 Found 312 hits of ic50 data for polymerid = 5624
Found 312 hits of ic50 data for polymerid = 5624 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458238
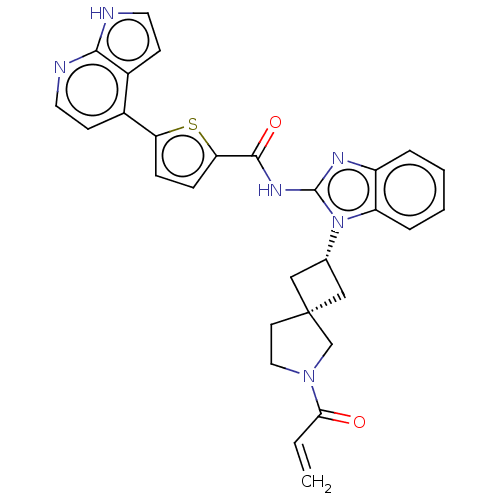
(US10752615, Compound 136)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3ccnc4[nH]ccc34)nc3ccccc23)C1 |r,wU:9.11,7.10,(-5.39,-6.68,;-4.99,-5.2,;-3.51,-4.8,;-2.42,-5.89,;-3.11,-3.31,;-1.64,-2.84,;-1.64,-1.3,;-3.11,-.82,;-2.34,.51,;-3.67,1.28,;-4.44,-.05,;-4.07,2.77,;-3.16,4.02,;-1.62,4.02,;-.85,5.35,;-1.62,6.68,;.69,5.35,;1.59,6.6,;3.06,6.12,;3.06,4.58,;1.59,4.11,;4.39,3.81,;4.39,2.27,;5.72,1.5,;7.06,2.27,;7.06,3.81,;8.2,4.84,;7.57,6.25,;6.04,6.09,;5.72,4.58,;-4.07,5.26,;-5.53,4.79,;-6.87,5.56,;-8.2,4.79,;-8.2,3.25,;-6.87,2.48,;-5.53,3.25,;-4.01,-2.07,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458600
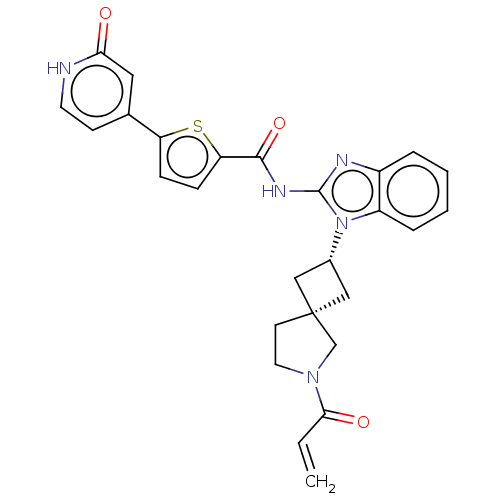
(CHEMBL4206765 | US10752615, Compound 97)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3cc[nH]c(=O)c3)nc3ccccc23)C1 |r,wU:7.6,9.11,(11.17,-39.5,;11.5,-38,;12.96,-37.53,;14.1,-38.57,;13.29,-36.02,;14.7,-35.41,;14.55,-33.87,;13.05,-33.54,;13.74,-32.16,;12.37,-31.47,;11.68,-32.85,;11.89,-30.01,;12.79,-28.75,;14.33,-28.74,;15.09,-27.41,;14.32,-26.08,;16.63,-27.39,;17.52,-26.14,;18.98,-26.6,;18.99,-28.14,;17.54,-28.62,;20.24,-29.02,;21.64,-28.38,;22.89,-29.29,;22.74,-30.82,;21.33,-31.45,;21.18,-32.99,;20.08,-30.56,;11.87,-27.5,;10.4,-27.99,;9.06,-27.23,;7.74,-28,;7.73,-29.54,;9.07,-30.31,;10.41,-29.54,;12.27,-34.87,)| Show InChI InChI=1S/C27H25N5O3S/c1-2-24(34)31-12-10-27(16-31)14-18(15-27)32-20-6-4-3-5-19(20)29-26(32)30-25(35)22-8-7-21(36-22)17-9-11-28-23(33)13-17/h2-9,11,13,18H,1,10,12,14-16H2,(H,28,33)(H,29,30,35)/t18-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458600

(CHEMBL4206765 | US10752615, Compound 97)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3cc[nH]c(=O)c3)nc3ccccc23)C1 |r,wU:7.6,9.11,(11.17,-39.5,;11.5,-38,;12.96,-37.53,;14.1,-38.57,;13.29,-36.02,;14.7,-35.41,;14.55,-33.87,;13.05,-33.54,;13.74,-32.16,;12.37,-31.47,;11.68,-32.85,;11.89,-30.01,;12.79,-28.75,;14.33,-28.74,;15.09,-27.41,;14.32,-26.08,;16.63,-27.39,;17.52,-26.14,;18.98,-26.6,;18.99,-28.14,;17.54,-28.62,;20.24,-29.02,;21.64,-28.38,;22.89,-29.29,;22.74,-30.82,;21.33,-31.45,;21.18,-32.99,;20.08,-30.56,;11.87,-27.5,;10.4,-27.99,;9.06,-27.23,;7.74,-28,;7.73,-29.54,;9.07,-30.31,;10.41,-29.54,;12.27,-34.87,)| Show InChI InChI=1S/C27H25N5O3S/c1-2-24(34)31-12-10-27(16-31)14-18(15-27)32-20-6-4-3-5-19(20)29-26(32)30-25(35)22-8-7-21(36-22)17-9-11-28-23(33)13-17/h2-9,11,13,18H,1,10,12,14-16H2,(H,28,33)(H,29,30,35)/t18-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458601
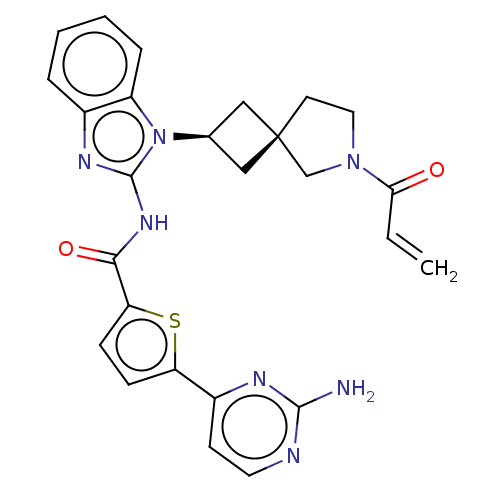
(CHEMBL4217959 | US10752615, Compound 138)Show SMILES Nc1nccc(n1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:26.32,24.27,(69.86,-5.85,;68.46,-6.5,;68.32,-8.04,;66.91,-8.67,;65.66,-7.78,;65.81,-6.25,;67.21,-5.61,;64.56,-5.35,;64.55,-3.82,;63.09,-3.36,;62.2,-4.61,;63.11,-5.84,;60.66,-4.63,;59.89,-3.3,;59.9,-5.96,;58.36,-5.97,;57.44,-4.72,;55.97,-5.21,;54.63,-4.45,;53.31,-5.22,;53.3,-6.76,;54.64,-7.53,;55.98,-6.76,;57.46,-7.23,;57.94,-8.69,;59.31,-9.38,;58.62,-10.76,;57.24,-10.07,;60.12,-11.09,;60.27,-12.63,;58.86,-13.24,;57.84,-12.09,;58.53,-14.75,;59.67,-15.79,;57.07,-15.22,;56.74,-16.72,)| Show InChI InChI=1S/C26H25N7O2S/c1-2-22(34)32-12-10-26(15-32)13-16(14-26)33-19-6-4-3-5-17(19)30-25(33)31-23(35)21-8-7-20(36-21)18-9-11-28-24(27)29-18/h2-9,11,16H,1,10,12-15H2,(H2,27,28,29)(H,30,31,35)/t16-,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458601

