

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM581978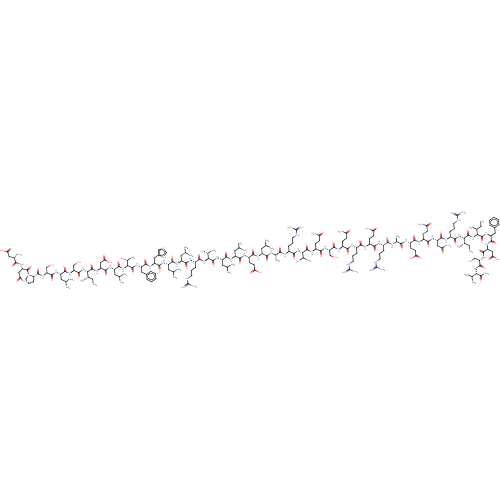 (DNPSLSIDLTFHLLRTLLELARTQSQRERAEQNRIIFDSV-NH2 (hUcn...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2023285347 | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Receptor SPA binding assays were performed in white 96-well plates in a total volume of 200 mI per well. Freeze dried analogues were dissolved in 80%... | Citation and Details BindingDB Entry DOI: 10.7270/Q23B63Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50026981 (CHEMBL2370939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Inhibition of recombinant corticotropin releasing factor receptor 1 assayed using nonselective [125I]-labeled agonist [Tyr0,Glu1,Nle17]-sauvagine | J Med Chem 45: 4737-47 (2002) BindingDB Entry DOI: 10.7270/Q2WQ04JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50370170 (CHEMBL1794009) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Inhibition of recombinant corticotropin releasing factor receptor 1 assayed using nonselective [125I]-labeled agonist [Tyr0,Glu1,Nle17]-sauvagine | J Med Chem 45: 4737-47 (2002) BindingDB Entry DOI: 10.7270/Q2WQ04JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50158983 (CHEMBL439883 | E G P P I S I D L S L E L L R K M I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Inhibition of recombinant corticotropin releasing factor receptor 1 assayed using nonselective [125I]-labeled agonist [Tyr0,Glu1,Nle17]-sauvagine | J Med Chem 45: 4737-47 (2002) BindingDB Entry DOI: 10.7270/Q2WQ04JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50119607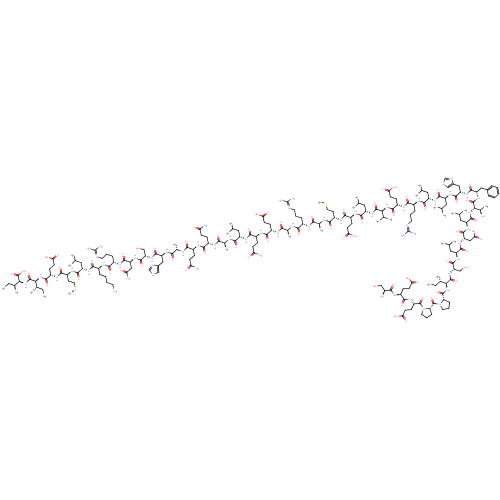 (CHEMBL440057 | E G P P I S I D L S L E L L R K M I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Inhibition of recombinant corticotropin releasing factor receptor 1 assayed using nonselective [125I]-labeled agonist [Tyr0,Glu1,Nle17]-sauvagine | J Med Chem 45: 4737-47 (2002) BindingDB Entry DOI: 10.7270/Q2WQ04JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50272638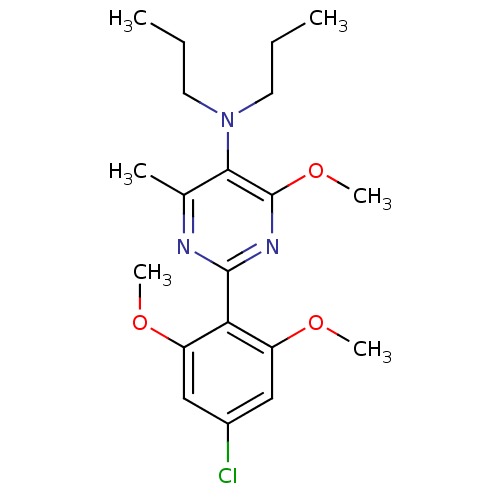 (2-(4-chloro-2,6-dimethoxyphenyl)-4-methoxy-6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at CRF1 receptor expressed in mouse AtT20 cells assessed as inhibition of sauvagine-stimulated cAMP accumulation | Bioorg Med Chem Lett 18: 4486-90 (2008) Article DOI: 10.1016/j.bmcl.2008.07.063 BindingDB Entry DOI: 10.7270/Q2NG4RJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50501032 (CHEMBL3799870) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at CRF1 receptor in human Y79 cells assessed as inhibition of CRF-stimulated cAMP production preincubated for 30 mins followed by... | Bioorg Med Chem Lett 26: 2184-7 (2016) Article DOI: 10.1016/j.bmcl.2016.03.067 BindingDB Entry DOI: 10.7270/Q2KK9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50300147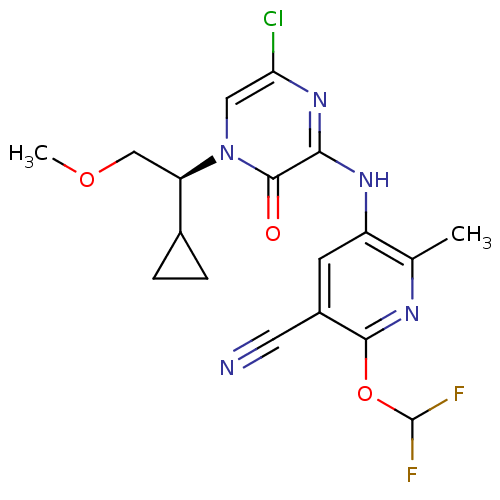 ((S)-4-(1-Cyclopropyl-2-methoxyethyl)-6-[6-(difluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at CRF1 receptor in human Y79 cells assessed as inhibition of CRF-stimulated cAMP production after 30 mins by HTRF analysis | J Med Chem 52: 7653-68 (2009) Article DOI: 10.1021/jm900716v BindingDB Entry DOI: 10.7270/Q2CN740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50026956 (CHEMBL2370938) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Inhibition of recombinant corticotropin releasing factor receptor 1 assayed using nonselective [125I]-labeled agonist [Tyr0,Glu1,Nle17]-sauvagine | J Med Chem 45: 4737-47 (2002) BindingDB Entry DOI: 10.7270/Q2WQ04JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50293912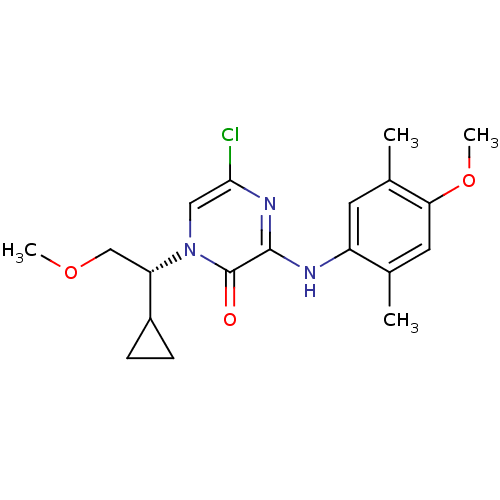 ((R)-5-chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at CRF1 receptor in human Y79 cells assessed as inhibition of CRF-stimulated cAMP production