 Found 2369 hits of ic50 data for polymerid = 5623
Found 2369 hits of ic50 data for polymerid = 5623 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM350321
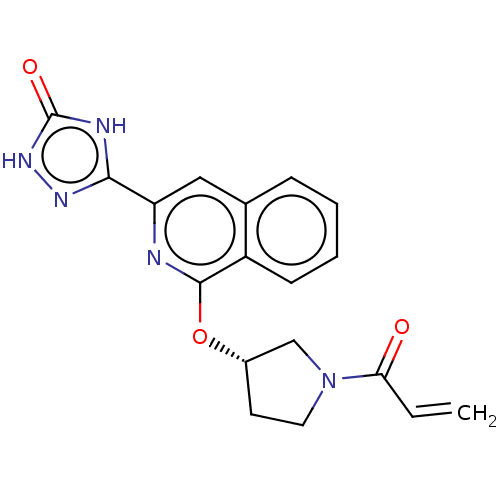
((S)-3-(1-((1-acryloylpyrrolidin-3-yl)oxy)isoquinol...)Show SMILES C=CC(=O)N1CC[C@@H](C1)Oc1nc(cc2ccccc12)-c1n[nH]c(=O)[nH]1 |r| Show InChI InChI=1S/C18H15N5O3/c1-2-15(24)23-8-7-12(10-23)26-17-13-6-4-3-5-11(13)9-14(19-17)16-20-18(25)22-21-16/h2-6,9,12H,1,7-8,10H2/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ITK |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM384541
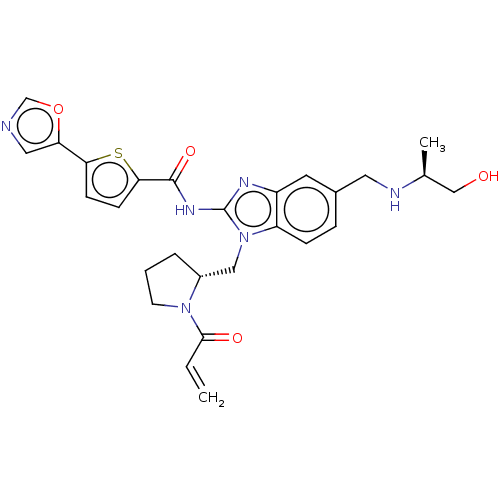
(N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...)Show SMILES C[C@@H](CO)NCc1ccc2n(C[C@H]3CCCN3C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r| Show InChI InChI=1S/C27H30N6O4S/c1-3-25(35)32-10-4-5-19(32)14-33-21-7-6-18(12-29-17(2)15-34)11-20(21)30-27(33)31-26(36)24-9-8-23(38-24)22-13-28-16-37-22/h3,6-9,11,13,16-17,19,29,34H,1,4-5,10,12,14-15H2,2H3,(H,30,31,36)/t17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... |
Bioorg Med Chem Lett 18: 2567-73 (2008)
BindingDB Entry DOI: 10.7270/Q21Z46RF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM384539
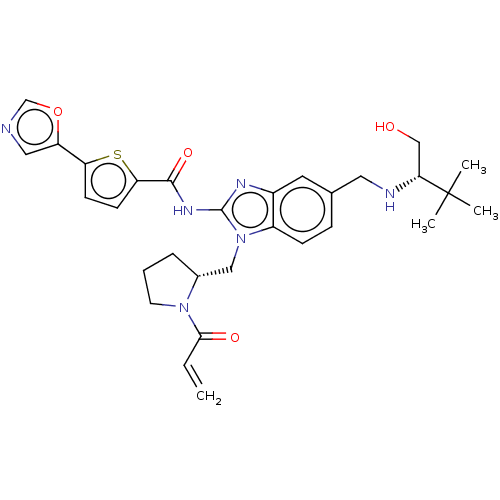
(N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...)Show SMILES CC(C)(C)[C@@H](CO)NCc1ccc2n(C[C@H]3CCCN3C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r| Show InChI InChI=1S/C30H36N6O4S/c1-5-27(38)35-12-6-7-20(35)16-36-22-9-8-19(14-32-26(17-37)30(2,3)4)13-21(22)33-29(36)34-28(39)25-11-10-24(41-25)23-15-31-18-40-23/h5,8-11,13,15,18,20,26,32,37H,1,6-7,12,14,16-17H2,2-4H3,(H,33,34,39)/t20-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... |
Bioorg Med Chem Lett 18: 2567-73 (2008)
BindingDB Entry DOI: 10.7270/Q21Z46RF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM384538
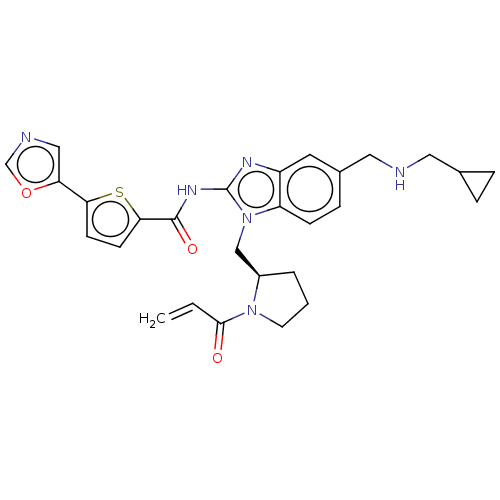
((R)-N-(1-((1-acryloylpyrrolidin-2-yl)methyl)-5-(((...)Show SMILES C=CC(=O)N1CCC[C@@H]1Cn1c(NC(=O)c2ccc(s2)-c2cnco2)nc2cc(CNCC3CC3)ccc12 |r| Show InChI InChI=1S/C28H30N6O3S/c1-2-26(35)33-11-3-4-20(33)16-34-22-8-7-19(14-29-13-18-5-6-18)12-21(22)31-28(34)32-27(36)25-10-9-24(38-25)23-15-30-17-37-23/h2,7-10,12,15,17-18,20,29H,1,3-6,11,13-14,16H2,(H,31,32,36)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... |
Bioorg Med Chem Lett 18: 2567-73 (2008)
BindingDB Entry DOI: 10.7270/Q21Z46RF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM384543
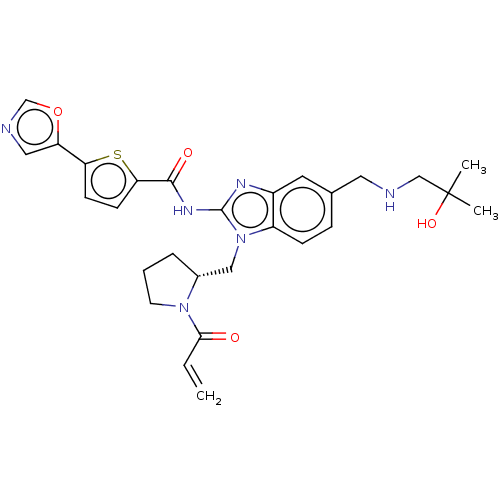
((R)-N-(1-((1-acryloylpyrrolidin-2-yl)methyl)-5-(((...)Show SMILES CC(C)(O)CNCc1ccc2n(C[C@H]3CCCN3C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r| Show InChI InChI=1S/C28H32N6O4S/c1-4-25(35)33-11-5-6-19(33)15-34-21-8-7-18(13-29-16-28(2,3)37)12-20(21)31-27(34)32-26(36)24-10-9-23(39-24)22-14-30-17-38-22/h4,7-10,12,14,17,19,29,37H,1,5-6,11,13,15-16H2,2-3H3,(H,31,32,36)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... |
Bioorg Med Chem Lett 18: 2567-73 (2008)
BindingDB Entry DOI: 10.7270/Q21Z46RF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM384542
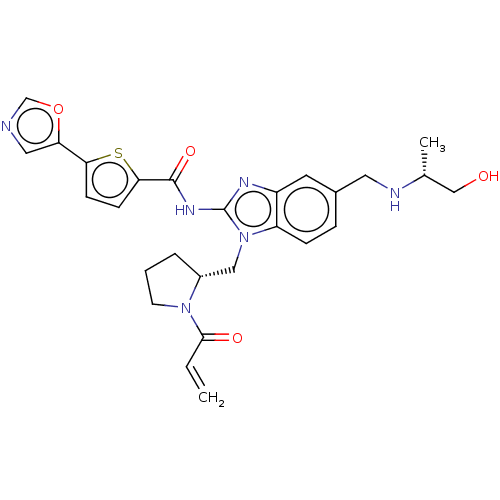
(N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...)Show SMILES C[C@H](CO)NCc1ccc2n(C[C@H]3CCCN3C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r| Show InChI InChI=1S/C27H30N6O4S/c1-3-25(35)32-10-4-5-19(32)14-33-21-7-6-18(12-29-17(2)15-34)11-20(21)30-27(33)31-26(36)24-9-8-23(38-24)22-13-28-16-37-22/h3,6-9,11,13,16-17,19,29,34H,1,4-5,10,12,14-15H2,2H3,(H,30,31,36)/t17-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... |
Bioorg Med Chem Lett 18: 2567-73 (2008)
BindingDB Entry DOI: 10.7270/Q21Z46RF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM384540
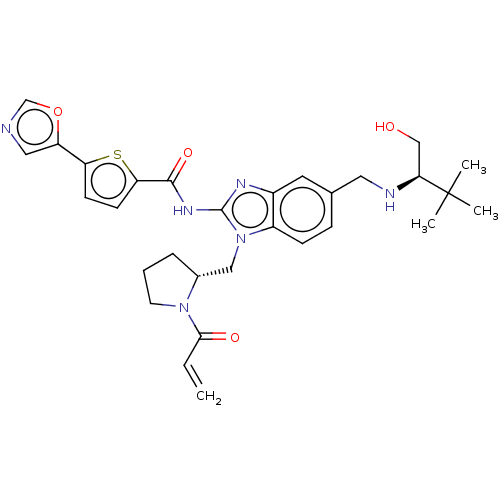
(N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...)Show SMILES CC(C)(C)[C@H](CO)NCc1ccc2n(C[C@H]3CCCN3C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r| Show InChI InChI=1S/C30H36N6O4S/c1-5-27(38)35-12-6-7-20(35)16-36-22-9-8-19(14-32-26(17-37)30(2,3)4)13-21(22)33-29(36)34-28(39)25-11-10-24(41-25)23-15-31-18-40-23/h5,8-11,13,15,18,20,26,32,37H,1,6-7,12,14,16-17H2,2-4H3,(H,33,34,39)/t20-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... |
Bioorg Med Chem Lett 18: 2567-73 (2008)
BindingDB Entry DOI: 10.7270/Q21Z46RF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM458115
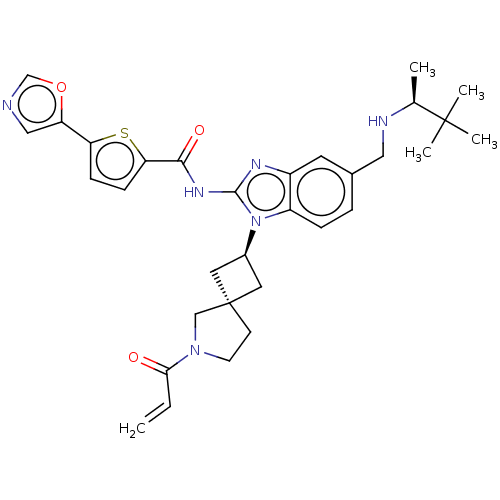
(US10752615, Compound 5)Show SMILES C[C@H](NCc1ccc2n([C@H]3C[C@@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1)C(C)(C)C |r,wU:9.8,wD:1.0,11.11,(-8.15,6.89,;-8.15,5.35,;-6.81,4.58,;-5.48,5.35,;-4.15,4.58,;-4.15,3.04,;-2.81,2.27,;-1.48,3.04,;-.01,2.57,;.38,1.08,;1.72,.31,;.95,-1.03,;-.39,-.26,;2.41,-1.5,;2.41,-3.04,;.95,-3.52,;.04,-2.27,;.55,-5.01,;1.64,-6.09,;-.94,-5.4,;-1.34,-6.89,;.89,3.81,;2.43,3.81,;3.2,5.14,;2.43,6.48,;4.74,5.14,;5.65,6.39,;7.11,5.91,;7.11,4.37,;5.65,3.9,;8.44,3.6,;9.91,4.08,;10.81,2.83,;9.91,1.59,;8.44,2.06,;-.01,5.06,;-1.48,4.58,;-2.81,5.35,;-9.48,4.58,;-10.81,5.35,;-8.71,3.25,;-10.25,3.25,)| Show InChI InChI=1S/C32H38N6O3S/c1-6-28(39)37-12-11-32(18-37)14-22(15-32)38-24-8-7-21(16-34-20(2)31(3,4)5)13-23(24)35-30(38)36-29(40)27-10-9-26(42-27)25-17-33-19-41-25/h6-10,13,17,19-20,22,34H,1,11-12,14-16,18H2,2-5H3,(H,35,36,40)/t20-,22-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM384544
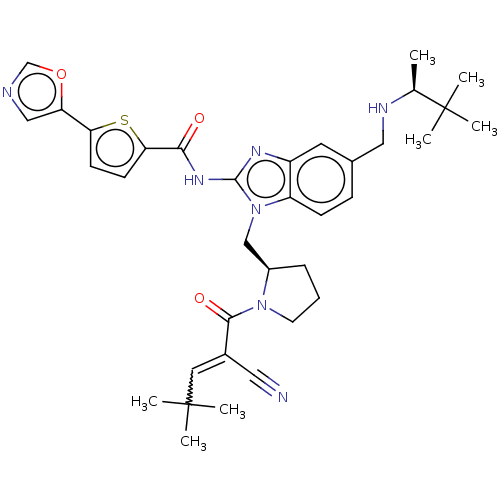
(N-(1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...)Show SMILES C[C@H](NCc1ccc2n(C[C@H]3CCCN3C(=O)C(=CC(C)(C)C)C#N)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1)C(C)(C)C |r,w:18.19| Show InChI InChI=1S/C35H43N7O3S/c1-22(35(5,6)7)38-18-23-10-11-27-26(15-23)39-33(40-31(43)30-13-12-29(46-30)28-19-37-21-45-28)42(27)20-25-9-8-14-41(25)32(44)24(17-36)16-34(2,3)4/h10-13,15-16,19,21-22,25,38H,8-9,14,18,20H2,1-7H3,(H,39,40,43)/t22-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... |
Bioorg Med Chem Lett 18: 2567-73 (2008)
BindingDB Entry DOI: 10.7270/Q21Z46RF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50381096
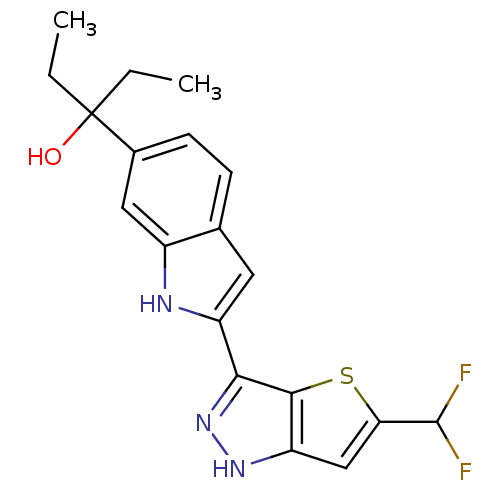
(CHEMBL2017556)Show SMILES CCC(O)(CC)c1ccc2cc([nH]c2c1)-c1n[nH]c2cc(sc12)C(F)F Show InChI InChI=1S/C19H19F2N3OS/c1-3-19(25,4-2)11-6-5-10-7-13(22-12(10)8-11)16-17-14(23-24-16)9-15(26-17)18(20)21/h5-9,18,22,25H,3-4H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminus His6 tagged Itk (357-620) autophosphorylation expressed in insect Sf9 cells using [33P]ATP by scintillation coun... |
Bioorg Med Chem Lett 22: 3296-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.016
BindingDB Entry DOI: 10.7270/Q2BG2Q00 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458597