(CHEMBL4217959 | US10752615, Compound 138)Show SMILES Nc1nccc(n1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:26.32,24.27,(69.86,-5.85,;68.46,-6.5,;68.32,-8.04,;66.91,-8.67,;65.66,-7.78,;65.81,-6.25,;67.21,-5.61,;64.56,-5.35,;64.55,-3.82,;63.09,-3.36,;62.2,-4.61,;63.11,-5.84,;60.66,-4.63,;59.89,-3.3,;59.9,-5.96,;58.36,-5.97,;57.44,-4.72,;55.97,-5.21,;54.63,-4.45,;53.31,-5.22,;53.3,-6.76,;54.64,-7.53,;55.98,-6.76,;57.46,-7.23,;57.94,-8.69,;59.31,-9.38,;58.62,-10.76,;57.24,-10.07,;60.12,-11.09,;60.27,-12.63,;58.86,-13.24,;57.84,-12.09,;58.53,-14.75,;59.67,-15.79,;57.07,-15.22,;56.74,-16.72,)| Show InChI InChI=1S/C26H25N7O2S/c1-2-22(34)32-12-10-26(15-32)13-16(14-26)33-19-6-4-3-5-17(19)30-25(33)31-23(35)21-8-7-20(36-21)18-9-11-28-24(27)29-18/h2-9,11,16H,1,10,12-15H2,(H2,27,28,29)(H,30,31,35)/t16-,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458604
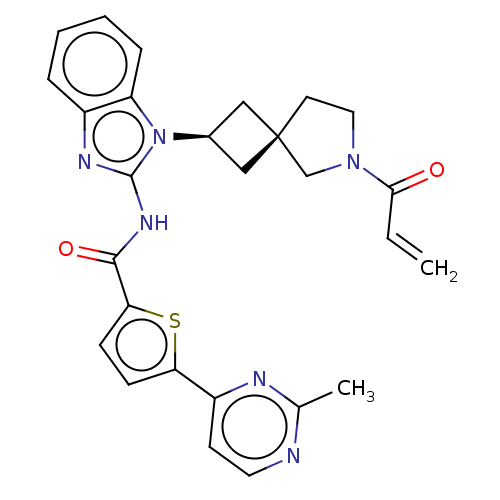
(CHEMBL4214683 | US10752615, Compound 149)Show SMILES Cc1nccc(n1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:26.32,24.27,(71.14,-29.4,;69.74,-30.04,;69.6,-31.58,;68.2,-32.22,;66.94,-31.32,;67.1,-29.79,;68.49,-29.15,;65.84,-28.9,;65.83,-27.36,;64.37,-26.9,;63.48,-28.16,;64.39,-29.38,;61.95,-28.17,;61.17,-26.84,;61.18,-29.51,;59.64,-29.52,;58.73,-28.27,;57.25,-28.76,;55.92,-27.99,;54.59,-28.76,;54.59,-30.31,;55.92,-31.08,;57.26,-30.31,;58.74,-30.78,;59.22,-32.24,;60.6,-32.93,;59.9,-34.31,;58.53,-33.61,;61.4,-34.64,;61.56,-36.17,;60.15,-36.79,;59.12,-35.64,;59.82,-38.29,;60.96,-39.33,;58.35,-38.76,;58.02,-40.26,)| Show InChI InChI=1S/C27H26N6O2S/c1-3-24(34)32-13-11-27(16-32)14-18(15-27)33-21-7-5-4-6-19(21)30-26(33)31-25(35)23-9-8-22(36-23)20-10-12-28-17(2)29-20/h3-10,12,18H,1,11,13-16H2,2H3,(H,30,31,35)/t18-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458604

(CHEMBL4214683 | US10752615, Compound 149)Show SMILES Cc1nccc(n1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:26.32,24.27,(71.14,-29.4,;69.74,-30.04,;69.6,-31.58,;68.2,-32.22,;66.94,-31.32,;67.1,-29.79,;68.49,-29.15,;65.84,-28.9,;65.83,-27.36,;64.37,-26.9,;63.48,-28.16,;64.39,-29.38,;61.95,-28.17,;61.17,-26.84,;61.18,-29.51,;59.64,-29.52,;58.73,-28.27,;57.25,-28.76,;55.92,-27.99,;54.59,-28.76,;54.59,-30.31,;55.92,-31.08,;57.26,-30.31,;58.74,-30.78,;59.22,-32.24,;60.6,-32.93,;59.9,-34.31,;58.53,-33.61,;61.4,-34.64,;61.56,-36.17,;60.15,-36.79,;59.12,-35.64,;59.82,-38.29,;60.96,-39.33,;58.35,-38.76,;58.02,-40.26,)| Show InChI InChI=1S/C27H26N6O2S/c1-3-24(34)32-13-11-27(16-32)14-18(15-27)33-21-7-5-4-6-19(21)30-26(33)31-25(35)23-9-8-22(36-23)20-10-12-28-17(2)29-20/h3-10,12,18H,1,11,13-16H2,2H3,(H,30,31,35)/t18-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458249
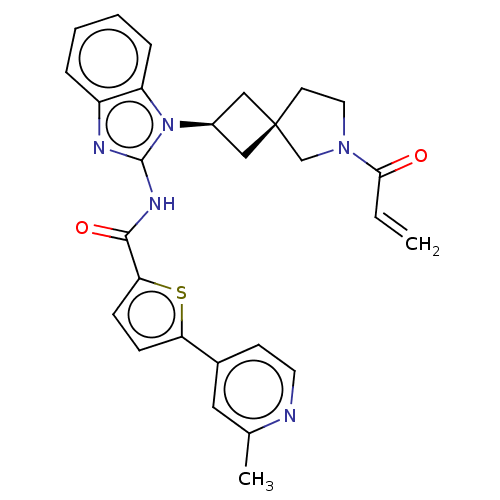
(US10752615, Compound 147)Show SMILES Cc1cc(ccn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:24.27,26.30,(8.3,4.58,;6.96,3.81,;5.63,4.58,;4.29,3.81,;4.29,2.27,;5.63,1.5,;6.96,2.27,;2.96,4.58,;2.96,6.12,;1.5,6.6,;.59,5.35,;1.5,4.11,;-.95,5.35,;-1.72,6.68,;-1.72,4.02,;-3.26,4.02,;-4.16,5.26,;-5.63,4.79,;-6.96,5.56,;-8.3,4.79,;-8.3,3.25,;-6.96,2.48,;-5.63,3.25,;-4.16,2.77,;-3.77,1.28,;-2.43,.51,;-3.2,-.82,;-4.54,-.05,;-1.74,-1.3,;-1.74,-2.84,;-3.2,-3.31,;-4.11,-2.07,;-3.6,-4.8,;-2.51,-5.89,;-5.09,-5.2,;-5.49,-6.68,)| Show InChI InChI=1S/C28H27N5O2S/c1-3-25(34)32-13-11-28(17-32)15-20(16-28)33-22-7-5-4-6-21(22)30-27(33)31-26(35)24-9-8-23(36-24)19-10-12-29-18(2)14-19/h3-10,12,14,20H,1,11,13,15-17H2,2H3,(H,30,31,35)/t20-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458200
