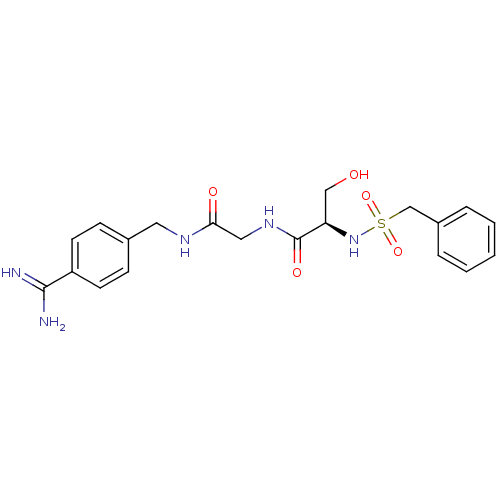 (CHEMBL158936 | N-(BENZYLSULFONYL)SERYL-N~1~-{4-[AM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | 1.10E+4 | -6.74 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98579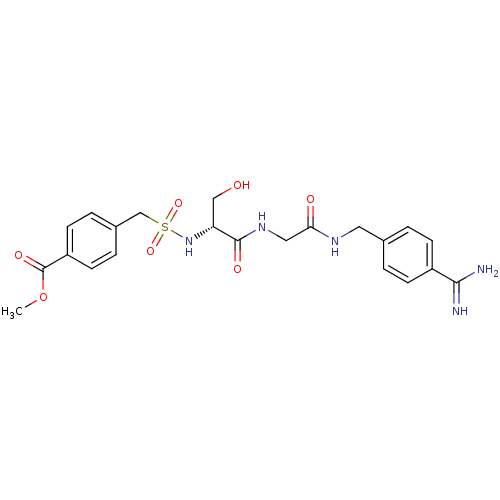 (US8476306, 6.4 | methyl 4-[[(1R)-2-[[2-[(4-carbami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.70E+4 | -6.48 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98595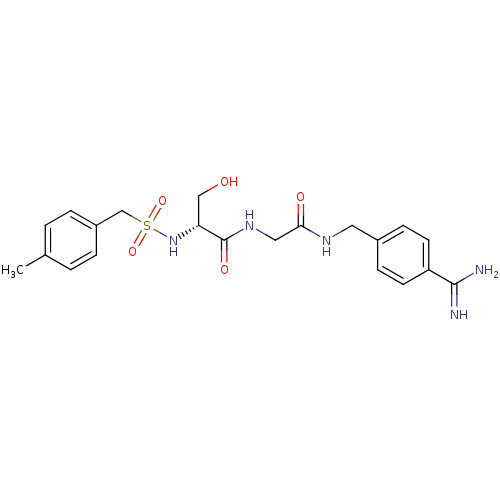 ((2R)-N-[2-[(4-carbamimidoylphenyl)methylamino]-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.80E+4 | -6.45 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98596 ((2R)-N-[2-[(4-carbamimidoylphenyl)methylamino]-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.00E+4 | -6.38 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM50069190 (CHEMBL287614 | N-(1-Carbamimidoyl-piperidin-4-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against serine protease plasmin | Bioorg Med Chem Lett 7: 1497-1500 (1997) Article DOI: 10.1016/S0960-894X(97)00257-6 BindingDB Entry DOI: 10.7270/Q2P55P15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98604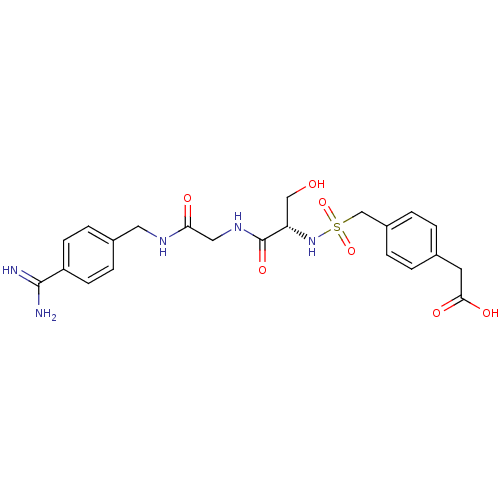 (2-[4-[[(1R)-2-[[2-[4-[(3-amino-4-methoxy-benzoyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 2.70E+4 | -6.21 