(CHEMBL4203077 | US10752615, Compound 4)Show SMILES C[C@H](NCc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1)C(C)(C)C |r,wU:11.13,9.8,wD:1.0,(5.27,-4.41,;5.27,-5.95,;6.6,-6.72,;7.93,-5.95,;9.27,-6.72,;9.27,-8.26,;10.6,-9.03,;11.94,-8.26,;13.42,-8.73,;13.9,-10.19,;15.27,-10.88,;14.58,-12.26,;13.21,-11.57,;16.08,-12.59,;16.24,-14.13,;14.83,-14.75,;13.8,-13.59,;14.5,-16.25,;15.64,-17.29,;13.03,-16.72,;12.7,-18.22,;14.32,-7.47,;15.86,-7.47,;16.63,-6.13,;15.85,-4.8,;18.16,-6.12,;19.06,-4.86,;20.53,-5.33,;20.53,-6.87,;19.07,-7.36,;21.78,-7.77,;23.25,-7.28,;24.16,-8.52,;23.26,-9.78,;21.79,-9.31,;13.4,-6.23,;11.93,-6.71,;10.6,-5.95,;3.93,-6.72,;2.6,-5.95,;3.93,-8.26,;2.59,-7.48,)| Show InChI InChI=1S/C32H38N6O3S/c1-6-28(39)37-12-11-32(18-37)14-22(15-32)38-24-8-7-21(16-34-20(2)31(3,4)5)13-23(24)35-30(38)36-29(40)27-10-9-26(42-27)25-17-33-19-41-25/h6-10,13,17,19-20,22,34H,1,11-12,14-16,18H2,2-5H3,(H,35,36,40)/t20-,22-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458597

(CHEMBL4203077 | US10752615, Compound 4)Show SMILES C[C@H](NCc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1)C(C)(C)C |r,wU:11.13,9.8,wD:1.0,(5.27,-4.41,;5.27,-5.95,;6.6,-6.72,;7.93,-5.95,;9.27,-6.72,;9.27,-8.26,;10.6,-9.03,;11.94,-8.26,;13.42,-8.73,;13.9,-10.19,;15.27,-10.88,;14.58,-12.26,;13.21,-11.57,;16.08,-12.59,;16.24,-14.13,;14.83,-14.75,;13.8,-13.59,;14.5,-16.25,;15.64,-17.29,;13.03,-16.72,;12.7,-18.22,;14.32,-7.47,;15.86,-7.47,;16.63,-6.13,;15.85,-4.8,;18.16,-6.12,;19.06,-4.86,;20.53,-5.33,;20.53,-6.87,;19.07,-7.36,;21.78,-7.77,;23.25,-7.28,;24.16,-8.52,;23.26,-9.78,;21.79,-9.31,;13.4,-6.23,;11.93,-6.71,;10.6,-5.95,;3.93,-6.72,;2.6,-5.95,;3.93,-8.26,;2.59,-7.48,)| Show InChI InChI=1S/C32H38N6O3S/c1-6-28(39)37-12-11-32(18-37)14-22(15-32)38-24-8-7-21(16-34-20(2)31(3,4)5)13-23(24)35-30(38)36-29(40)27-10-9-26(42-27)25-17-33-19-41-25/h6-10,13,17,19-20,22,34H,1,11-12,14-16,18H2,2-5H3,(H,35,36,40)/t20-,22-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458588
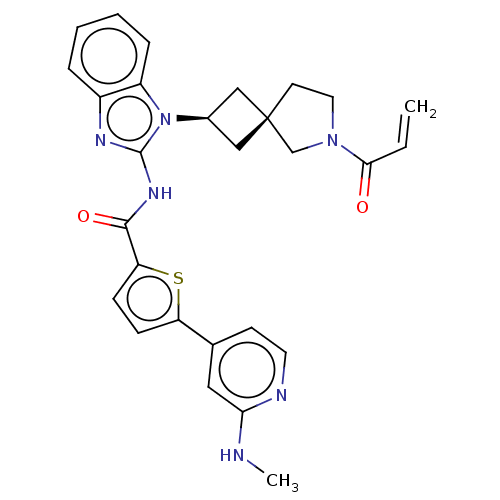
(CHEMBL4219011 | US10752615, Compound 131)Show SMILES CNc1cc(ccn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:27.33,25.28,(47.22,-29.18,;45.97,-28.29,;44.57,-28.94,;43.31,-28.05,;41.92,-28.68,;41.77,-30.22,;43.02,-31.11,;44.42,-30.48,;40.67,-27.79,;40.66,-26.26,;39.19,-25.8,;38.3,-27.05,;39.21,-28.28,;36.77,-27.07,;35.99,-25.74,;36.01,-28.4,;34.47,-28.41,;33.55,-27.16,;32.08,-27.65,;30.74,-26.89,;29.41,-27.66,;29.41,-29.2,;30.74,-29.97,;32.09,-29.2,;33.56,-29.67,;34.04,-31.13,;35.42,-31.82,;34.72,-33.2,;33.35,-32.51,;36.23,-33.53,;36.38,-35.06,;34.97,-35.68,;33.95,-34.53,;34.64,-37.19,;35.78,-38.22,;33.17,-37.66,;32.85,-39.16,)| Show InChI InChI=1S/C28H28N6O2S/c1-3-25(35)33-13-11-28(17-33)15-19(16-28)34-21-7-5-4-6-20(21)31-27(34)32-26(36)23-9-8-22(37-23)18-10-12-30-24(14-18)29-2/h3-10,12,14,19H,1,11,13,15-17H2,2H3,(H,29,30)(H,31,32,36)/t19-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458588