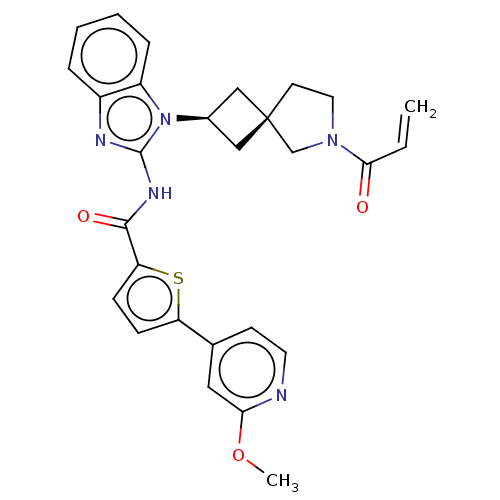
(US10752615, Compound 96)Show SMILES COc1cc(ccn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:25.28,27.31,(7.62,-.83,;6.29,-.06,;6.3,1.48,;4.95,2.27,;4.95,3.81,;6.28,4.58,;7.64,3.81,;7.64,2.26,;3.62,4.58,;3.62,6.12,;2.15,6.6,;1.25,5.35,;2.15,4.11,;-.29,5.35,;-1.06,6.68,;-1.06,4.02,;-2.6,4.02,;-3.51,5.26,;-4.97,4.79,;-6.31,5.56,;-7.64,4.79,;-7.64,3.25,;-6.31,2.48,;-4.97,3.25,;-3.51,2.77,;-3.11,1.28,;-1.78,.51,;-2.55,-.82,;-3.88,-.05,;-1.08,-1.3,;-1.08,-2.84,;-2.55,-3.31,;-3.45,-2.07,;-2.94,-4.8,;-1.86,-5.89,;-4.43,-5.2,;-4.83,-6.68,)| Show InChI InChI=1S/C28H27N5O3S/c1-3-25(34)32-13-11-28(17-32)15-19(16-28)33-21-7-5-4-6-20(21)30-27(33)31-26(35)23-9-8-22(37-23)18-10-12-29-24(14-18)36-2/h3-10,12,14,19H,1,11,13,15-17H2,2H3,(H,30,31,35)/t19-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458588
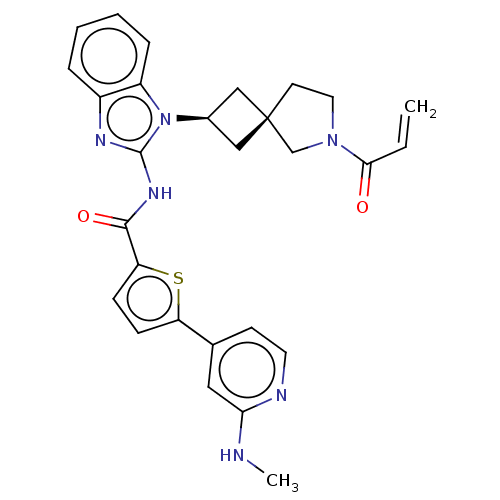
(CHEMBL4219011 | US10752615, Compound 131)Show SMILES CNc1cc(ccn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:27.33,25.28,(47.22,-29.18,;45.97,-28.29,;44.57,-28.94,;43.31,-28.05,;41.92,-28.68,;41.77,-30.22,;43.02,-31.11,;44.42,-30.48,;40.67,-27.79,;40.66,-26.26,;39.19,-25.8,;38.3,-27.05,;39.21,-28.28,;36.77,-27.07,;35.99,-25.74,;36.01,-28.4,;34.47,-28.41,;33.55,-27.16,;32.08,-27.65,;30.74,-26.89,;29.41,-27.66,;29.41,-29.2,;30.74,-29.97,;32.09,-29.2,;33.56,-29.67,;34.04,-31.13,;35.42,-31.82,;34.72,-33.2,;33.35,-32.51,;36.23,-33.53,;36.38,-35.06,;34.97,-35.68,;33.95,-34.53,;34.64,-37.19,;35.78,-38.22,;33.17,-37.66,;32.85,-39.16,)| Show InChI InChI=1S/C28H28N6O2S/c1-3-25(35)33-13-11-28(17-33)15-19(16-28)34-21-7-5-4-6-20(21)31-27(34)32-26(36)23-9-8-22(37-23)18-10-12-30-24(14-18)29-2/h3-10,12,14,19H,1,11,13,15-17H2,2H3,(H,29,30)(H,31,32,36)/t19-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458588

(CHEMBL4219011 | US10752615, Compound 131)Show SMILES CNc1cc(ccn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:27.33,25.28,(47.22,-29.18,;45.97,-28.29,;44.57,-28.94,;43.31,-28.05,;41.92,-28.68,;41.77,-30.22,;43.02,-31.11,;44.42,-30.48,;40.67,-27.79,;40.66,-26.26,;39.19,-25.8,;38.3,-27.05,;39.21,-28.28,;36.77,-27.07,;35.99,-25.74,;36.01,-28.4,;34.47,-28.41,;33.55,-27.16,;32.08,-27.65,;30.74,-26.89,;29.41,-27.66,;29.41,-29.2,;30.74,-29.97,;32.09,-29.2,;33.56,-29.67,;34.04,-31.13,;35.42,-31.82,;34.72,-33.2,;33.35,-32.51,;36.23,-33.53,;36.38,-35.06,;34.97,-35.68,;33.95,-34.53,;34.64,-37.19,;35.78,-38.22,;33.17,-37.66,;32.85,-39.16,)| Show InChI InChI=1S/C28H28N6O2S/c1-3-25(35)33-13-11-28(17-33)15-19(16-28)34-21-7-5-4-6-20(21)31-27(34)32-26(36)23-9-8-22(37-23)18-10-12-30-24(14-18)29-2/h3-10,12,14,19H,1,11,13,15-17H2,2H3,(H,29,30)(H,31,32,36)/t19-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458248
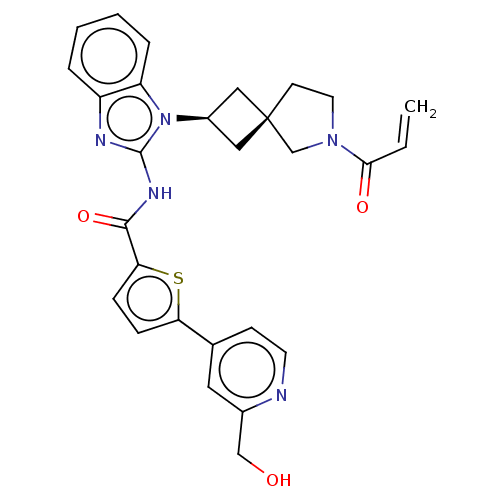
(US10752615, Compound 146)Show SMILES OCc1cc(ccn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:25.28,27.31,(8.96,3.81,;7.63,4.58,;6.3,3.81,;4.96,4.58,;3.63,3.81,;3.63,2.27,;4.96,1.5,;6.3,2.27,;2.29,4.58,;2.29,6.12,;.83,6.6,;-.08,5.35,;.83,4.11,;-1.62,5.35,;-2.39,6.68,;-2.39,4.02,;-3.93,4.02,;-4.83,5.26,;-6.3,4.79,;-7.63,5.56,;-8.96,4.79,;-8.96,3.25,;-7.63,2.48,;-6.3,3.25,;-4.83,2.77,;-4.43,1.28,;-3.1,.51,;-3.87,-.82,;-5.2,-.05,;-2.4,-1.3,;-2.4,-2.84,;-3.87,-3.31,;-4.77,-2.07,;-4.27,-4.8,;-3.18,-5.89,;-5.75,-5.2,;-6.15,-6.68,)| Show InChI InChI=1S/C28H27N5O3S/c1-2-25(35)32-12-10-28(17-32)14-20(15-28)33-22-6-4-3-5-21(22)30-27(33)31-26(36)24-8-7-23(37-24)18-9-11-29-19(13-18)16-34/h2-9,11,13,20,34H,1,10,12,14-17H2,(H,30,31,36)/t20-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458247