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98585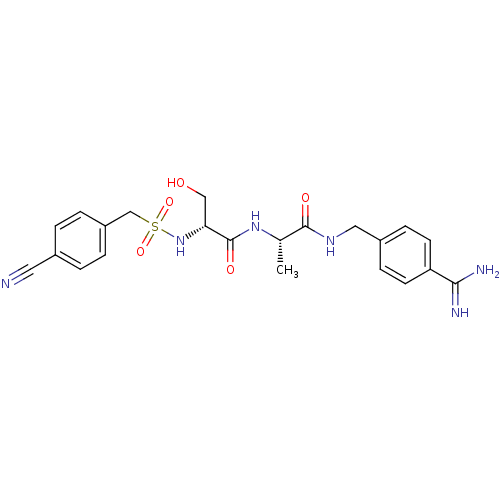 ((2R)-N-[(1S)-2-[(4-carbamimidoylphenyl)methylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.70E+4 | -6.21 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM50110015 (CHEMBL158936 | N-(BENZYLSULFONYL)SERYL-N~1~-{4-[AM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | 2.80E+4 | -6.19 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98594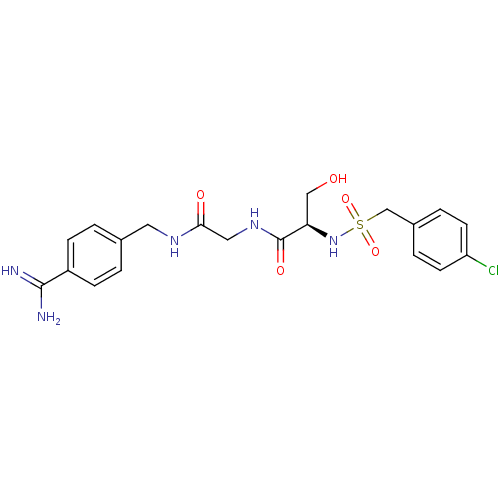 ((2R)-N-[2-[(4-carbamimidoylphenyl)methylamino]-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.20E+4 | -6.11 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98597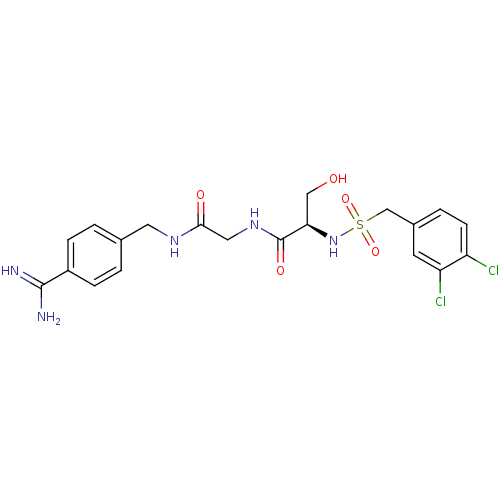 ((2R)-N-[2-[(4-carbamimidoylphenyl)methylamino]-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.20E+4 | -6.11 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98580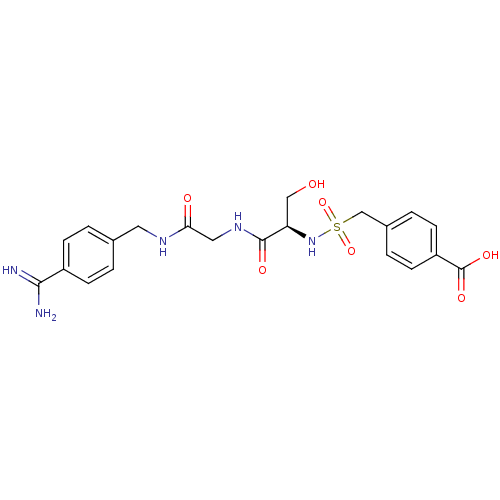 (4-[[(1R)-2-[[2-[(4-carbamimidoylphenyl)methylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.50E+4 | -6.05 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98598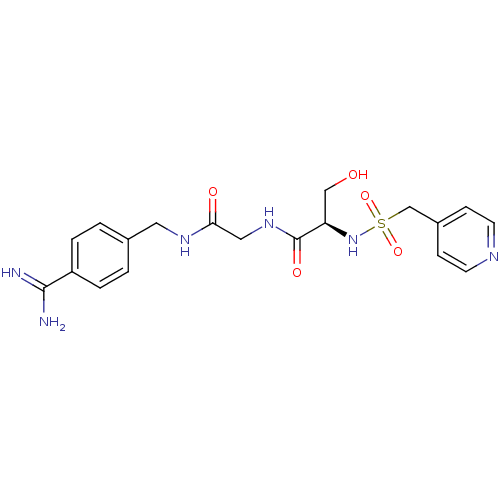 ((2R)-N-[2-[(4-carbamimidoylphenyl)methylamino]-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.70E+4 | -6.02 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98581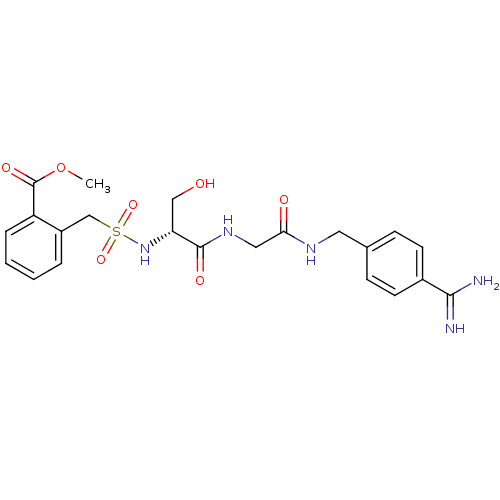 (US8476306, 6.6 | methyl 