(CHEMBL4219011 | US10752615, Compound 131)Show SMILES CNc1cc(ccn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:27.33,25.28,(47.22,-29.18,;45.97,-28.29,;44.57,-28.94,;43.31,-28.05,;41.92,-28.68,;41.77,-30.22,;43.02,-31.11,;44.42,-30.48,;40.67,-27.79,;40.66,-26.26,;39.19,-25.8,;38.3,-27.05,;39.21,-28.28,;36.77,-27.07,;35.99,-25.74,;36.01,-28.4,;34.47,-28.41,;33.55,-27.16,;32.08,-27.65,;30.74,-26.89,;29.41,-27.66,;29.41,-29.2,;30.74,-29.97,;32.09,-29.2,;33.56,-29.67,;34.04,-31.13,;35.42,-31.82,;34.72,-33.2,;33.35,-32.51,;36.23,-33.53,;36.38,-35.06,;34.97,-35.68,;33.95,-34.53,;34.64,-37.19,;35.78,-38.22,;33.17,-37.66,;32.85,-39.16,)| Show InChI InChI=1S/C28H28N6O2S/c1-3-25(35)33-13-11-28(17-33)15-19(16-28)34-21-7-5-4-6-20(21)31-27(34)32-26(36)23-9-8-22(37-23)18-10-12-30-24(14-18)29-2/h3-10,12,14,19H,1,11,13,15-17H2,2H3,(H,29,30)(H,31,32,36)/t19-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50512346

(CHEMBL4464404)Show SMILES C=CC(=O)Nc1cccc(Oc2nc(Nc3ccc(cc3)N3CCOCC3)nc3[nH]cc(-c4ccncc4)c23)c1 Show InChI InChI=1S/C30H27N7O3/c1-2-26(38)33-22-4-3-5-24(18-22)40-29-27-25(20-10-12-31-13-11-20)19-32-28(27)35-30(36-29)34-21-6-8-23(9-7-21)37-14-16-39-17-15-37/h2-13,18-19H,1,14-17H2,(H,33,38)(H2,32,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged ITK (352 to 617 residues) expressed in baculovirus infected Sf21 insect cells using STK substr... |
Eur J Med Chem 173: 167-183 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.055
BindingDB Entry DOI: 10.7270/Q2G1645G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM458162
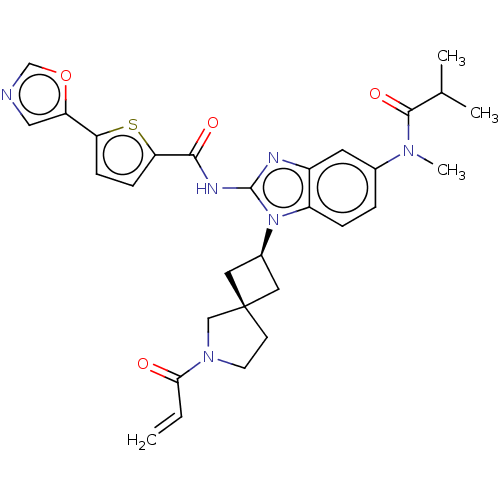
(US10752615, Compound 54)Show SMILES CC(C)C(=O)N(C)c1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:12.11,14.14,(-10.15,4.58,;-8.81,5.35,;-8.81,6.89,;-7.48,4.58,;-7.48,3.04,;-6.15,5.35,;-6.15,6.89,;-4.81,4.58,;-4.81,3.04,;-3.48,2.27,;-2.15,3.04,;-.68,2.57,;-.28,1.08,;1.05,.31,;.28,-1.03,;-1.05,-.26,;1.75,-1.5,;1.75,-3.04,;.28,-3.52,;-.62,-2.27,;-.12,-5.01,;.97,-6.09,;-1.6,-5.4,;-2,-6.89,;.22,3.81,;1.76,3.81,;2.53,5.14,;1.76,6.48,;4.07,5.14,;4.98,6.39,;6.44,5.91,;6.44,4.37,;4.98,3.9,;7.78,3.6,;9.24,4.08,;10.15,2.83,;9.24,1.59,;7.78,2.06,;-.68,5.06,;-2.15,4.58,;-3.48,5.35,)| Show InChI InChI=1S/C30H32N6O4S/c1-5-26(37)35-11-10-30(16-35)13-20(14-30)36-22-7-6-19(34(4)28(39)18(2)3)12-21(22)32-29(36)33-27(38)25-9-8-24(41-25)23-15-31-17-40-23/h5-9,12,15,17-18,20H,1,10-11,13-14,16H2,2-4H3,(H,32,33,38)/t20-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM458232
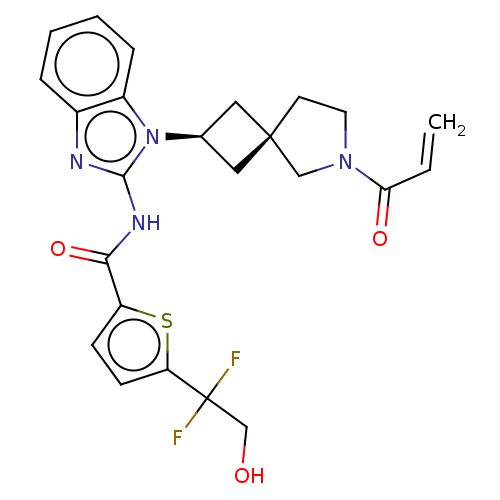
(US10752615, Compound 130)Show SMILES OCC(F)(F)c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:22.24,24.27,(6.89,1.5,;5.55,2.27,;5.55,3.81,;7.04,3.41,;6.64,4.9,;4.22,4.58,;4.22,6.12,;2.75,6.6,;1.85,5.35,;2.75,4.11,;.31,5.35,;-.46,6.68,;-.46,4.02,;-2,4.02,;-2.91,5.26,;-4.37,4.79,;-5.71,5.56,;-7.04,4.79,;-7.04,3.25,;-5.71,2.48,;-4.37,3.25,;-2.91,2.77,;-2.51,1.28,;-1.17,.51,;-1.94,-.82,;-3.28,-.05,;-.48,-1.3,;-.48,-2.84,;-1.94,-3.31,;-2.85,-2.07,;-2.34,-4.8,;-1.25,-5.89,;-3.83,-5.2,;-4.23,-6.68,)| Show InChI InChI=1S/C24H24F2N4O3S/c1-2-20(32)29-10-9-23(13-29)11-15(12-23)30-17-6-4-3-5-16(17)27-22(30)28-21(33)18-7-8-19(34-18)24(25,26)14-31/h2-8,15,31H,1,9-14H2,(H,27,28,33)/t15-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458600
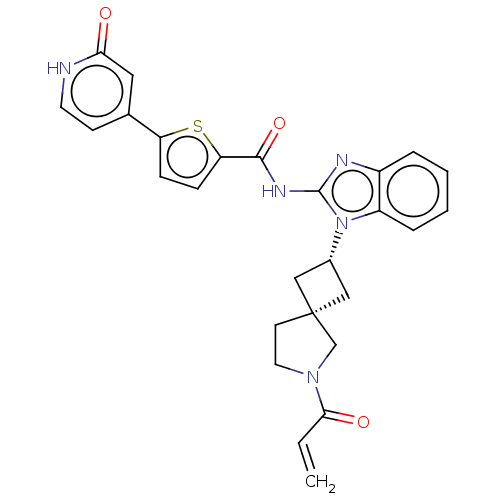
(CHEMBL4206765 | US10752615, Compound 97)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3cc[nH]c(=O)c3)nc3ccccc23)C1 |r,wU:7.6,9.11,(11.17,-39.5,;11.5,-38,;12.96,-37.53,;14.1,-38.57,;13.29,-36.02,;14.7,-35.41,;14.55,-33.87,;13.05,-33.54,;13.74,-32.16,;12.37,-31.47,;11.68,-32.85,;11.89,-30.01,;12.79,-28.75,;14.33,-28.74,;15.09,-27.41,;14.32,-26.08,;16.63,-27.39,;17.52,-26.14,;18.98,-26.6,;18.99,-28.14,;17.54,-28.62,;20.24,-29.02,;21.64,-28.38,;22.89,-29.29,;22.74,-30.82,;21.33,-31.45,;21.18,-32.99,;20.08,-30.56,;11.87,-27.5,;10.4,-27.99,;9.06,-27.23,;7.74,-28,;7.73,-29.54,;9.07,-30.31,;10.41,-29.54,;12.27,-34.87,)| Show InChI InChI=1S/C27H25N5O3S/c1-2-24(34)31-12-10-27(16-31)14-18(15-27)32-20-6-4-3-5-19(20)29-26(32)30-25(35)22-8-7-21(36-22)17-9-11-28-23(33)13-17/h2-9,11,13,18H,1,10,12,14-16H2,(H,28,33)(H,29,30,35)/t18-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458600

(CHEMBL4206765 | US10752615, Compound 97)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3cc[nH]c(=O)c3)nc3ccccc23)C1 |r,wU:7.6,9.11,(11.17,-39.5,;11.5,-38,;12.96,-37.53,;14.1,-38.57,;13.29,-36.02,;14.7,-35.41,;14.55,-33.87,;13.05,-33.54,;13.74,-32.16,;12.37,-31.47,;11.68,-32.85,;11.89,-30.01,;12.79,-28.75,;14.33,-28.74,;15.09,-27.41,;14.32,-26.08,;16.63,-27.39,;17.52,-26.14,;18.98,-26.6,;18.99,-28.14,;17.54,-28.62,;20.24,-29.02,;21.64,-28.38,;22.89,-29.29,;22.74,-30.82,;21.33,-31.45,;21.18,-32.99,;20.08,-30.56,;11.87,-27.5,;10.4,-27.99,;9.06,-27.23,;7.74,-28,;7.73,-29.54,;9.07,-30.31,;10.41,-29.54,;12.27,-34.87,)| Show InChI InChI=1S/C27H25N5O3S/c1-2-24(34)31-12-10-27(16-31)14-18(15-27)32-20-6-4-3-5-19(20)29-26(32)30-25(35)22-8-7-21(36-22)17-9-11-28-23(33)13-17/h2-9,11,13,18H,1,10,12,14-16H2,(H,28,33)(H,29,30,35)/t18-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458604
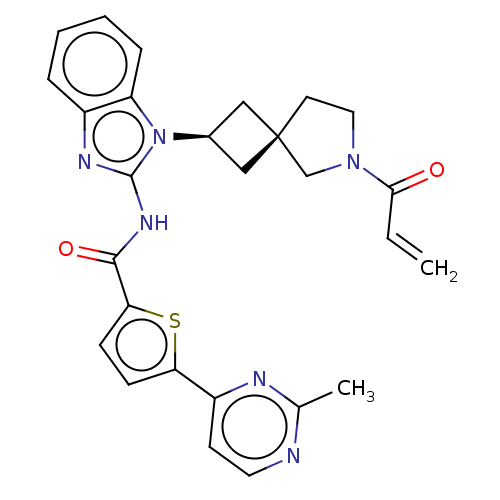
(CHEMBL4214683 | US10752615, Compound 149)Show SMILES Cc1nccc(n1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:26.32,24.27,(71.14,-29.4,;69.74,-30.04,;69.6,-31.58,;68.2,-32.22,;66.94,-31.32,;67.1,-29.79,;68.49,-29.15,;65.84,-28.9,;65.83,-27.36,;64.37,-26.9,;63.48,-28.16,;64.39,-29.38,;61.95,-28.17,;61.17,-26.84,;61.18,-29.51,;59.64,-29.52,;58.73,-28.27,;57.25,-28.76,;55.92,-27.99,;54.59,-28.76,;54.59,-30.31,;55.92,-31.08,;57.26,-30.31,;58.74,-30.78,;59.22,-32.24,;60.6,-32.93,;59.9,-34.31,;58.53,-33.61,;61.4,-34.64,;61.56,-36.17,;60.15,-36.79,;59.12,-35.64,;59.82,-38.29,;60.96,-39.33,;58.35,-38.76,;58.02,-40.26,)| Show InChI InChI=1S/C27H26N6O2S/c1-3-24(34)32-13-11-27(16-32)14-18(15-27)33-21-7-5-4-6-19(21)30-26(33)31-25(35)23-9-8-22(36-23)20-10-12-28-17(2)29-20/h3-10,12,18H,1,11,13-16H2,2H3,(H,30,31,35)/t18-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458604