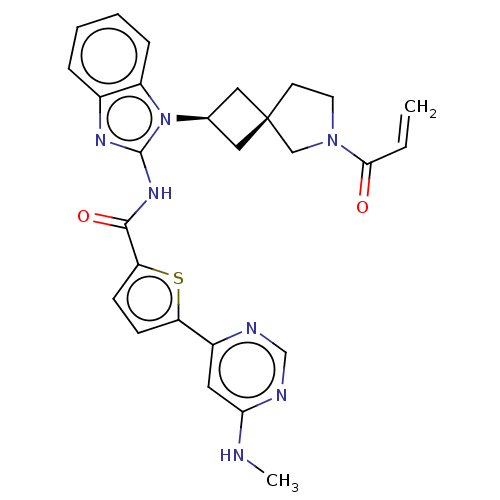
(US10752615, Compound 145)Show SMILES CNc1cc(ncn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:25.28,27.31,(8.96,3.81,;7.63,4.58,;6.3,3.81,;4.96,4.58,;3.63,3.81,;3.63,2.27,;4.96,1.5,;6.3,2.27,;2.29,4.58,;2.29,6.12,;.83,6.6,;-.08,5.35,;.83,4.11,;-1.62,5.35,;-2.39,6.68,;-2.39,4.02,;-3.93,4.02,;-4.83,5.26,;-6.3,4.79,;-7.63,5.56,;-8.96,4.79,;-8.96,3.25,;-7.63,2.48,;-6.3,3.25,;-4.83,2.77,;-4.43,1.28,;-3.1,.51,;-3.87,-.82,;-5.2,-.05,;-2.4,-1.3,;-2.4,-2.84,;-3.87,-3.31,;-4.77,-2.07,;-4.27,-4.8,;-3.18,-5.89,;-5.75,-5.2,;-6.15,-6.68,)| Show InChI InChI=1S/C27H27N7O2S/c1-3-24(35)33-11-10-27(15-33)13-17(14-27)34-20-7-5-4-6-18(20)31-26(34)32-25(36)22-9-8-21(37-22)19-12-23(28-2)30-16-29-19/h3-9,12,16-17H,1,10-11,13-15H2,2H3,(H,28,29,30)(H,31,32,36)/t17-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458242
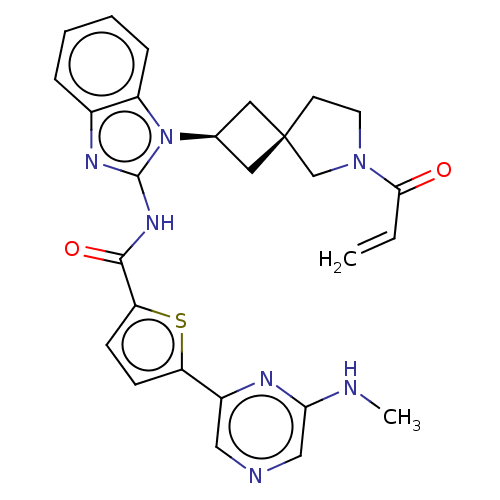
(US10752615, Compound 140)Show SMILES CNc1cncc(n1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:25.28,27.31,(8.96,3.81,;7.63,4.58,;6.3,3.81,;6.3,2.27,;4.96,1.5,;3.63,2.27,;3.63,3.81,;4.96,4.58,;2.29,4.58,;2.29,6.12,;.83,6.6,;-.08,5.35,;.83,4.11,;-1.62,5.35,;-2.39,6.68,;-2.39,4.02,;-3.93,4.02,;-4.83,5.26,;-6.3,4.79,;-7.63,5.56,;-8.96,4.79,;-8.96,3.25,;-7.63,2.48,;-6.3,3.25,;-4.83,2.77,;-4.43,1.28,;-3.1,.51,;-3.87,-.82,;-5.2,-.05,;-2.4,-1.3,;-2.4,-2.84,;-3.87,-3.31,;-4.77,-2.07,;-4.27,-4.8,;-3.18,-5.89,;-5.75,-5.2,;-6.15,-6.68,)| Show InChI InChI=1S/C27H27N7O2S/c1-3-24(35)33-11-10-27(16-33)12-17(13-27)34-20-7-5-4-6-18(20)31-26(34)32-25(36)22-9-8-21(37-22)19-14-29-15-23(28-2)30-19/h3-9,14-15,17H,1,10-13,16H2,2H3,(H,28,30)(H,31,32,36)/t17-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458213
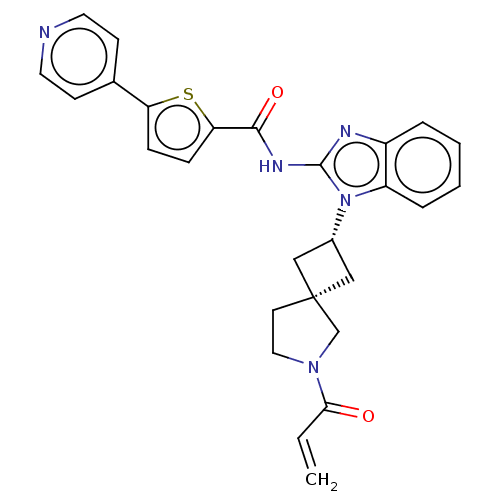
(US10752615, Compound 110)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3ccncc3)nc3ccccc23)C1 |r,wU:9.11,7.10,(-4.82,-6.68,;-4.42,-5.2,;-2.93,-4.8,;-1.84,-5.89,;-2.53,-3.31,;-1.07,-2.84,;-1.07,-1.3,;-2.53,-.82,;-1.76,.51,;-3.1,1.28,;-3.87,-.05,;-3.5,2.77,;-2.59,4.02,;-1.05,4.02,;-.28,5.35,;-1.05,6.68,;1.26,5.35,;2.16,6.6,;3.63,6.12,;3.63,4.58,;2.16,4.11,;4.96,3.81,;4.96,2.27,;6.3,1.5,;7.63,2.27,;7.63,3.81,;6.3,4.58,;-3.5,5.26,;-4.96,4.79,;-6.3,5.56,;-7.63,4.79,;-7.63,3.25,;-6.3,2.48,;-4.96,3.25,;-3.44,-2.07,)| Show InChI InChI=1S/C27H25N5O2S/c1-2-24(33)31-14-11-27(17-31)15-19(16-27)32-21-6-4-3-5-20(21)29-26(32)30-25(34)23-8-7-22(35-23)18-9-12-28-13-10-18/h2-10,12-13,19H,1,11,14-17H2,(H,29,30,34)/t19-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458230
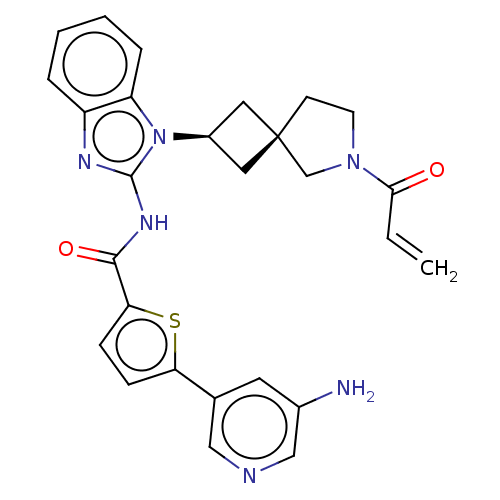
(US10752615, Compound 128)Show SMILES Nc1cncc(c1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:24.27,26.30,(8.3,4.58,;6.96,3.81,;6.96,2.27,;5.63,1.5,;4.29,2.27,;4.29,3.81,;5.63,4.58,;2.96,4.58,;2.96,6.12,;1.5,6.6,;.59,5.35,;1.5,4.11,;-.95,5.35,;-1.72,6.68,;-1.72,4.02,;-3.26,4.02,;-4.16,5.26,;-5.63,4.79,;-6.96,5.56,;-8.3,4.79,;-8.3,3.25,;-6.96,2.48,;-5.63,3.25,;-4.16,2.77,;-3.77,1.28,;-2.43,.51,;-3.2,-.82,;-4.54,-.05,;-1.74,-1.3,;-1.74,-2.84,;-3.2,-3.31,;-4.11,-2.07,;-3.6,-4.8,;-2.51,-5.89,;-5.09,-5.2,;-5.49,-6.68,)| Show InChI InChI=1S/C27H26N6O2S/c1-2-24(34)32-10-9-27(16-32)12-19(13-27)33-21-6-4-3-5-20(21)30-26(33)31-25(35)23-8-7-22(36-23)17-11-18(28)15-29-14-17/h2-8,11,14-15,19H,1,9-10,12-13,16,28H2,(H,30,31,35)/t19-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458587