by HTRF method | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50362277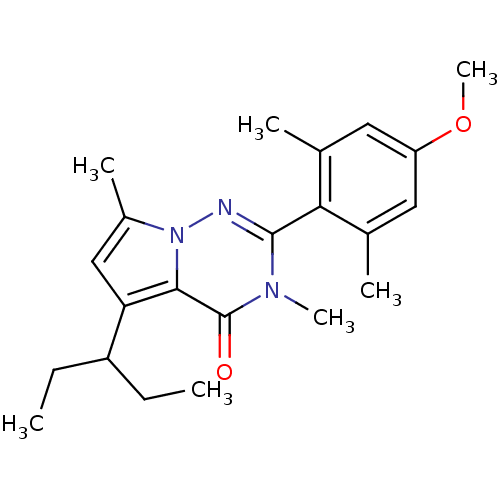 (CHEMBL1939601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counter | Bioorg Med Chem 20: 1122-38 (2012) Article DOI: 10.1016/j.bmc.2011.11.015 BindingDB Entry DOI: 10.7270/Q2B27VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50354028 (CHEMBL1830524) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5955-66 (2011) Article DOI: 10.1016/j.bmc.2011.08.055 BindingDB Entry DOI: 10.7270/Q24X5862 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50362259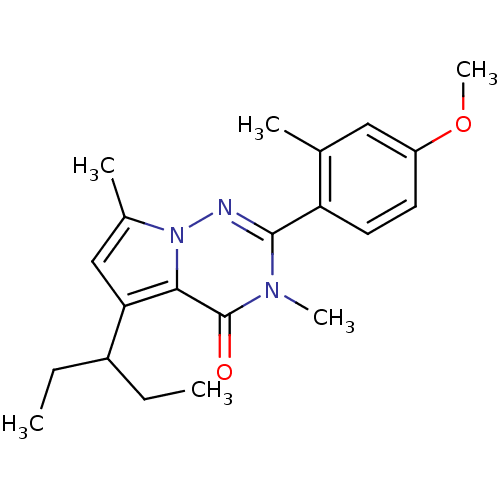 (CHEMBL1939570) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counter | Bioorg Med Chem 20: 1122-38 (2012) Article DOI: 10.1016/j.bmc.2011.11.015 BindingDB Entry DOI: 10.7270/Q2B27VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50132927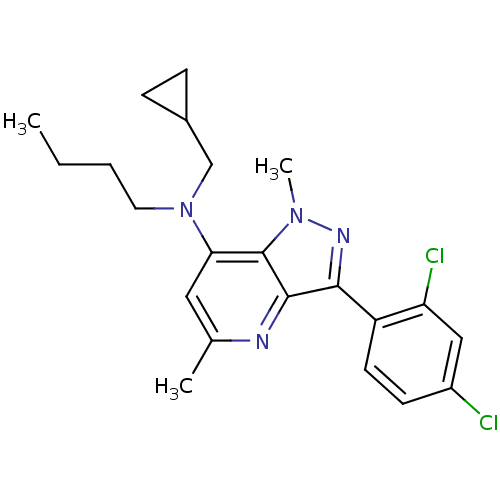 (Butyl-cyclopropylmethyl-[3-(2,4-dichloro-phenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Corticotropin releasing factor receptor 1-stimulated cAMP production | Bioorg Med Chem Lett 13: 3371-4 (2003) BindingDB Entry DOI: 10.7270/Q21C1W8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50351393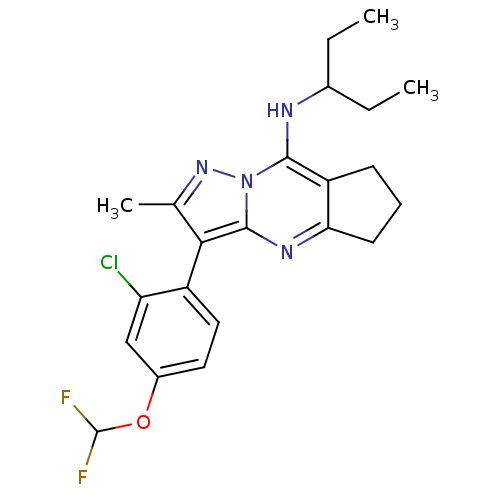 (CHEMBL1819083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50354027 (CHEMBL1830523) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5955-66 (2011) Article DOI: 10.1016/j.bmc.2011.08.055 BindingDB Entry DOI: 10.7270/Q24X5862 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50362291 (CHEMBL1939590) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counter | Bioorg Med Chem 20: 1122-38 (2012) Article DOI: 10.1016/j.bmc.2011.11.015 BindingDB Entry DOI: 10.7270/Q2B27VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50026954 (CHEMBL2370929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Inhibition of