(CHEMBL4214683 | US10752615, Compound 149)Show SMILES Cc1nccc(n1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:26.32,24.27,(71.14,-29.4,;69.74,-30.04,;69.6,-31.58,;68.2,-32.22,;66.94,-31.32,;67.1,-29.79,;68.49,-29.15,;65.84,-28.9,;65.83,-27.36,;64.37,-26.9,;63.48,-28.16,;64.39,-29.38,;61.95,-28.17,;61.17,-26.84,;61.18,-29.51,;59.64,-29.52,;58.73,-28.27,;57.25,-28.76,;55.92,-27.99,;54.59,-28.76,;54.59,-30.31,;55.92,-31.08,;57.26,-30.31,;58.74,-30.78,;59.22,-32.24,;60.6,-32.93,;59.9,-34.31,;58.53,-33.61,;61.4,-34.64,;61.56,-36.17,;60.15,-36.79,;59.12,-35.64,;59.82,-38.29,;60.96,-39.33,;58.35,-38.76,;58.02,-40.26,)| Show InChI InChI=1S/C27H26N6O2S/c1-3-24(34)32-13-11-27(16-32)14-18(15-27)33-21-7-5-4-6-19(21)30-26(33)31-25(35)23-9-8-22(36-23)20-10-12-28-17(2)29-20/h3-10,12,18H,1,11,13-16H2,2H3,(H,30,31,35)/t18-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM458231
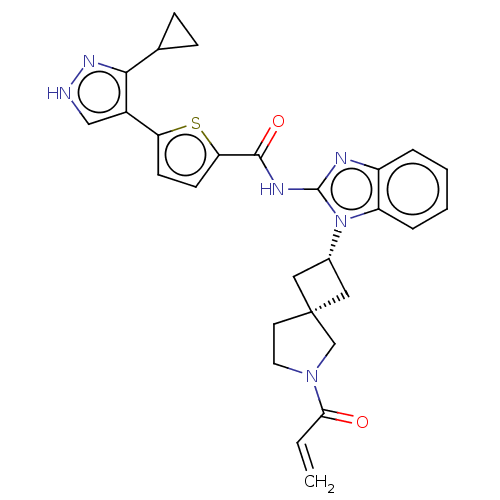
(US10752615, Compound 129)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3c[nH]nc3C3CC3)nc3ccccc23)C1 |r,wU:9.11,7.10,(-4.96,-6.97,;-4.56,-5.49,;-3.08,-5.09,;-1.99,-6.18,;-2.68,-3.6,;-1.21,-3.12,;-1.21,-1.58,;-2.68,-1.11,;-1.91,.23,;-3.24,1,;-4.01,-.34,;-3.64,2.48,;-2.73,3.73,;-1.19,3.73,;-.42,5.06,;-1.19,6.4,;1.12,5.06,;2.02,6.31,;3.49,5.83,;3.49,4.29,;2.02,3.82,;4.82,3.52,;4.82,1.98,;6.28,1.51,;7.19,2.75,;6.28,4,;6.68,5.49,;7.77,6.57,;6.28,6.97,;-3.64,4.97,;-5.1,4.5,;-6.44,5.27,;-7.77,4.5,;-7.77,2.96,;-6.44,2.19,;-5.1,2.96,;-3.58,-2.35,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM384534
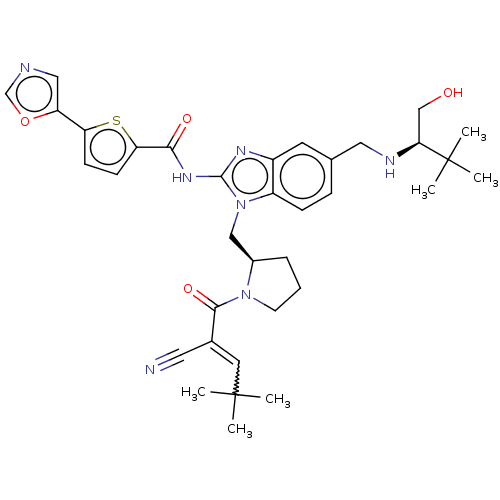
(N-(1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...)Show SMILES CC(C)(C)C=C(C#N)C(=O)N1CCC[C@@H]1Cn1c(NC(=O)c2ccc(s2)-c2cnco2)nc2cc(CN[C@@H](CO)C(C)(C)C)ccc12 |r,w:4.3| Show InChI InChI=1S/C35H43N7O4S/c1-34(2,3)15-23(16-36)32(45)41-13-7-8-24(41)19-42-26-10-9-22(17-38-30(20-43)35(4,5)6)14-25(26)39-33(42)40-31(44)29-12-11-28(47-29)27-18-37-21-46-27/h9-12,14-15,18,21,24,30,38,43H,7-8,13,17,19-20H2,1-6H3,(H,39,40,44)/t24-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... |
Bioorg Med Chem Lett 18: 2567-73 (2008)
BindingDB Entry DOI: 10.7270/Q21Z46RF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM290222

(US9573958, Compound 20)Show SMILES C=CC(=O)N1CCCC(C1)n1c2ccccc2[nH]c1=NC(=O)c1ccc(s1)-c1cn[nH]c1 |w:19.22| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC.
US Patent
| Assay Description
The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... |
US Patent US9573958 (2017)
BindingDB Entry DOI: 10.7270/Q2M047GW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM290224
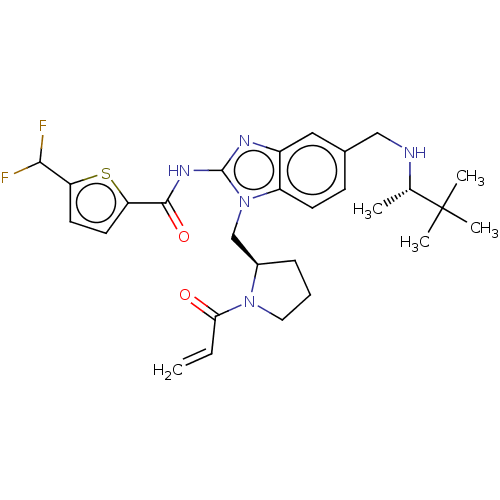
(US9573958, Compound 59)Show SMILES C[C@H](NCc1ccc2n(C[C@H]3CCCN3C(=O)C=C)c(NC(=O)c3ccc(s3)C(F)F)nc2c1)C(C)(C)C Show InChI InChI=1S/C28H35F2N5O2S/c1-6-24(36)34-13-7-8-19(34)16-35-21-10-9-18(15-31-17(2)28(3,4)5)14-20(21)32-27(35)33-26(37)23-12-11-22(38-23)25(29)30/h6,9-12,14,17,19,25,31H,1,7-8,13,15-16H2,2-5H3,(H,32,33,37)/t17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | n/a |
PRINCIPIA BIOPHARMA, INC.
US Patent
| Assay Description
The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... |
US Patent US9573958 (2017)
BindingDB Entry DOI: 10.7270/Q2M047GW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458603
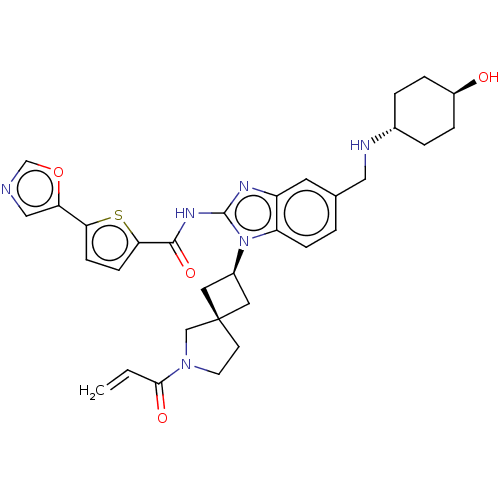
(CHEMBL4216529 | US10752615, Compound 42)Show SMILES O[C@H]1CC[C@@H](CC1)NCc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:16.19,14.14,4.7,wD:1.0,(27.35,-24.1,;28.68,-24.87,;28.67,-26.42,;30.01,-27.19,;31.34,-26.42,;31.34,-24.88,;30.02,-24.11,;32.67,-27.19,;34,-26.42,;35.34,-27.19,;35.34,-28.73,;36.67,-29.51,;38.01,-28.73,;39.49,-29.21,;39.97,-30.66,;41.34,-31.36,;40.65,-32.73,;39.28,-32.04,;42.15,-33.06,;42.31,-34.6,;40.9,-35.22,;39.87,-34.06,;40.57,-36.72,;41.71,-37.76,;39.1,-37.19,;38.77,-38.69,;40.39,-27.94,;41.93,-27.94,;42.7,-26.6,;41.92,-25.27,;44.23,-26.59,;45.12,-25.33,;46.58,-25.79,;46.59,-27.33,;45.14,-27.81,;47.84,-28.22,;49.3,-27.73,;50.21,-28.96,;49.32,-30.21,;47.86,-29.75,;39.47,-26.7,;38,-27.18,;36.67,-26.42,)| Show InChI InChI=1S/C32H36N6O4S/c1-2-29(40)37-12-11-32(18-37)14-22(15-32)38-25-8-3-20(16-34-21-4-6-23(39)7-5-21)13-24(25)35-31(38)36-30(41)28-10-9-27(43-28)26-17-33-19-42-26/h2-3,8-10,13,17,19,21-23,34,39H,1,4-7,11-12,14-16,18H2,(H,35,36,41)/t21-,22-,23-,32- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM458137
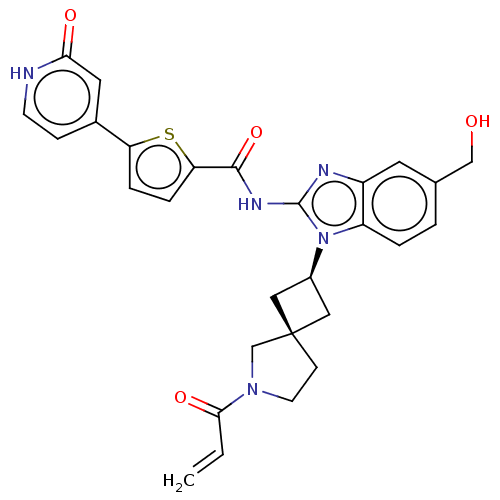
(US10752615, Compound 29)Show SMILES OCc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cc[nH]c(=O)c3)nc2c1 |r,wU:7.6,9.9,(-8.96,4.79,;-7.63,5.56,;-6.3,4.79,;-6.3,3.25,;-4.96,2.48,;-3.63,3.25,;-2.16,2.77,;-1.76,1.28,;-.43,.51,;-1.2,-.82,;-2.53,-.05,;.26,-1.3,;.26,-2.84,;-1.2,-3.31,;-2.11,-2.07,;-1.6,-4.8,;-.51,-5.89,;-3.09,-5.2,;-3.49,-6.68,;-1.26,4.02,;.28,4.02,;1.05,5.35,;.28,6.68,;2.59,5.35,;3.5,6.6,;4.96,6.12,;4.96,4.58,;3.5,4.11,;6.3,3.81,;7.63,4.58,;8.96,3.81,;8.96,2.27,;7.63,1.5,;7.63,-.04,;6.3,2.27,;-2.16,5.26,;-3.63,4.79,;-4.96,5.56,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458603