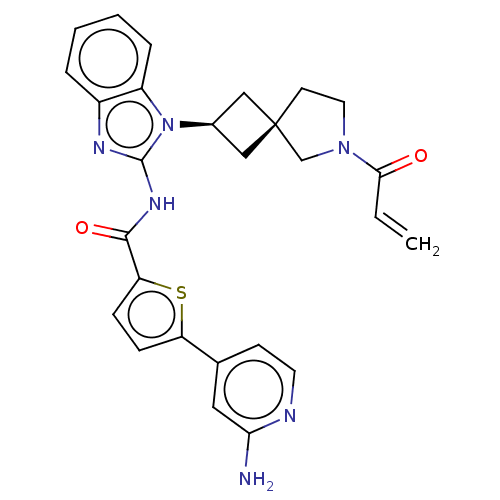
(CHEMBL4207292)Show SMILES Nc1cc(ccn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:26.32,24.27,(45.45,-6.32,;44.05,-6.96,;42.79,-6.07,;41.4,-6.71,;41.25,-8.25,;42.5,-9.14,;43.9,-8.5,;40.15,-5.82,;40.13,-4.29,;38.67,-3.83,;37.78,-5.08,;38.69,-6.31,;36.25,-5.09,;35.47,-3.76,;35.49,-6.43,;33.95,-6.44,;33.03,-5.19,;31.56,-5.68,;30.22,-4.91,;28.89,-5.69,;28.89,-7.23,;30.22,-8,;31.56,-7.23,;33.04,-7.7,;33.52,-9.16,;34.9,-9.85,;34.2,-11.23,;32.83,-10.54,;35.71,-11.56,;35.86,-13.09,;34.45,-13.71,;33.42,-12.56,;34.12,-15.22,;35.26,-16.25,;32.65,-15.68,;32.32,-17.19,)| Show InChI InChI=1S/C27H26N6O2S/c1-2-24(34)32-12-10-27(16-32)14-18(15-27)33-20-6-4-3-5-19(20)30-26(33)31-25(35)22-8-7-21(36-22)17-9-11-29-23(28)13-17/h2-9,11,13,18H,1,10,12,14-16H2,(H2,28,29)(H,30,31,35)/t18-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458216
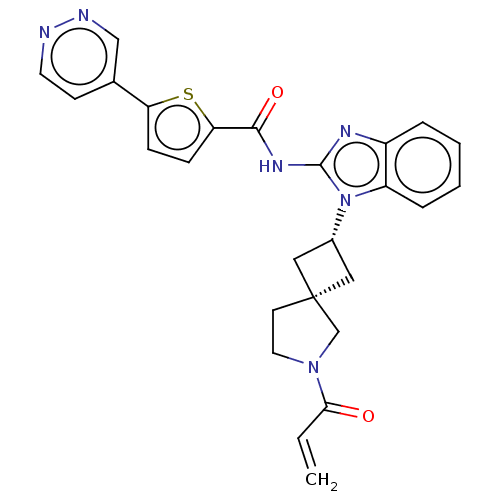
(US10752615, Compound 114)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3ccnnc3)nc3ccccc23)C1 |r,wU:9.11,7.10,(-4.82,-6.68,;-4.42,-5.2,;-2.93,-4.8,;-1.84,-5.89,;-2.53,-3.31,;-1.07,-2.84,;-1.07,-1.3,;-2.53,-.82,;-1.76,.51,;-3.1,1.28,;-3.87,-.05,;-3.5,2.77,;-2.59,4.02,;-1.05,4.02,;-.28,5.35,;-1.05,6.68,;1.26,5.35,;2.16,6.6,;3.63,6.12,;3.63,4.58,;2.16,4.11,;4.96,3.81,;4.96,2.27,;6.3,1.5,;7.63,2.27,;7.63,3.81,;6.3,4.58,;-3.5,5.26,;-4.96,4.79,;-6.3,5.56,;-7.63,4.79,;-7.63,3.25,;-6.3,2.48,;-4.96,3.25,;-3.44,-2.07,)| Show InChI InChI=1S/C26H24N6O2S/c1-2-23(33)31-12-10-26(16-31)13-18(14-26)32-20-6-4-3-5-19(20)29-25(32)30-24(34)22-8-7-21(35-22)17-9-11-27-28-15-17/h2-9,11,15,18H,1,10,12-14,16H2,(H,29,30,34)/t18-,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458227
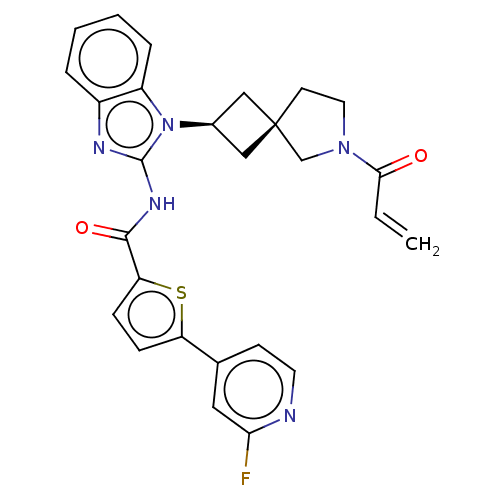
(US10752615, Compound 125)Show SMILES Fc1cc(ccn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:24.27,26.30,(6.3,-.04,;6.3,1.5,;4.96,2.27,;4.96,3.81,;6.3,4.58,;7.63,3.81,;7.63,2.27,;3.63,4.58,;3.63,6.12,;2.16,6.6,;1.26,5.35,;2.16,4.11,;-.28,5.35,;-1.05,6.68,;-1.05,4.02,;-2.59,4.02,;-3.5,5.26,;-4.96,4.79,;-6.3,5.56,;-7.63,4.79,;-7.63,3.25,;-6.3,2.48,;-4.96,3.25,;-3.5,2.77,;-3.1,1.28,;-1.76,.51,;-2.53,-.82,;-3.87,-.05,;-1.07,-1.3,;-1.07,-2.84,;-2.53,-3.31,;-3.44,-2.07,;-2.93,-4.8,;-1.84,-5.89,;-4.42,-5.2,;-4.82,-6.68,)| Show InChI InChI=1S/C27H24FN5O2S/c1-2-24(34)32-12-10-27(16-32)14-18(15-27)33-20-6-4-3-5-19(20)30-26(33)31-25(35)22-8-7-21(36-22)17-9-11-29-23(28)13-17/h2-9,11,13,18H,1,10,12,14-16H2,(H,30,31,35)/t18-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458243
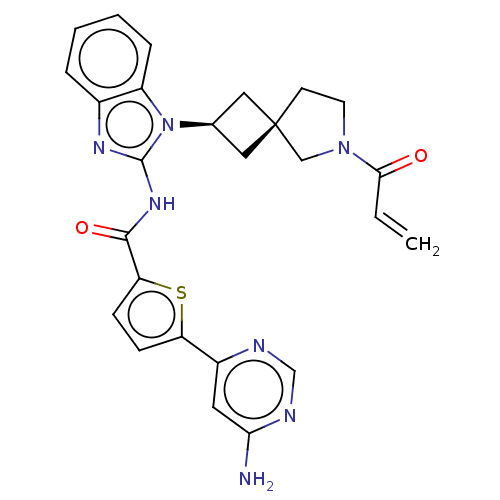
(US10752615, Compound 141)Show SMILES Nc1cc(ncn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:24.27,26.30,(8.3,4.58,;6.96,3.81,;5.63,4.58,;4.29,3.81,;4.29,2.27,;5.63,1.5,;6.96,2.27,;2.96,4.58,;2.96,6.12,;1.5,6.6,;.59,5.35,;1.5,4.11,;-.95,5.35,;-1.72,6.68,;-1.72,4.02,;-3.26,4.02,;-4.16,5.26,;-5.63,4.79,;-6.96,5.56,;-8.3,4.79,;-8.3,3.25,;-6.96,2.48,;-5.63,3.25,;-4.16,2.77,;-3.77,1.28,;-2.43,.51,;-3.2,-.82,;-4.54,-.05,;-1.74,-1.3,;-1.74,-2.84,;-3.2,-3.31,;-4.11,-2.07,;-3.6,-4.8,;-2.51,-5.89,;-5.09,-5.2,;-5.49,-6.68,)| Show InChI InChI=1S/C26H25N7O2S/c1-2-23(34)32-10-9-26(14-32)12-16(13-26)33-19-6-4-3-5-17(19)30-25(33)31-24(35)21-8-7-20(36-21)18-11-22(27)29-15-28-18/h2-8,11,15-16H,1,9-10,12-14H2,(H2,27,28,29)(H,30,31,35)/t16-,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458235
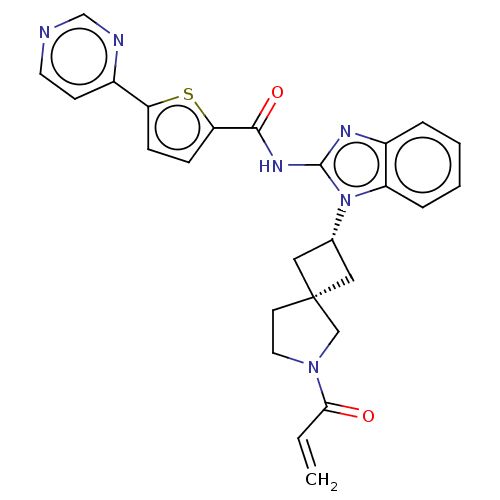