recombinant corticotropin releasing factor receptor 1 assayed using nonselective [125I]-labeled agonist [Tyr0,Glu1,Nle17]-sauvagine | J Med Chem 45: 4737-47 (2002) BindingDB Entry DOI: 10.7270/Q2WQ04JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50362260 (CHEMBL1939571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counter | Bioorg Med Chem 20: 1122-38 (2012) Article DOI: 10.1016/j.bmc.2011.11.015 BindingDB Entry DOI: 10.7270/Q2B27VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50362269 (CHEMBL1939593) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counter | Bioorg Med Chem 20: 1122-38 (2012) Article DOI: 10.1016/j.bmc.2011.11.015 BindingDB Entry DOI: 10.7270/Q2B27VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50423382 (CHEMBL251386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human recombinant CRF1 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 5218-21 (2007) Article DOI: 10.1016/j.bmcl.2007.06.077 BindingDB Entry DOI: 10.7270/Q2P55PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50351387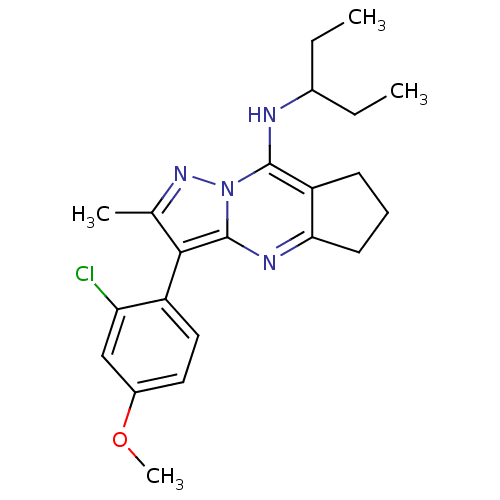 (CHEMBL1819077) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50116105 (3-(6-(dimethylamino)-4-methylpyridin-3-yl)-2,5-dim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at CRF1 receptor in human IMR32 cells assessed as inhibition of CRF-induced intracellular cAMP accumulation after 30 mins by immu... | J Med Chem 55: 8450-63 (2012) Article DOI: 10.1021/jm300864p BindingDB Entry DOI: 10.7270/Q2KD202P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50234480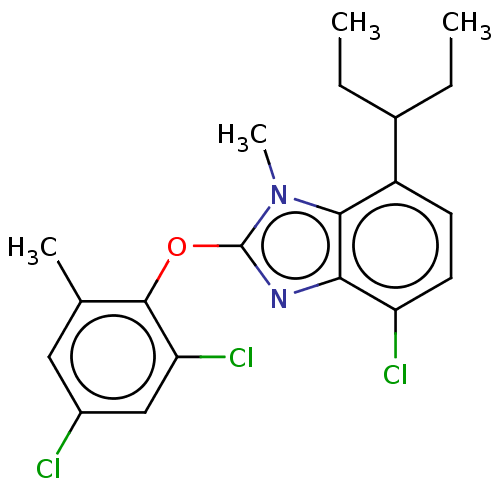 (CHEMBL4069551) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes preincubated for 1 hr followed by [125I]-CRF addition meas... | Bioorg Med Chem 25: 1556-1570 (2017) Article DOI: 10.1016/j.bmc.2016.11.011 BindingDB Entry DOI: 10.7270/Q23R0W4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50354036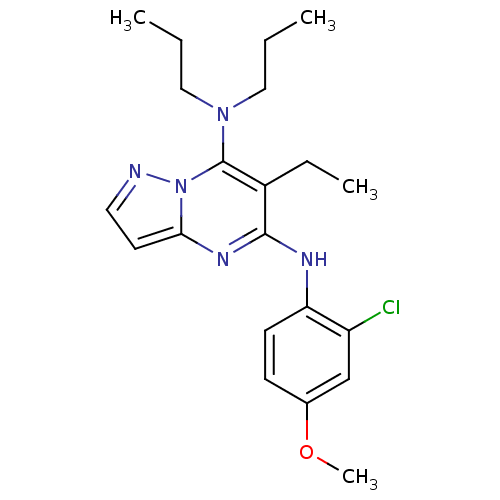 (CHEMBL1828890) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5955-66 (2011) Article DOI: 10.1016/j.bmc.2011.08.055 BindingDB Entry DOI: 10.7270/Q24X5862 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50362279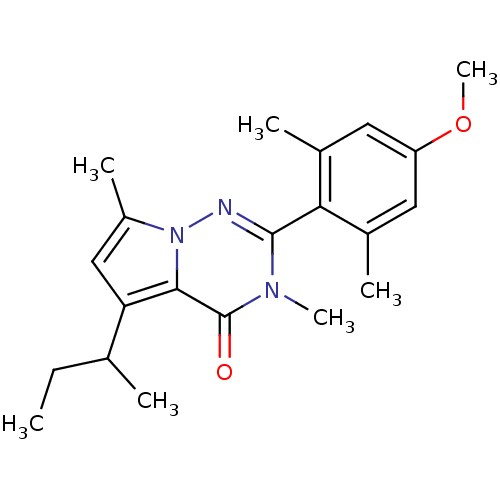 (CHEMBL1939603) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counter | Bioorg Med Chem 20: 1122-38 (2012) Article DOI: 10.1016/j.bmc.2011.11.015 BindingDB Entry DOI: 10.7270/Q2B27VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50362286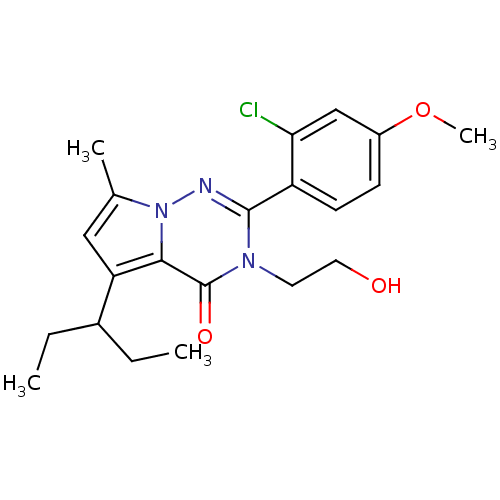 (CHEMBL1939585) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counter | Bioorg Med Chem 20: 1122-38 (2012) Article DOI: 10.1016/j.bmc.2011.11.015 BindingDB Entry DOI: 10.7270/Q2B27VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50460165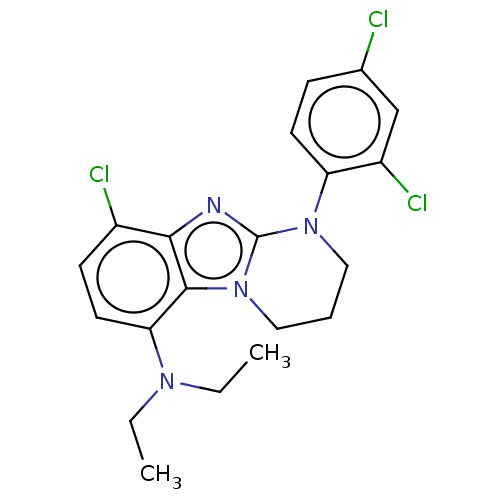 (CHEMBL4225254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human CRF1 receptor expressed in CHO cells assessed as inhibition of human CRF-stimulated cAMP accumulation after 4 hrs by luc... | Bioorg Med Chem 26: 2229-2250 (2018) Article DOI: 10.1016/j.bmc.2018.01.020 BindingDB Entry DOI: 10.7270/Q2474DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50354015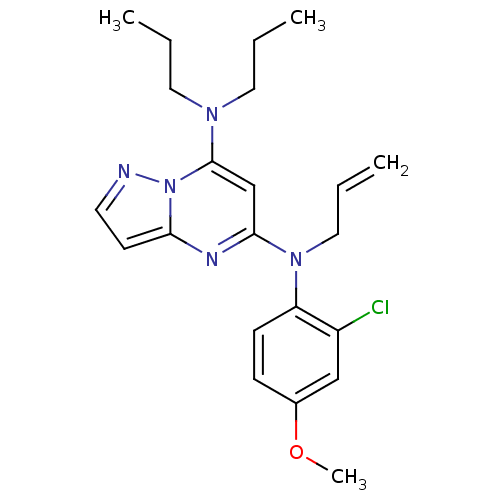 (CHEMBL1828900) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5955-66 (2011) Article DOI: 10.1016/j.bmc.2011.08.055 BindingDB Entry DOI: 10.7270/Q24X5862 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50362270 (CHEMBL1939594) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counter | Bioorg Med Chem 20: 1122-38 (2012) Article DOI: 10.1016/j.bmc.2011.11.015 BindingDB Entry DOI: 10.7270/Q2B27VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50362284 (CHEMBL1939583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counter | Bioorg Med Chem 20: 1122-38 (2012) Article DOI: 