(CHEMBL4216529 | US10752615, Compound 42)Show SMILES O[C@H]1CC[C@@H](CC1)NCc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:16.19,14.14,4.7,wD:1.0,(27.35,-24.1,;28.68,-24.87,;28.67,-26.42,;30.01,-27.19,;31.34,-26.42,;31.34,-24.88,;30.02,-24.11,;32.67,-27.19,;34,-26.42,;35.34,-27.19,;35.34,-28.73,;36.67,-29.51,;38.01,-28.73,;39.49,-29.21,;39.97,-30.66,;41.34,-31.36,;40.65,-32.73,;39.28,-32.04,;42.15,-33.06,;42.31,-34.6,;40.9,-35.22,;39.87,-34.06,;40.57,-36.72,;41.71,-37.76,;39.1,-37.19,;38.77,-38.69,;40.39,-27.94,;41.93,-27.94,;42.7,-26.6,;41.92,-25.27,;44.23,-26.59,;45.12,-25.33,;46.58,-25.79,;46.59,-27.33,;45.14,-27.81,;47.84,-28.22,;49.3,-27.73,;50.21,-28.96,;49.32,-30.21,;47.86,-29.75,;39.47,-26.7,;38,-27.18,;36.67,-26.42,)| Show InChI InChI=1S/C32H36N6O4S/c1-2-29(40)37-12-11-32(18-37)14-22(15-32)38-25-8-3-20(16-34-21-4-6-23(39)7-5-21)13-24(25)35-31(38)36-30(41)28-10-9-27(43-28)26-17-33-19-42-26/h2-3,8-10,13,17,19,21-23,34,39H,1,4-7,11-12,14-16,18H2,(H,35,36,41)/t21-,22-,23-,32- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458587
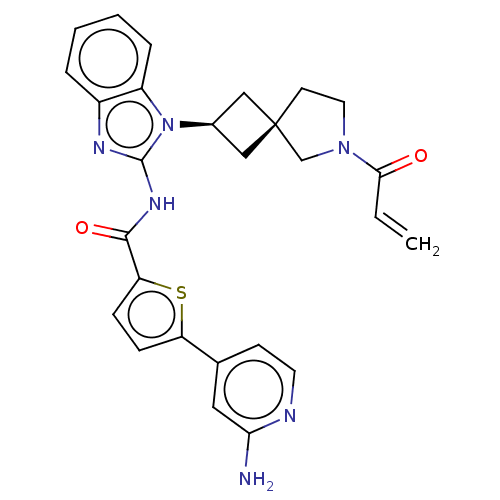
(CHEMBL4207292)Show SMILES Nc1cc(ccn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:26.32,24.27,(45.45,-6.32,;44.05,-6.96,;42.79,-6.07,;41.4,-6.71,;41.25,-8.25,;42.5,-9.14,;43.9,-8.5,;40.15,-5.82,;40.13,-4.29,;38.67,-3.83,;37.78,-5.08,;38.69,-6.31,;36.25,-5.09,;35.47,-3.76,;35.49,-6.43,;33.95,-6.44,;33.03,-5.19,;31.56,-5.68,;30.22,-4.91,;28.89,-5.69,;28.89,-7.23,;30.22,-8,;31.56,-7.23,;33.04,-7.7,;33.52,-9.16,;34.9,-9.85,;34.2,-11.23,;32.83,-10.54,;35.71,-11.56,;35.86,-13.09,;34.45,-13.71,;33.42,-12.56,;34.12,-15.22,;35.26,-16.25,;32.65,-15.68,;32.32,-17.19,)| Show InChI InChI=1S/C27H26N6O2S/c1-2-24(34)32-12-10-27(16-32)14-18(15-27)33-20-6-4-3-5-19(20)30-26(33)31-25(35)22-8-7-21(36-22)17-9-11-29-23(28)13-17/h2-9,11,13,18H,1,10,12,14-16H2,(H2,28,29)(H,30,31,35)/t18-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM458230
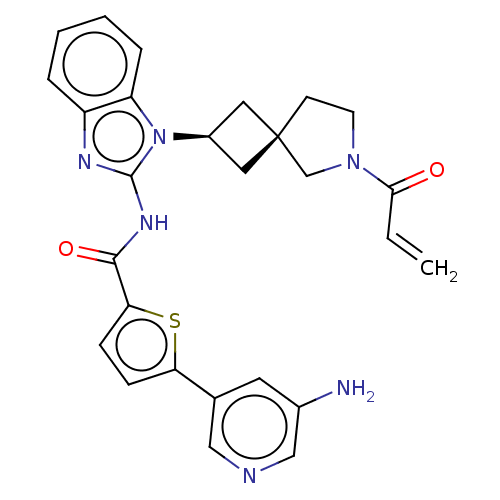
(US10752615, Compound 128)Show SMILES Nc1cncc(c1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:24.27,26.30,(8.3,4.58,;6.96,3.81,;6.96,2.27,;5.63,1.5,;4.29,2.27,;4.29,3.81,;5.63,4.58,;2.96,4.58,;2.96,6.12,;1.5,6.6,;.59,5.35,;1.5,4.11,;-.95,5.35,;-1.72,6.68,;-1.72,4.02,;-3.26,4.02,;-4.16,5.26,;-5.63,4.79,;-6.96,5.56,;-8.3,4.79,;-8.3,3.25,;-6.96,2.48,;-5.63,3.25,;-4.16,2.77,;-3.77,1.28,;-2.43,.51,;-3.2,-.82,;-4.54,-.05,;-1.74,-1.3,;-1.74,-2.84,;-3.2,-3.31,;-4.11,-2.07,;-3.6,-4.8,;-2.51,-5.89,;-5.09,-5.2,;-5.49,-6.68,)| Show InChI InChI=1S/C27H26N6O2S/c1-2-24(34)32-10-9-27(16-32)12-19(13-27)33-21-6-4-3-5-20(21)30-26(33)31-25(35)23-8-7-22(36-23)17-11-18(28)15-29-14-17/h2-8,11,14-15,19H,1,9-10,12-13,16,28H2,(H,30,31,35)/t19-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM458119
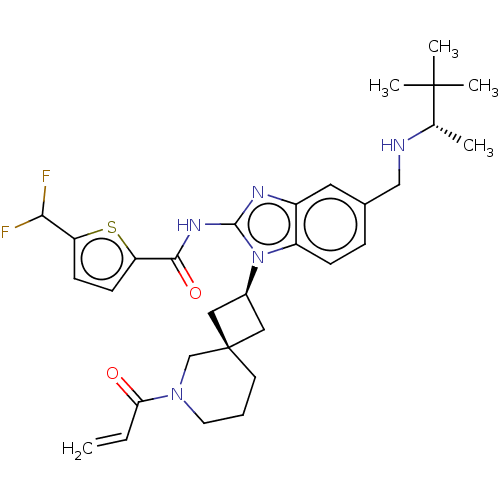
(US10752615, Compound 9)Show SMILES C[C@H](NCc1ccc2n([C@H]3C[C@]4(C3)CCCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)C(F)F)nc2c1)C(C)(C)C |r,wU:9.8,11.11,wD:1.0,(-7.63,6.54,;-7.63,5,;-6.3,4.23,;-4.96,5,;-3.63,4.23,;-3.63,2.69,;-2.29,1.92,;-.96,2.69,;.5,2.21,;.9,.72,;2.24,-.05,;1.47,-1.38,;.13,-.61,;2.95,-1.78,;3.35,-3.27,;2.26,-4.36,;.78,-3.96,;.38,-2.47,;-.31,-5.05,;.09,-6.54,;-1.8,-4.65,;-2.89,-5.74,;1.41,3.46,;2.95,3.46,;3.72,4.79,;2.95,6.12,;5.26,4.79,;6.16,6.03,;7.63,5.56,;7.63,4.02,;6.16,3.54,;8.96,3.25,;10.3,4.02,;8.96,1.71,;.5,4.7,;-.96,4.23,;-2.29,5,;-8.96,4.23,;-10.3,5,;-8.96,2.69,;-10.3,3.46,)| Show InChI InChI=1S/C31H39F2N5O2S/c1-6-26(39)37-13-7-12-31(18-37)15-21(16-31)38-23-9-8-20(17-34-19(2)30(3,4)5)14-22(23)35-29(38)36-28(40)25-11-10-24(41-25)27(32)33/h6,8-11,14,19,21,27,34H,1,7,12-13,15-18H2,2-5H3,(H,35,36,40)/t19-,21-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458595

(CHEMBL4215147 | US10752615, Compound 44)Show SMILES CC(C)(CO)CNCc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:15.17,13.12,(57.21,-6.44,;56.45,-5.11,;55.68,-6.44,;55.12,-4.34,;53.79,-5.11,;57.79,-4.34,;59.13,-5.11,;60.46,-4.34,;61.79,-5.11,;61.79,-6.65,;63.13,-7.43,;64.47,-6.65,;65.94,-7.13,;66.42,-8.58,;67.8,-9.28,;67.1,-10.66,;65.73,-9.96,;68.61,-10.98,;68.76,-12.52,;67.35,-13.14,;66.33,-11.99,;67.02,-14.64,;68.16,-15.68,;65.55,-15.11,;65.23,-16.61,;66.85,-5.86,;68.39,-5.86,;69.15,-4.52,;68.37,-3.19,;70.69,-4.51,;71.57,-3.25,;73.04,-3.71,;73.05,-5.25,;71.59,-5.73,;74.3,-6.14,;75.76,-5.65,;76.67,-6.88,;75.78,-8.13,;74.32,-7.67,;65.93,-4.62,;64.46,-5.1,;63.12,-4.34,)| Show InChI InChI=1S/C31H36N6O4S/c1-4-27(39)36-10-9-31(17-36)12-21(13-31)37-23-6-5-20(14-32-16-30(2,3)18-38)11-22(23)34-29(37)35-28(40)26-8-7-25(42-26)24-15-33-19-41-24/h4-8,11,15,19,21,32,38H,1,9-10,12-14,16-18H2,2-3H3,(H,34,35,40)/t21-,31- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458595