(US10752615, Compound 133)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3ccncn3)nc3ccccc23)C1 |r,wU:9.11,7.10,(-4.82,-6.68,;-4.42,-5.2,;-2.93,-4.8,;-1.84,-5.89,;-2.53,-3.31,;-1.07,-2.84,;-1.07,-1.3,;-2.53,-.82,;-1.76,.51,;-3.1,1.28,;-3.87,-.05,;-3.5,2.77,;-2.59,4.02,;-1.05,4.02,;-.28,5.35,;-1.05,6.68,;1.26,5.35,;2.16,6.6,;3.63,6.12,;3.63,4.58,;2.16,4.11,;4.96,3.81,;4.96,2.27,;6.3,1.5,;7.63,2.27,;7.63,3.81,;6.3,4.58,;-3.5,5.26,;-4.96,4.79,;-6.3,5.56,;-7.63,4.79,;-7.63,3.25,;-6.3,2.48,;-4.96,3.25,;-3.44,-2.07,)| Show InChI InChI=1S/C26H24N6O2S/c1-2-23(33)31-12-10-26(15-31)13-17(14-26)32-20-6-4-3-5-18(20)29-25(32)30-24(34)22-8-7-21(35-22)19-9-11-27-16-28-19/h2-9,11,16-17H,1,10,12-15H2,(H,29,30,34)/t17-,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458244
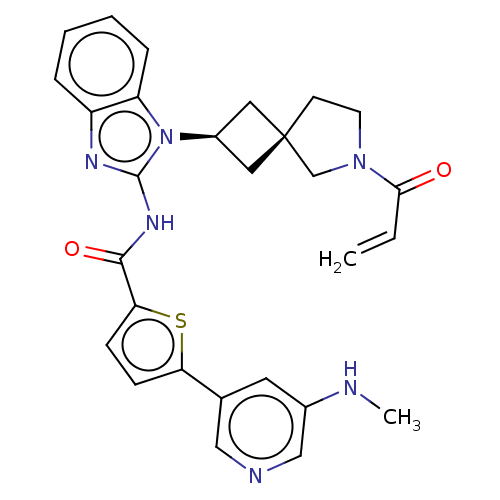
(US10752615, Compound 142)Show SMILES CNc1cncc(c1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:25.28,27.31,(8.96,3.81,;7.63,4.58,;6.3,3.81,;6.3,2.27,;4.96,1.5,;3.63,2.27,;3.63,3.81,;4.96,4.58,;2.29,4.58,;2.29,6.12,;.83,6.6,;-.08,5.35,;.83,4.11,;-1.62,5.35,;-2.39,6.68,;-2.39,4.02,;-3.93,4.02,;-4.83,5.26,;-6.3,4.79,;-7.63,5.56,;-8.96,4.79,;-8.96,3.25,;-7.63,2.48,;-6.3,3.25,;-4.83,2.77,;-4.43,1.28,;-3.1,.51,;-3.87,-.82,;-5.2,-.05,;-2.4,-1.3,;-2.4,-2.84,;-3.87,-3.31,;-4.77,-2.07,;-4.27,-4.8,;-3.18,-5.89,;-5.75,-5.2,;-6.15,-6.68,)| Show InChI InChI=1S/C28H28N6O2S/c1-3-25(35)33-11-10-28(17-33)13-20(14-28)34-22-7-5-4-6-21(22)31-27(34)32-26(36)24-9-8-23(37-24)18-12-19(29-2)16-30-15-18/h3-9,12,15-16,20,29H,1,10-11,13-14,17H2,2H3,(H,31,32,36)/t20-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458239
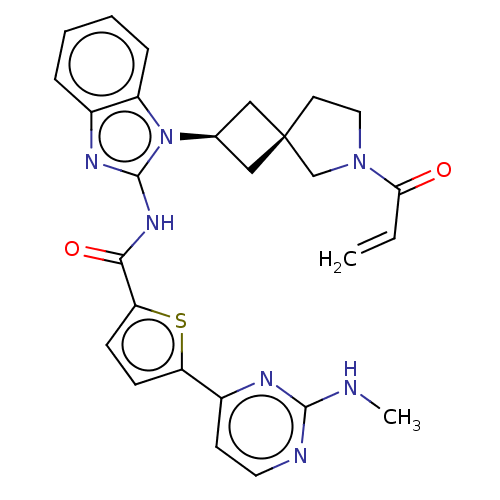
(US10752615, Compound 137)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:25.28,27.31,(8.96,3.81,;7.63,4.58,;6.3,3.81,;6.3,2.27,;4.96,1.5,;3.63,2.27,;3.63,3.81,;4.96,4.58,;2.29,4.58,;2.29,6.12,;.83,6.6,;-.08,5.35,;.83,4.11,;-1.62,5.35,;-2.39,6.68,;-2.39,4.02,;-3.93,4.02,;-4.83,5.26,;-6.3,4.79,;-7.63,5.56,;-8.96,4.79,;-8.96,3.25,;-7.63,2.48,;-6.3,3.25,;-4.83,2.77,;-4.43,1.28,;-3.1,.51,;-3.87,-.82,;-5.2,-.05,;-2.4,-1.3,;-2.4,-2.84,;-3.87,-3.31,;-4.77,-2.07,;-4.27,-4.8,;-3.18,-5.89,;-5.75,-5.2,;-6.15,-6.68,)| Show InChI InChI=1S/C27H27N7O2S/c1-3-23(35)33-13-11-27(16-33)14-17(15-27)34-20-7-5-4-6-18(20)31-26(34)32-24(36)22-9-8-21(37-22)19-10-12-29-25(28-2)30-19/h3-10,12,17H,1,11,13-16H2,2H3,(H,28,29,30)(H,31,32,36)/t17-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458241
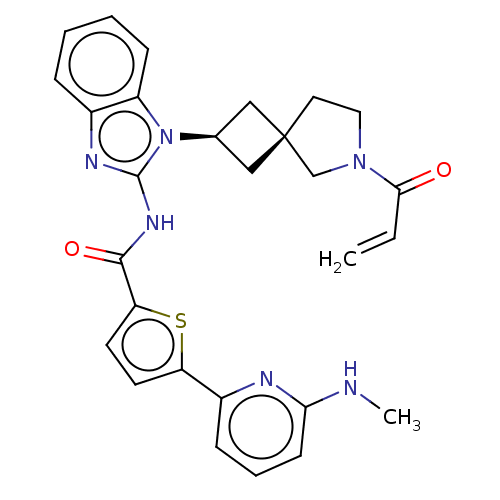
(US10752615, Compound 139)Show SMILES CNc1cccc(n1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:25.28,27.31,(8.96,3.81,;7.63,4.58,;6.3,3.81,;6.3,2.27,;4.96,1.5,;3.63,2.27,;3.63,3.81,;4.96,4.58,;2.29,4.58,;2.29,6.12,;.83,6.6,;-.08,5.35,;.83,4.11,;-1.62,5.35,;-2.39,6.68,;-2.39,4.02,;-3.93,4.02,;-4.83,5.26,;-6.3,4.79,;-7.63,5.56,;-8.96,4.79,;-8.96,3.25,;-7.63,2.48,;-6.3,3.25,;-4.83,2.77,;-4.43,1.28,;-3.1,.51,;-3.87,-.82,;-5.2,-.05,;-2.4,-1.3,;-2.4,-2.84,;-3.87,-3.31,;-4.77,-2.07,;-4.27,-4.8,;-3.18,-5.89,;-5.75,-5.2,;-6.15,-6.68,)| Show InChI InChI=1S/C28H28N6O2S/c1-3-25(35)33-14-13-28(17-33)15-18(16-28)34-21-9-5-4-7-19(21)31-27(34)32-26(36)23-12-11-22(37-23)20-8-6-10-24(29-2)30-20/h3-12,18H,1,13-17H2,2H3,(H,29,30)(H,31,32,36)/t18-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TEC using Poly(Glu, Tyr) 4:1 as substrate after 1 hr by ELISA |
J Med Chem 61: 4608-4627 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00441
BindingDB Entry DOI: 10.7270/Q2B85BRC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50589186
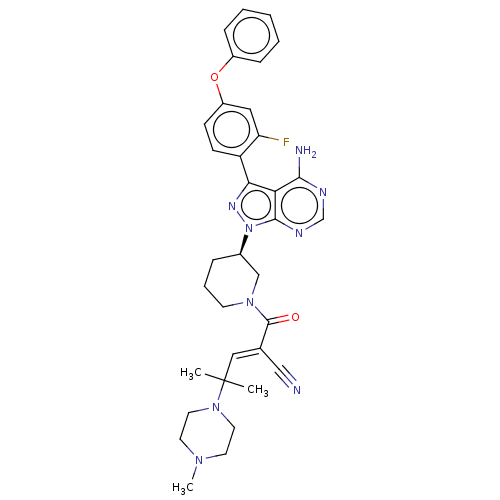