2-[[(1R)-2-[[2-[(4-carbami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.80E+4 | -6.01 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98578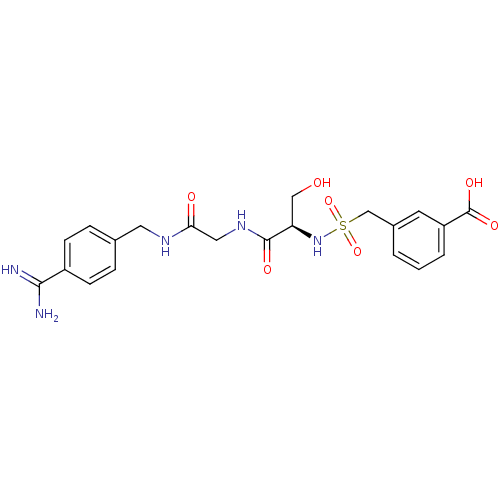 (3-[[(1R)-2-[[2-[(4-carbamimidoylphenyl)methylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.90E+4 | -5.75 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM50074919 (Morpholine-4-carboxylic acid [(R)-2,2-dimethyl-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Procter& Gamble Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against Plasmin (HP) | Bioorg Med Chem Lett 9: 301-6 (1999) BindingDB Entry DOI: 10.7270/Q2C24WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM50368379 (CHEMBL1202120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against plasmin | J Med Chem 35: 3364-9 (1992) BindingDB Entry DOI: 10.7270/Q2RX9CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Rattus norvegicus) | BDBM98582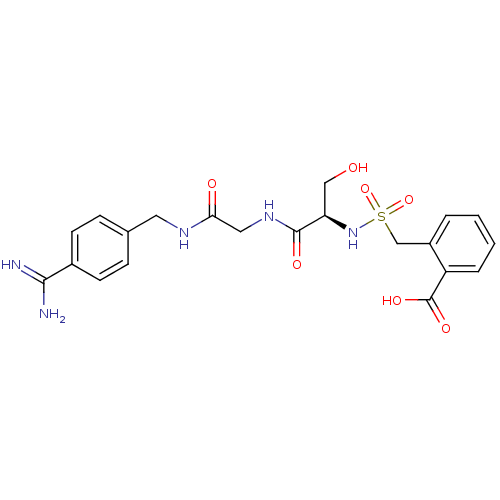 (2-[[(1R)-2-[[2-[(4-carbamimidoylphenyl)methylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.20E+5 | -4.97 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company US Patent | Assay Description Inhibition constant of the compound against Plasmin | US Patent US8476306 (2013) BindingDB Entry DOI: 10.7270/Q2154FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||