(CHEMBL4215147 | US10752615, Compound 44)Show SMILES CC(C)(CO)CNCc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:15.17,13.12,(57.21,-6.44,;56.45,-5.11,;55.68,-6.44,;55.12,-4.34,;53.79,-5.11,;57.79,-4.34,;59.13,-5.11,;60.46,-4.34,;61.79,-5.11,;61.79,-6.65,;63.13,-7.43,;64.47,-6.65,;65.94,-7.13,;66.42,-8.58,;67.8,-9.28,;67.1,-10.66,;65.73,-9.96,;68.61,-10.98,;68.76,-12.52,;67.35,-13.14,;66.33,-11.99,;67.02,-14.64,;68.16,-15.68,;65.55,-15.11,;65.23,-16.61,;66.85,-5.86,;68.39,-5.86,;69.15,-4.52,;68.37,-3.19,;70.69,-4.51,;71.57,-3.25,;73.04,-3.71,;73.05,-5.25,;71.59,-5.73,;74.3,-6.14,;75.76,-5.65,;76.67,-6.88,;75.78,-8.13,;74.32,-7.67,;65.93,-4.62,;64.46,-5.1,;63.12,-4.34,)| Show InChI InChI=1S/C31H36N6O4S/c1-4-27(39)36-10-9-31(17-36)12-21(13-31)37-23-6-5-20(14-32-16-30(2,3)18-38)11-22(23)34-29(37)35-28(40)26-8-7-25(42-26)24-15-33-19-41-24/h4-8,11,15,19,21,32,38H,1,9-10,12-14,16-18H2,2-3H3,(H,34,35,40)/t21-,31- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458596
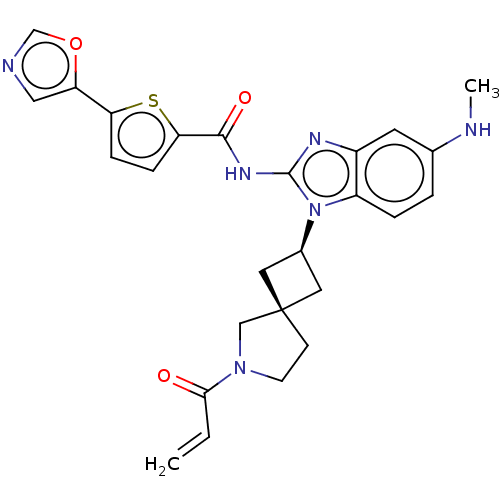
(CHEMBL4216187 | US10752615, Compound 50)Show SMILES CNc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:9.11,7.6,(58.28,-26.3,;59.61,-25.53,;60.95,-26.3,;60.94,-27.84,;62.28,-28.61,;63.62,-27.84,;65.1,-28.31,;65.58,-29.77,;66.95,-30.46,;66.26,-31.84,;64.89,-31.15,;67.76,-32.17,;67.91,-33.7,;66.5,-34.32,;65.48,-33.17,;66.18,-35.83,;67.31,-36.87,;64.71,-36.3,;64.38,-37.8,;66,-27.05,;67.54,-27.04,;68.3,-25.71,;67.53,-24.38,;69.84,-25.69,;70.73,-24.44,;72.19,-24.9,;72.2,-26.43,;70.75,-26.92,;73.45,-27.32,;74.91,-26.84,;75.82,-28.07,;74.93,-29.32,;73.47,-28.86,;65.08,-25.8,;63.61,-26.29,;62.28,-25.53,)| Show InChI InChI=1S/C26H26N6O3S/c1-3-23(33)31-9-8-26(14-31)11-17(12-26)32-19-5-4-16(27-2)10-18(19)29-25(32)30-24(34)22-7-6-21(36-22)20-13-28-15-35-20/h3-7,10,13,15,17,27H,1,8-9,11-12,14H2,2H3,(H,29,30,34)/t17-,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458596

(CHEMBL4216187 | US10752615, Compound 50)Show SMILES CNc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:9.11,7.6,(58.28,-26.3,;59.61,-25.53,;60.95,-26.3,;60.94,-27.84,;62.28,-28.61,;63.62,-27.84,;65.1,-28.31,;65.58,-29.77,;66.95,-30.46,;66.26,-31.84,;64.89,-31.15,;67.76,-32.17,;67.91,-33.7,;66.5,-34.32,;65.48,-33.17,;66.18,-35.83,;67.31,-36.87,;64.71,-36.3,;64.38,-37.8,;66,-27.05,;67.54,-27.04,;68.3,-25.71,;67.53,-24.38,;69.84,-25.69,;70.73,-24.44,;72.19,-24.9,;72.2,-26.43,;70.75,-26.92,;73.45,-27.32,;74.91,-26.84,;75.82,-28.07,;74.93,-29.32,;73.47,-28.86,;65.08,-25.8,;63.61,-26.29,;62.28,-25.53,)| Show InChI InChI=1S/C26H26N6O3S/c1-3-23(33)31-9-8-26(14-31)11-17(12-26)32-19-5-4-16(27-2)10-18(19)29-25(32)30-24(34)22-7-6-21(36-22)20-13-28-15-35-20/h3-7,10,13,15,17,27H,1,8-9,11-12,14H2,2H3,(H,29,30,34)/t17-,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM458151
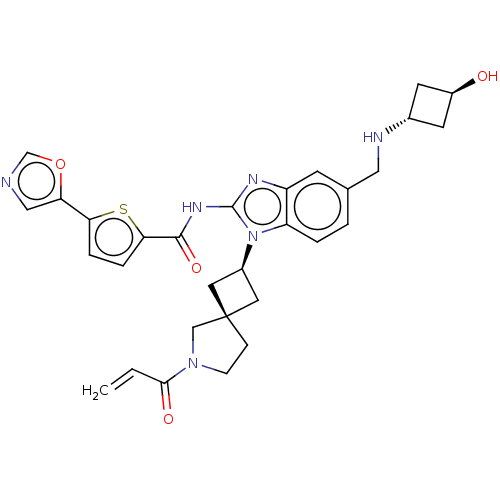
(US10752615, Compound 43)Show SMILES O[C@H]1C[C@@H](C1)NCc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:12.12,14.15,3.5,wD:1.0,(-11.09,7.05,;-9.76,6.28,;-9.36,4.79,;-7.87,5.19,;-8.27,6.68,;-6.54,4.42,;-5.2,5.19,;-3.87,4.42,;-3.87,2.88,;-2.54,2.11,;-1.2,2.88,;.26,2.41,;.66,.92,;1.99,.15,;1.22,-1.19,;-.11,-.42,;2.69,-1.66,;2.69,-3.2,;1.22,-3.68,;.32,-2.43,;.83,-5.16,;1.91,-6.25,;-.66,-5.56,;-1.06,-7.05,;1.17,3.65,;2.71,3.65,;3.48,4.99,;2.71,6.32,;5.02,4.99,;5.92,6.23,;7.39,5.76,;7.39,4.22,;5.92,3.74,;8.72,3.45,;10.19,3.92,;11.09,2.68,;10.19,1.43,;8.72,1.91,;.26,4.9,;-1.2,4.42,;-2.54,5.19,)| Show InChI InChI=1S/C30H32N6O4S/c1-2-27(38)35-8-7-30(16-35)12-20(13-30)36-23-4-3-18(14-32-19-10-21(37)11-19)9-22(23)33-29(36)34-28(39)26-6-5-25(41-26)24-15-31-17-40-24/h2-6,9,15,17,19-21,32,37H,1,7-8,10-14,16H2,(H,33,34,39)/t19-,20-,21-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM458136
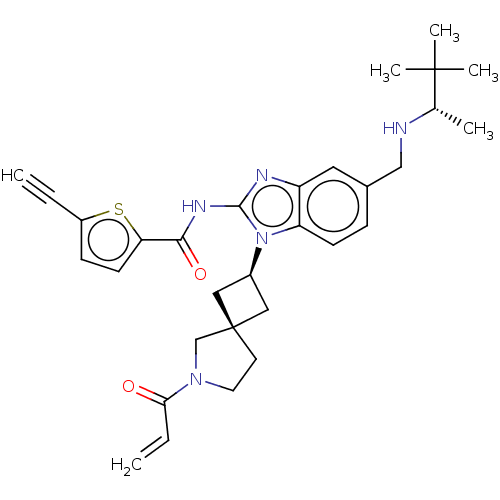
(US10752615, Compound 28)Show SMILES C[C@H](NCc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)C#C)nc2c1)C(C)(C)C |r,wU:9.8,11.11,wD:1.0,(-7.63,6.89,;-7.63,5.35,;-6.3,4.58,;-4.96,5.35,;-3.63,4.58,;-3.63,3.04,;-2.29,2.27,;-.96,3.04,;.5,2.57,;.9,1.08,;2.24,.31,;1.47,-1.03,;.13,-.26,;2.93,-1.5,;2.93,-3.04,;1.47,-3.52,;.56,-2.27,;1.07,-5.01,;2.16,-6.09,;-.42,-5.4,;-.82,-6.89,;1.41,3.81,;2.95,3.81,;3.72,5.14,;2.95,6.48,;5.26,5.14,;6.16,6.39,;7.63,5.91,;7.63,4.37,;6.16,3.9,;8.96,3.6,;10.3,2.83,;.5,5.06,;-.96,4.58,;-2.29,5.35,;-8.96,4.58,;-10.3,5.35,;-8.96,3.04,;-10.3,3.81,)| Show InChI InChI=1S/C31H37N5O2S/c1-7-23-10-12-26(39-23)28(38)34-29-33-24-15-21(18-32-20(3)30(4,5)6)9-11-25(24)36(29)22-16-31(17-22)13-14-35(19-31)27(37)8-2/h1,8-12,15,20,22,32H,2,13-14,16-19H2,3-6H3,(H,33,34,38)/t20-,22-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM458258