(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) preincubated for 1 hr followed by Biotin-AVLESEEELYSSARQ-NH2 substrate addition in presence of ATP and measured af... |
J Med Chem 62: 7923-7940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00687
BindingDB Entry DOI: 10.7270/Q2RJ4NXF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) preincubated for 1 hr followed by Biotin-AVLESEEELYSSARQ-NH2 substrate addition in presence of ATP and measured af... |
J Med Chem 62: 7923-7940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00687
BindingDB Entry DOI: 10.7270/Q2RJ4NXF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458252
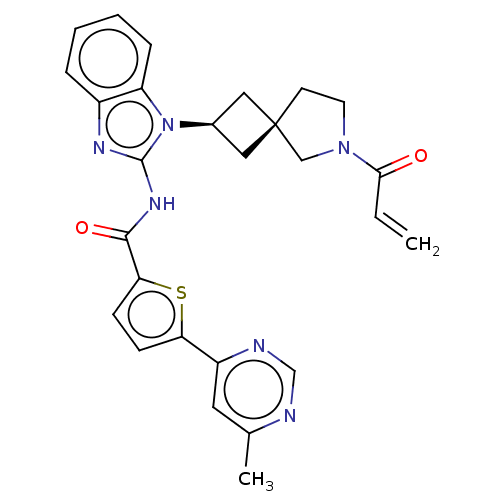
(US10752615, Compound 150)Show SMILES Cc1cc(ncn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:24.27,26.30,(6.3,-.04,;6.3,1.5,;4.96,2.27,;4.96,3.81,;6.3,4.58,;7.63,3.81,;7.63,2.27,;3.63,4.58,;3.63,6.12,;2.16,6.6,;1.26,5.35,;2.16,4.11,;-.28,5.35,;-1.05,6.68,;-1.05,4.02,;-2.59,4.02,;-3.5,5.26,;-4.96,4.79,;-6.3,5.56,;-7.63,4.79,;-7.63,3.25,;-6.3,2.48,;-4.96,3.25,;-3.5,2.77,;-3.1,1.28,;-1.76,.51,;-2.53,-.82,;-3.87,-.05,;-1.07,-1.3,;-1.07,-2.84,;-2.53,-3.31,;-3.44,-2.07,;-2.93,-4.8,;-1.84,-5.89,;-4.42,-5.2,;-4.82,-6.68,)| Show InChI InChI=1S/C27H26N6O2S/c1-3-24(34)32-11-10-27(15-32)13-18(14-27)33-21-7-5-4-6-19(21)30-26(33)31-25(35)23-9-8-22(36-23)20-12-17(2)28-16-29-20/h3-9,12,16,18H,1,10-11,13-15H2,2H3,(H,30,31,35)/t18-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458592
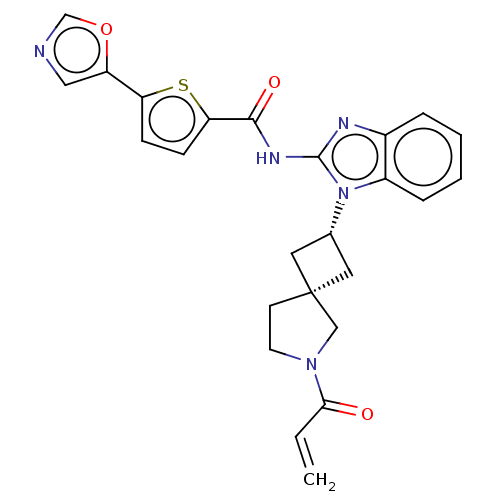
(CHEMBL4211372 | US10752615, Compound 16)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3cnco3)nc3ccccc23)C1 |r,wU:7.6,9.11,(34.53,-42.18,;34.86,-40.68,;36.33,-40.21,;37.47,-41.25,;36.66,-38.71,;38.07,-38.09,;37.92,-36.56,;36.41,-36.23,;37.11,-34.85,;35.73,-34.16,;35.04,-35.53,;35.25,-32.7,;36.16,-31.44,;37.7,-31.43,;38.46,-30.09,;37.68,-28.76,;39.99,-30.08,;40.89,-28.83,;42.36,-29.29,;42.37,-30.83,;40.9,-31.32,;43.62,-31.73,;45.08,-31.25,;45.99,-32.49,;45.09,-33.74,;43.63,-33.27,;35.24,-30.19,;33.76,-30.68,;32.43,-29.91,;31.1,-30.68,;31.1,-32.23,;32.43,-33,;33.77,-32.23,;35.63,-37.56,)| Show InChI InChI=1S/C25H23N5O3S/c1-2-22(31)29-10-9-25(14-29)11-16(12-25)30-18-6-4-3-5-17(18)27-24(30)28-23(32)21-8-7-20(34-21)19-13-26-15-33-19/h2-8,13,15-16H,1,9-12,14H2,(H,27,28,32)/t16-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458592

(CHEMBL4211372 | US10752615, Compound 16)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3cnco3)nc3ccccc23)C1 |r,wU:7.6,9.11,(34.53,-42.18,;34.86,-40.68,;36.33,-40.21,;37.47,-41.25,;36.66,-38.71,;38.07,-38.09,;37.92,-36.56,;36.41,-36.23,;37.11,-34.85,;35.73,-34.16,;35.04,-35.53,;35.25,-32.7,;36.16,-31.44,;37.7,-31.43,;38.46,-30.09,;37.68,-28.76,;39.99,-30.08,;40.89,-28.83,;42.36,-29.29,;42.37,-30.83,;40.9,-31.32,;43.62,-31.73,;45.08,-31.25,;45.99,-32.49,;45.09,-33.74,;43.63,-33.27,;35.24,-30.19,;33.76,-30.68,;32.43,-29.91,;31.1,-30.68,;31.1,-32.23,;32.43,-33,;33.77,-32.23,;35.63,-37.56,)| Show InChI InChI=1S/C25H23N5O3S/c1-2-22(31)29-10-9-25(14-29)11-16(12-25)30-18-6-4-3-5-17(18)27-24(30)28-23(32)21-8-7-20(34-21)19-13-26-15-33-19/h2-8,13,15-16H,1,9-12,14H2,(H,27,28,32)/t16-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458194
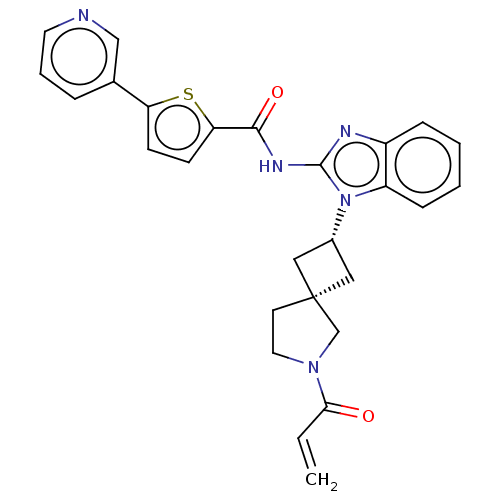