10.1016/j.bmc.2011.11.015 BindingDB Entry DOI: 10.7270/Q2B27VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50354029 (CHEMBL1830525) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5955-66 (2011) Article DOI: 10.1016/j.bmc.2011.08.055 BindingDB Entry DOI: 10.7270/Q24X5862 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50362273 (CHEMBL1939597) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counter | Bioorg Med Chem 20: 1122-38 (2012) Article DOI: 10.1016/j.bmc.2011.11.015 BindingDB Entry DOI: 10.7270/Q2B27VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50293910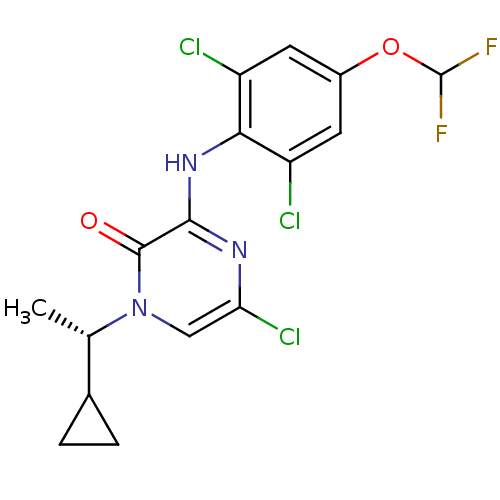 ((S)-5-chloro-1-(1-cyclopropylethyl)-3-(2,6-dichlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at CRF1 receptor in human Y-79 cells assessed as inhibition of CRF-induced cAMP production after 30 mins by HTRF analysis | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50362271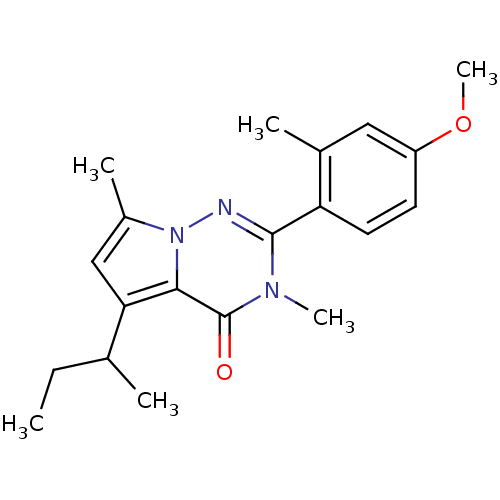 (CHEMBL1939595) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counter | Bioorg Med Chem 20: 1122-38 (2012) Article DOI: 10.1016/j.bmc.2011.11.015 BindingDB Entry DOI: 10.7270/Q2B27VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50132898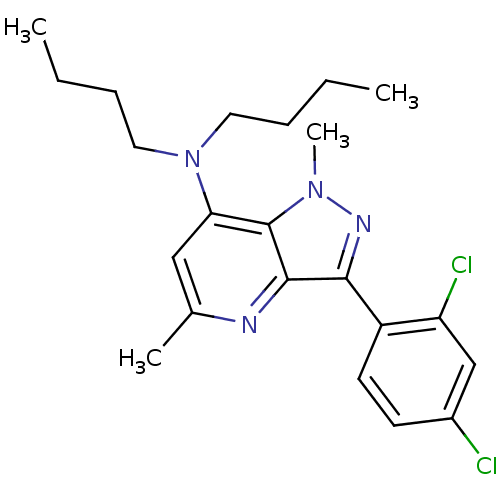 (CHEMBL115142 | Dibutyl-[3-(2,4-dichloro-phenyl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Corticotropin releasing factor receptor 1-stimulated cAMP production | Bioorg Med Chem Lett 13: 3371-4 (2003) BindingDB Entry DOI: 10.7270/Q21C1W8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50132901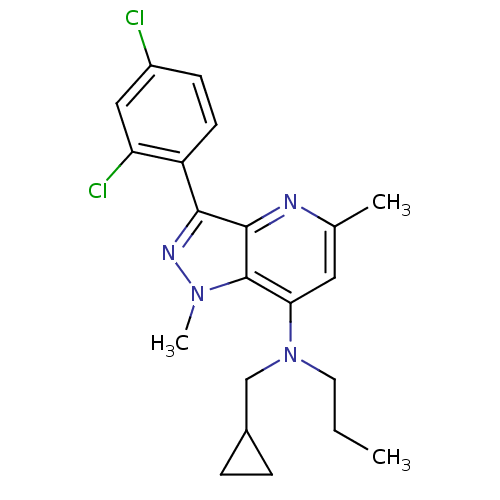 (CHEMBL445075 | Cyclopropylmethyl-[3-(2,4-dichloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Compound was tested to inhibit Corticotropin releasing factor receptor 1-stimulated c-AMP production | Bioorg Med Chem Lett 13: 3367-70 (2003) BindingDB Entry DOI: 10.7270/Q2542N05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50362280 (CHEMBL1939592) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counter | Bioorg Med Chem 20: 1122-38 (2012) Article DOI: 10.1016/j.bmc.2011.11.015 BindingDB Entry DOI: 10.7270/Q2B27VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50362288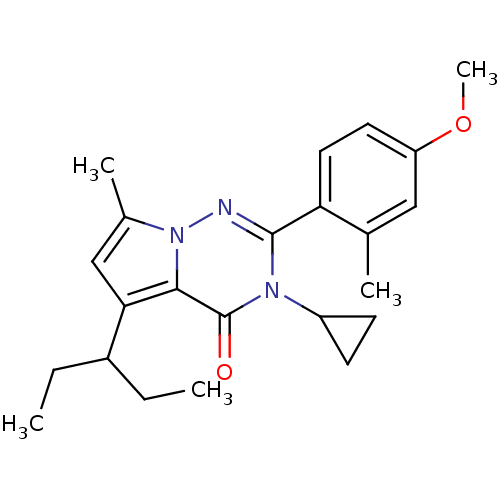 (CHEMBL1939587) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counter | Bioorg Med Chem 20: 1122-38 (2012) Article DOI: 10.1016/j.bmc.2011.11.015 BindingDB Entry DOI: 10.7270/Q2B27VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50026976 (CHEMBL2370933) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Inhibition of recombinant corticotropin releasing factor receptor 1 assayed using nonselective [125I]-labeled agonist [Tyr0,Glu1,Nle17]-sauvagine | J Med Chem 45: 4737-47 (2002) BindingDB Entry DOI: 10.7270/Q2WQ04JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50362289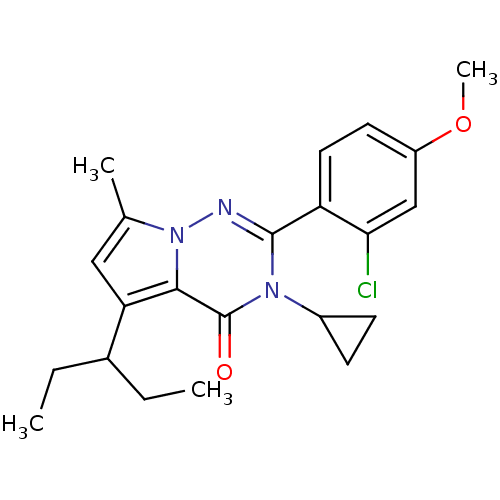 (CHEMBL1939588) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counter | Bioorg Med Chem 20: 1122-38 (2012) Article DOI: 10.1016/j.bmc.2011.11.015 BindingDB Entry DOI: 10.7270/Q2B27VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50351394 (CHEMBL1819084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM29490 (Pyrazolo[1,5-a]-1,3,5-triazine, 13-15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at human CRF1 receptor | Drug Metab Dispos 40: 1093-103 (2012) Article DOI: 10.1124/dmd.111.043596 BindingDB Entry DOI: 10.7270/Q2C24Z52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50354038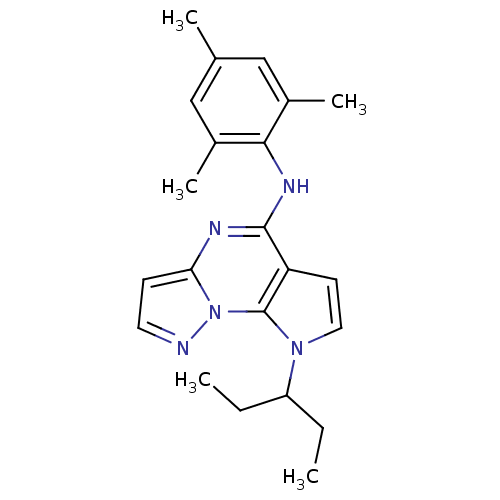 (CHEMBL1830527) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5955-66 (2011) Article DOI: 10.1016/j.bmc.2011.08.055 BindingDB Entry DOI: 10.7270/Q24X5862 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50272704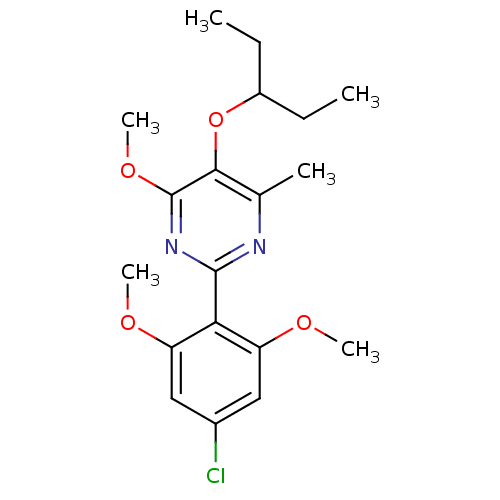 (2-(4-chloro-2,6-dimethoxyphenyl)-4-methoxy-6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at CRF1 receptor expressed in mouse AtT20 cells assessed as inhibition of sauvagine-stimulated cAMP accumulation | Bioorg Med Chem Lett 18: 4486-90 (2008) Article DOI: 10.1016/j.bmcl.2008.07.063 BindingDB Entry DOI: 10.7270/Q2NG4RJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50354031 (CHEMBL1830528) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5955-66 (2011) Article DOI: 10.1016/j.bmc.2011.08.055 BindingDB Entry DOI: 10.7270/Q24X5862 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50351396 (CHEMBL1819086) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5432-45 (2011) Article DOI: 10.1016/j.bmc.2011.07.055 BindingDB Entry DOI: 10.7270/Q2Z038JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50354030 (CHEMBL1830526) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-CRF from human CRF1 receptor expressed in CHO-K1 cells after 2 hrs by gamma counting | Bioorg Med Chem 19: 5955-66 (2011) Article DOI: 10.1016/j.bmc.2011.08.055 BindingDB Entry DOI: 10.7270/Q24X5862 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50398565 (CHEMBL2179195) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at CRF1 receptor in human IMR32 cells assessed as inhibition of CRF-induced intracellular cAMP accumulation after 30 mins by immu... | J Med Chem 55: 8450-63 (2012) Article DOI: 10.1021/jm300864p BindingDB Entry DOI: 10.7270/Q2KD202P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50460165 (CHEMBL4225254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Displacement of ovine [125-I]-CRF from human CRF1 receptor expressed in CHO cell membranes after 1.5 hrs by liquid scintillation counting method | Bioorg Med Chem 26: 2229-2250 (2018) Article DOI: 10.1016/j.bmc.2018.01.020 BindingDB Entry DOI: 10.7270/Q2474DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 762 total ) | Next | Last >> |