(US10752615, Compound 156)Show SMILES Cc1n[nH]cc1-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:23.26,25.29,(6.97,5.77,;6.58,4.29,;7.48,3.04,;6.58,1.8,;5.11,2.27,;5.11,3.81,;3.78,4.58,;3.78,6.12,;2.31,6.6,;1.41,5.35,;2.31,4.11,;-.13,5.35,;-.9,6.68,;-.9,4.02,;-2.44,4.02,;-3.35,5.26,;-4.81,4.79,;-6.15,5.56,;-7.48,4.79,;-7.48,3.25,;-6.15,2.48,;-4.81,3.25,;-3.35,2.77,;-2.95,1.28,;-1.62,.51,;-2.39,-.82,;-3.72,-.05,;-.92,-1.3,;-.92,-2.84,;-2.39,-3.31,;-3.29,-2.07,;-2.78,-4.8,;-1.7,-5.89,;-4.27,-5.2,;-4.67,-6.68,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM458160
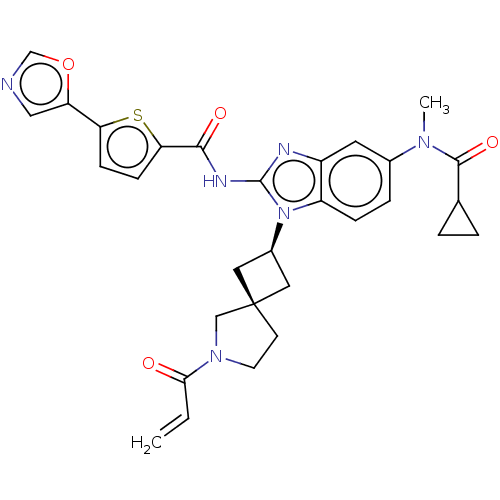
(US10752615, Compound 52)Show SMILES CN(C(=O)C1CC1)c1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:12.12,14.15,(-6.04,6.89,;-6.04,5.35,;-7.38,4.58,;-7.38,3.04,;-8.71,5.35,;-9.48,6.68,;-10.25,5.35,;-4.71,4.58,;-4.71,3.04,;-3.38,2.27,;-2.04,3.04,;-.58,2.57,;-.18,1.08,;1.15,.31,;.38,-1.03,;-.95,-.26,;1.85,-1.5,;1.85,-3.04,;.38,-3.52,;-.52,-2.27,;-.01,-5.01,;1.07,-6.09,;-1.5,-5.4,;-1.9,-6.89,;.33,3.81,;1.87,3.81,;2.64,5.14,;1.87,6.48,;4.18,5.14,;5.08,6.39,;6.55,5.91,;6.55,4.37,;5.08,3.9,;7.88,3.6,;9.35,4.08,;10.25,2.83,;9.35,1.59,;7.88,2.06,;-.58,5.06,;-2.04,4.58,;-3.38,5.35,)| Show InChI InChI=1S/C30H30N6O4S/c1-3-26(37)35-11-10-30(16-35)13-20(14-30)36-22-7-6-19(34(2)28(39)18-4-5-18)12-21(22)32-29(36)33-27(38)25-9-8-24(41-25)23-15-31-17-40-23/h3,6-9,12,15,17-18,20H,1,4-5,10-11,13-14,16H2,2H3,(H,32,33,38)/t20-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM458249
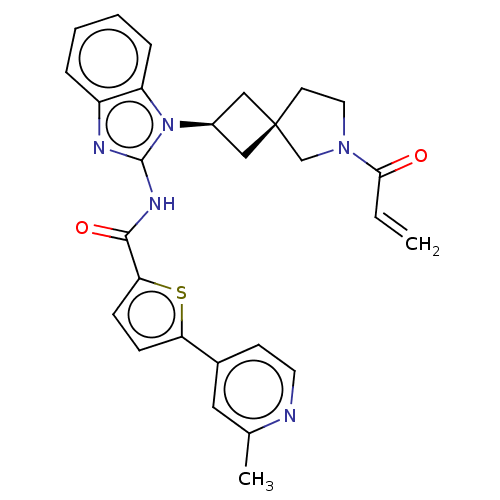
(US10752615, Compound 147)Show SMILES Cc1cc(ccn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:24.27,26.30,(8.3,4.58,;6.96,3.81,;5.63,4.58,;4.29,3.81,;4.29,2.27,;5.63,1.5,;6.96,2.27,;2.96,4.58,;2.96,6.12,;1.5,6.6,;.59,5.35,;1.5,4.11,;-.95,5.35,;-1.72,6.68,;-1.72,4.02,;-3.26,4.02,;-4.16,5.26,;-5.63,4.79,;-6.96,5.56,;-8.3,4.79,;-8.3,3.25,;-6.96,2.48,;-5.63,3.25,;-4.16,2.77,;-3.77,1.28,;-2.43,.51,;-3.2,-.82,;-4.54,-.05,;-1.74,-1.3,;-1.74,-2.84,;-3.2,-3.31,;-4.11,-2.07,;-3.6,-4.8,;-2.51,-5.89,;-5.09,-5.2,;-5.49,-6.68,)| Show InChI InChI=1S/C28H27N5O2S/c1-3-25(34)32-13-11-28(17-32)15-20(16-28)33-22-7-5-4-6-21(22)30-27(33)31-26(35)24-9-8-23(36-24)19-10-12-29-18(2)14-19/h3-10,12,14,20H,1,11,13,15-17H2,2H3,(H,30,31,35)/t20-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM384535
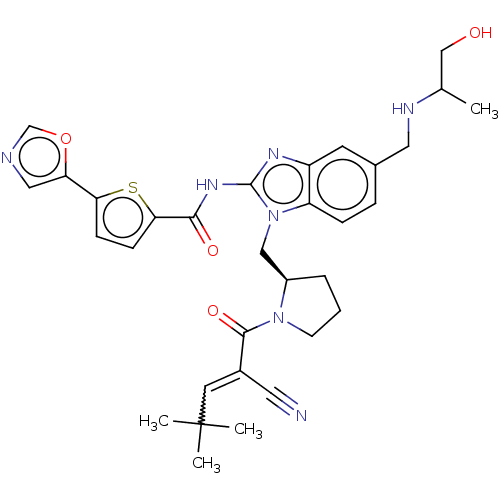
(N-(1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...)Show SMILES CC(CO)NCc1ccc2n(C[C@H]3CCCN3C(=O)C(=CC(C)(C)C)C#N)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,w:20.21| Show InChI InChI=1S/C32H37N7O4S/c1-20(18-40)35-15-21-7-8-25-24(12-21)36-31(37-29(41)28-10-9-27(44-28)26-16-34-19-43-26)39(25)17-23-6-5-11-38(23)30(42)22(14-33)13-32(2,3)4/h7-10,12-13,16,19-20,23,35,40H,5-6,11,15,17-18H2,1-4H3,(H,36,37,41)/t20?,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... |
Bioorg Med Chem Lett 18: 2567-73 (2008)
BindingDB Entry DOI: 10.7270/Q21Z46RF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM384537
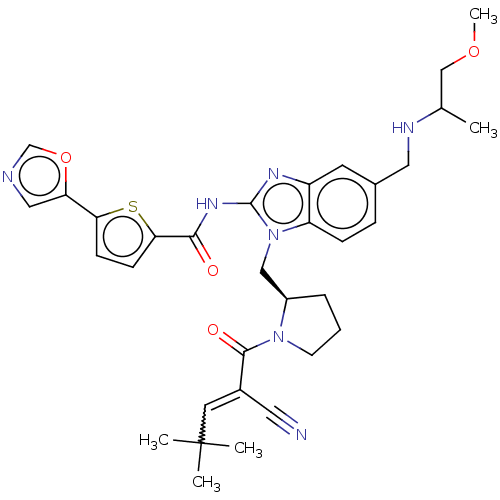
(N-1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyrr...)Show SMILES COCC(C)NCc1ccc2n(C[C@H]3CCCN3C(=O)C(=CC(C)(C)C)C#N)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,w:21.22| Show InChI InChI=1S/C33H39N7O4S/c1-21(19-43-5)36-16-22-8-9-26-25(13-22)37-32(38-30(41)29-11-10-28(45-29)27-17-35-20-44-27)40(26)18-24-7-6-12-39(24)31(42)23(15-34)14-33(2,3)4/h8-11,13-14,17,20-21,24,36H,6-7,12,16,18-19H2,1-5H3,(H,37,38,41)/t21?,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... |
Bioorg Med Chem Lett 18: 2567-73 (2008)
BindingDB Entry DOI: 10.7270/Q21Z46RF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM384533
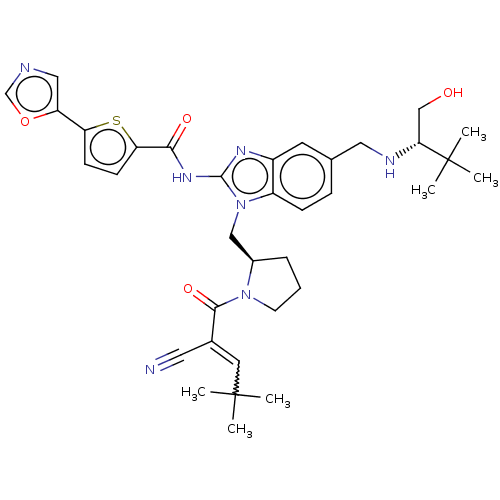
(N-(1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...)Show SMILES CC(C)(C)C=C(C#N)C(=O)N1CCC[C@@H]1Cn1c(NC(=O)c2ccc(s2)-c2cnco2)nc2cc(CN[C@H](CO)C(C)(C)C)ccc12 |r,w:4.3| Show InChI InChI=1S/C35H43N7O4S/c1-34(2,3)15-23(16-36)32(45)41-13-7-8-24(41)19-42-26-10-9-22(17-38-30(20-43)35(4,5)6)14-25(26)39-33(42)40-31(44)29-12-11-28(47-29)27-18-37-21-46-27/h9-12,14-15,18,21,24,30,38,43H,7-8,13,17,19-20H2,1-6H3,(H,39,40,44)/t24-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... |
Bioorg Med Chem Lett 18: 2567-73 (2008)
BindingDB Entry DOI: 10.7270/Q21Z46RF |
More data for this
Ligand-Target Pair | |
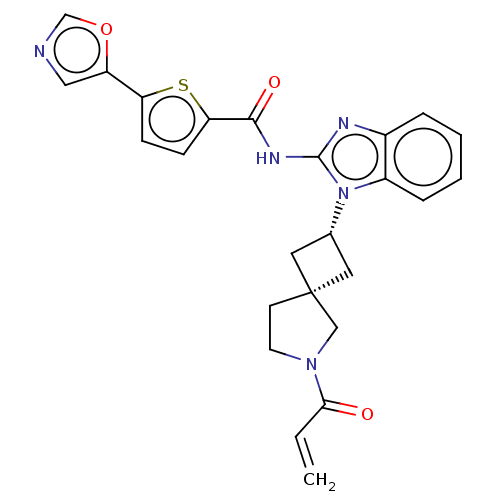
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458592
(CHEMBL4211372 | US10752615, Compound 16)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3cnco3)nc3ccccc23)C1 |r,wU:7.6,9.11,(34.53,-42.18,;34.86,-40.68,;36.33,-40.21,;37.47,-41.25,;36.66,-38.71,;38.07,-38.09,;37.92,-36.56,;36.41,-36.23,;37.11,-34.85,;35.73,-34.16,;35.04,-35.53,;35.25,-32.7,;36.16,-31.44,;37.7,-31.43,;38.46,-30.09,;37.68,-28.76,;39.99,-30.08,;40.89,-28.83,;42.36,-29.29,;42.37,-30.83,;40.9,-31.32,;43.62,-31.73,;45.08,-31.25,;45.99,-32.49,;45.09,-33.74,;43.63,-33.27,;35.24,-30.19,;33.76,-30.68,;32.43,-29.91,;31.1,-30.68,;31.1,-32.23,;32.43,-33,;33.77,-32.23,;35.63,-37.56,)| Show InChI InChI=1S/C25H23N5O3S/c1-2-22(31)29-10-9-25(14-29)11-16(12-25)30-18-6-4-3-5-17(18)27-24(30)28-23(32)21-8-7-20(34-21)19-13-26-15-33-19/h2-8,13,15-16H,1,9-12,14H2,(H,27,28,32)/t16-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458592

(CHEMBL4211372 | US10752615, Compound 16)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3cnco3)nc3ccccc23)C1 |r,wU:7.6,9.11,(34.53,-42.18,;34.86,-40.68,;36.33,-40.21,;37.47,-41.25,;36.66,-38.71,;38.07,-38.09,;37.92,-36.56,;36.41,-36.23,;37.11,-34.85,;35.73,-34.16,;35.04,-35.53,;35.25,-32.7,;36.16,-31.44,;37.7,-31.43,;38.46,-30.09,;37.68,-28.76,;39.99,-30.08,;40.89,-28.83,;42.36,-29.29,;42.37,-30.83,;40.9,-31.32,;43.62,-31.73,;45.08,-31.25,;45.99,-32.49,;45.09,-33.74,;43.63,-33.27,;35.24,-30.19,;33.76,-30.68,;32.43,-29.91,;31.1,-30.68,;31.1,-32.23,;32.43,-33,;33.77,-32.23,;35.63,-37.56,)| Show InChI InChI=1S/C25H23N5O3S/c1-2-22(31)29-10-9-25(14-29)11-16(12-25)30-18-6-4-3-5-17(18)27-24(30)28-23(32)21-8-7-20(34-21)19-13-26-15-33-19/h2-8,13,15-16H,1,9-12,14H2,(H,27,28,32)/t16-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM458184
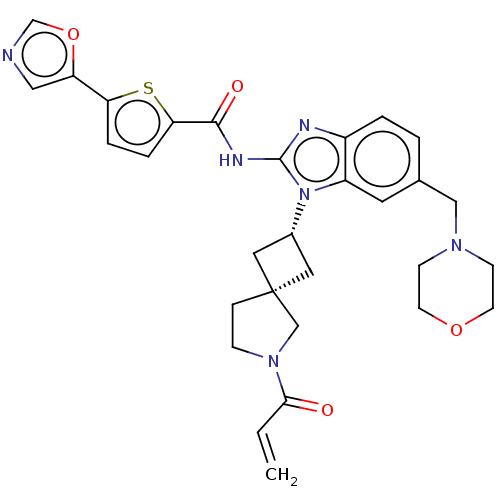
(US10752615, Compound 77)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3cnco3)nc3ccc(CN4CCOCC4)cc23)C1 |r,wU:9.11,7.10,(-2,-6.68,;-1.6,-5.2,;-.12,-4.8,;.97,-5.89,;.28,-3.31,;1.75,-2.84,;1.75,-1.3,;.28,-.82,;1.05,.51,;-.28,1.28,;-1.05,-.05,;-.68,2.77,;.22,4.02,;1.76,4.02,;2.53,5.35,;1.76,6.68,;4.07,5.35,;4.98,6.6,;6.44,6.12,;6.44,4.58,;4.98,4.11,;7.78,3.81,;9.24,4.29,;10.15,3.04,;9.24,1.8,;7.78,2.27,;-.68,5.26,;-2.15,4.79,;-3.48,5.56,;-4.81,4.79,;-4.81,3.25,;-6.15,2.48,;-7.48,3.25,;-7.48,4.79,;-8.81,5.56,;-10.15,4.79,;-10.15,3.25,;-8.81,2.48,;-3.48,2.48,;-2.15,3.25,;-.62,-2.07,)| Show InChI InChI=1S/C30H32N6O4S/c1-2-27(37)35-8-7-30(18-35)14-21(15-30)36-23-13-20(17-34-9-11-39-12-10-34)3-4-22(23)32-29(36)33-28(38)26-6-5-25(41-26)24-16-31-19-40-24/h2-6,13,16,19,21H,1,7-12,14-15,17-18H2,(H,32,33,38)/t21-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458591

(CHEMBL4206487 | US10752615, Compound 2)Show SMILES C[C@H](NCc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)C(F)F)nc2c1)C(C)(C)C |r,wU:11.13,9.8,wD:1.0,(29.61,-4,;29.61,-5.54,;30.95,-6.31,;32.28,-5.54,;33.61,-6.31,;33.61,-7.85,;34.94,-8.62,;36.29,-7.85,;37.76,-8.32,;38.24,-9.78,;39.62,-10.47,;38.92,-11.85,;37.55,-11.16,;40.43,-12.18,;40.58,-13.71,;39.17,-14.33,;38.15,-13.18,;38.84,-15.84,;39.98,-16.87,;37.37,-16.31,;37.05,-17.81,;38.67,-7.06,;40.21,-7.05,;40.97,-5.72,;40.19,-4.39,;42.5,-5.7,;43.4,-4.45,;44.87,-4.92,;44.88,-6.46,;43.42,-6.94,;46.13,-7.36,;45.98,-8.89,;47.53,-6.72,;37.75,-5.81,;36.28,-6.3,;34.94,-5.54,;28.28,-6.31,;26.94,-5.54,;28.28,-7.85,;26.93,-7.07,)| Show InChI InChI=1S/C30H37F2N5O2S/c1-6-25(38)36-12-11-30(17-36)14-20(15-30)37-22-8-7-19(16-33-18(2)29(3,4)5)13-21(22)34-28(37)35-27(39)24-10-9-23(40-24)26(31)32/h6-10,13,18,20,26,33H,1,11-12,14-17H2,2-5H3,(H,34,35,39)/t18-,20-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458594
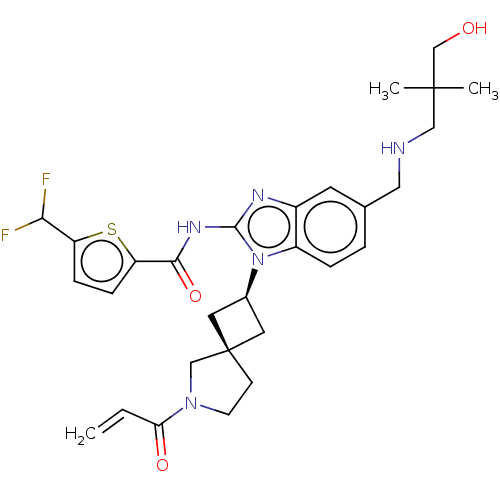
(CHEMBL4202941 | US10752615, Compound 37)Show SMILES CC(C)(CO)CNCc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)C(F)F)nc2c1 |r,wU:15.17,13.12,(31.69,-7.25,;30.92,-5.92,;30.15,-7.24,;29.6,-5.15,;28.26,-5.92,;32.26,-5.15,;33.6,-5.92,;34.93,-5.15,;36.26,-5.92,;36.26,-7.46,;37.6,-8.23,;38.94,-7.46,;40.42,-7.93,;40.9,-9.39,;42.27,-10.08,;41.58,-11.46,;40.2,-10.77,;43.08,-11.79,;43.23,-13.33,;41.82,-13.94,;40.8,-12.79,;41.49,-15.45,;42.63,-16.49,;40.03,-15.92,;39.7,-17.42,;41.32,-6.67,;42.86,-6.66,;43.62,-5.33,;42.84,-4,;45.16,-5.31,;46.05,-4.06,;47.51,-4.52,;47.52,-6.05,;46.07,-6.54,;48.77,-6.95,;50.17,-6.31,;48.62,-8.48,;40.4,-5.42,;38.93,-5.91,;37.59,-5.15,)| Show InChI InChI=1S/C29H35F2N5O3S/c1-4-24(38)35-10-9-29(16-35)12-19(13-29)36-21-6-5-18(14-32-15-28(2,3)17-37)11-20(21)33-27(36)34-26(39)23-8-7-22(40-23)25(30)31/h4-8,11,19,25,32,37H,1,9-10,12-17H2,2-3H3,(H,33,34,39)/t19-,29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50458594

(CHEMBL4202941 | US10752615, Compound 37)Show SMILES CC(C)(CO)CNCc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)C(F)F)nc2c1 |r,wU:15.17,13.12,(31.69,-7.25,;30.92,-5.92,;30.15,-7.24,;29.6,-5.15,;28.26,-5.92,;32.26,-5.15,;33.6,-5.92,;34.93,-5.15,;36.26,-5.92,;36.26,-7.46,;37.6,-8.23,;38.94,-7.46,;40.42,-7.93,;40.9,-9.39,;42.27,-10.08,;41.58,-11.46,;40.2,-10.77,;43.08,-11.79,;43.23,-13.33,;41.82,-13.94,;40.8,-12.79,;41.49,-15.45,;42.63,-16.49,;40.03,-15.92,;39.7,-17.42,;41.32,-6.67,;42.86,-6.66,;43.62,-5.33,;42.84,-4,;45.16,-5.31,;46.05,-4.06,;47.51,-4.52,;47.52,-6.05,;46.07,-6.54,;48.77,-6.95,;50.17,-6.31,;48.62,-8.48,;40.4,-5.42,;38.93,-5.91,;37.59,-5.15,)| Show InChI InChI=1S/C29H35F2N5O3S/c1-4-24(38)35-10-9-29(16-35)12-19(13-29)36-21-6-5-18(14-32-15-28(2,3)17-37)11-20(21)33-27(36)34-26(39)23-8-7-22(40-23)25(30)31/h4-8,11,19,25,32,37H,1,9-10,12-17H2,2-3H3,(H,33,34,39)/t19-,29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM458248
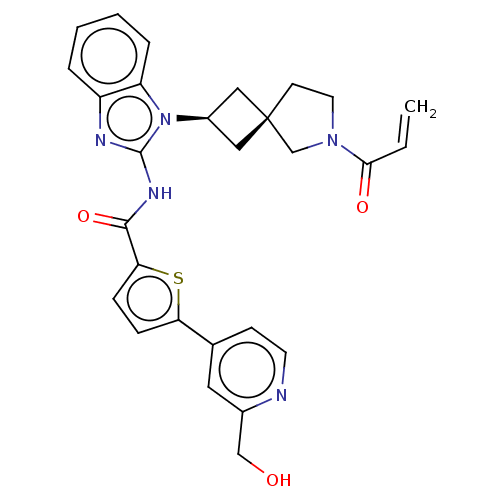
(US10752615, Compound 146)Show SMILES OCc1cc(ccn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:25.28,27.31,(8.96,3.81,;7.63,4.58,;6.3,3.81,;4.96,4.58,;3.63,3.81,;3.63,2.27,;4.96,1.5,;6.3,2.27,;2.29,4.58,;2.29,6.12,;.83,6.6,;-.08,5.35,;.83,4.11,;-1.62,5.35,;-2.39,6.68,;-2.39,4.02,;-3.93,4.02,;-4.83,5.26,;-6.3,4.79,;-7.63,5.56,;-8.96,4.79,;-8.96,3.25,;-7.63,2.48,;-6.3,3.25,;-4.83,2.77,;-4.43,1.28,;-3.1,.51,;-3.87,-.82,;-5.2,-.05,;-2.4,-1.3,;-2.4,-2.84,;-3.87,-3.31,;-4.77,-2.07,;-4.27,-4.8,;-3.18,-5.89,;-5.75,-5.2,;-6.15,-6.68,)| Show InChI InChI=1S/C28H27N5O3S/c1-2-25(35)32-12-10-28(17-32)14-20(15-28)33-22-6-4-3-5-21(22)30-27(33)31-26(36)24-8-7-23(37-24)18-9-11-29-19(13-18)16-34/h2-9,11,13,20,34H,1,10,12,14-17H2,(H,30,31,36)/t20-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