(US10752615, Compound 90)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3cccnc3)nc3ccccc23)C1 |r,wU:9.11,7.10,(-4.82,-6.68,;-4.42,-5.2,;-2.93,-4.8,;-1.84,-5.89,;-2.53,-3.31,;-1.07,-2.84,;-1.07,-1.3,;-2.53,-.82,;-1.76,.51,;-3.1,1.28,;-3.87,-.05,;-3.5,2.77,;-2.59,4.02,;-1.05,4.02,;-.28,5.35,;-1.05,6.68,;1.26,5.35,;2.16,6.6,;3.63,6.12,;3.63,4.58,;2.16,4.11,;4.96,3.81,;4.96,2.27,;6.3,1.5,;7.63,2.27,;7.63,3.81,;6.3,4.58,;-3.5,5.26,;-4.96,4.79,;-6.3,5.56,;-7.63,4.79,;-7.63,3.25,;-6.3,2.48,;-4.96,3.25,;-3.44,-2.07,)| Show InChI InChI=1S/C27H25N5O2S/c1-2-24(33)31-13-11-27(17-31)14-19(15-27)32-21-8-4-3-7-20(21)29-26(32)30-25(34)23-10-9-22(35-23)18-6-5-12-28-16-18/h2-10,12,16,19H,1,11,13-15,17H2,(H,29,30,34)/t19-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458596
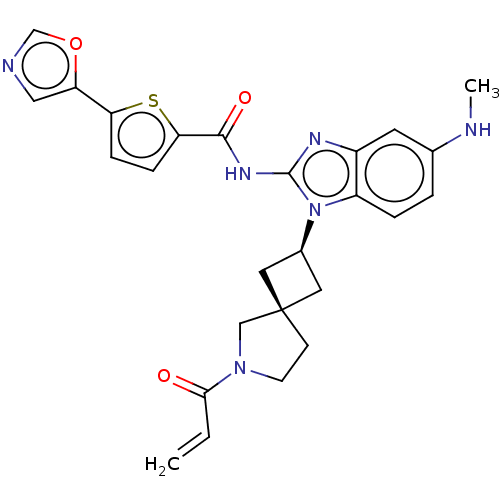
(CHEMBL4216187 | US10752615, Compound 50)Show SMILES CNc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:9.11,7.6,(58.28,-26.3,;59.61,-25.53,;60.95,-26.3,;60.94,-27.84,;62.28,-28.61,;63.62,-27.84,;65.1,-28.31,;65.58,-29.77,;66.95,-30.46,;66.26,-31.84,;64.89,-31.15,;67.76,-32.17,;67.91,-33.7,;66.5,-34.32,;65.48,-33.17,;66.18,-35.83,;67.31,-36.87,;64.71,-36.3,;64.38,-37.8,;66,-27.05,;67.54,-27.04,;68.3,-25.71,;67.53,-24.38,;69.84,-25.69,;70.73,-24.44,;72.19,-24.9,;72.2,-26.43,;70.75,-26.92,;73.45,-27.32,;74.91,-26.84,;75.82,-28.07,;74.93,-29.32,;73.47,-28.86,;65.08,-25.8,;63.61,-26.29,;62.28,-25.53,)| Show InChI InChI=1S/C26H26N6O3S/c1-3-23(33)31-9-8-26(14-31)11-17(12-26)32-19-5-4-16(27-2)10-18(19)29-25(32)30-24(34)22-7-6-21(36-22)20-13-28-15-35-20/h3-7,10,13,15,17,27H,1,8-9,11-12,14H2,2H3,(H,29,30,34)/t17-,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458596

(CHEMBL4216187 | US10752615, Compound 50)Show SMILES CNc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:9.11,7.6,(58.28,-26.3,;59.61,-25.53,;60.95,-26.3,;60.94,-27.84,;62.28,-28.61,;63.62,-27.84,;65.1,-28.31,;65.58,-29.77,;66.95,-30.46,;66.26,-31.84,;64.89,-31.15,;67.76,-32.17,;67.91,-33.7,;66.5,-34.32,;65.48,-33.17,;66.18,-35.83,;67.31,-36.87,;64.71,-36.3,;64.38,-37.8,;66,-27.05,;67.54,-27.04,;68.3,-25.71,;67.53,-24.38,;69.84,-25.69,;70.73,-24.44,;72.19,-24.9,;72.2,-26.43,;70.75,-26.92,;73.45,-27.32,;74.91,-26.84,;75.82,-28.07,;74.93,-29.32,;73.47,-28.86,;65.08,-25.8,;63.61,-26.29,;62.28,-25.53,)| Show InChI InChI=1S/C26H26N6O3S/c1-3-23(33)31-9-8-26(14-31)11-17(12-26)32-19-5-4-16(27-2)10-18(19)29-25(32)30-24(34)22-7-6-21(36-22)20-13-28-15-35-20/h3-7,10,13,15,17,27H,1,8-9,11-12,14H2,2H3,(H,29,30,34)/t17-,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458589
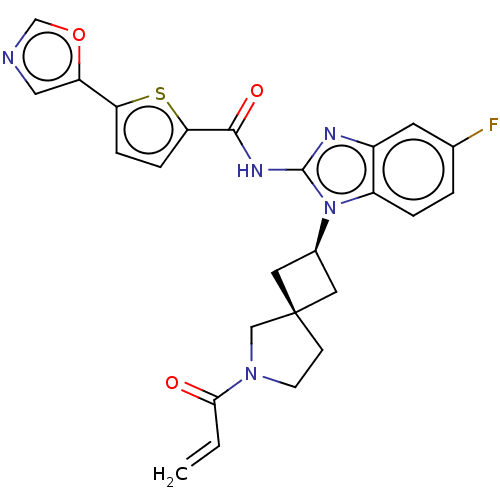
(CHEMBL4208358 | US10752615, Compound 57)Show SMILES Fc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:8.10,6.5,(6.64,-6.08,;7.97,-6.84,;7.97,-8.39,;9.3,-9.16,;10.64,-8.39,;12.12,-8.86,;12.6,-10.32,;13.98,-11.01,;13.28,-12.39,;11.91,-11.7,;14.79,-12.72,;14.94,-14.25,;13.53,-14.87,;12.5,-13.72,;13.2,-16.38,;14.34,-17.41,;11.73,-16.84,;11.4,-18.35,;13.03,-7.6,;14.57,-7.59,;15.33,-6.25,;14.55,-4.92,;16.86,-6.24,;17.75,-4.98,;19.21,-5.45,;19.23,-6.98,;17.77,-7.47,;20.47,-7.87,;21.93,-7.39,;22.84,-8.62,;21.95,-9.87,;20.49,-9.4,;12.11,-6.35,;10.63,-6.84,;9.3,-6.07,)| Show InChI InChI=1S/C25H22FN5O3S/c1-2-22(32)30-8-7-25(13-30)10-16(11-25)31-18-4-3-15(26)9-17(18)28-24(31)29-23(33)21-6-5-20(35-21)19-12-27-14-34-19/h2-6,9,12,14,16H,1,7-8,10-11,13H2,(H,28,29,33)/t16-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458589

(CHEMBL4208358 | US10752615, Compound 57)Show SMILES Fc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:8.10,6.5,(6.64,-6.08,;7.97,-6.84,;7.97,-8.39,;9.3,-9.16,;10.64,-8.39,;12.12,-8.86,;12.6,-10.32,;13.98,-11.01,;13.28,-12.39,;11.91,-11.7,;14.79,-12.72,;14.94,-14.25,;13.53,-14.87,;12.5,-13.72,;13.2,-16.38,;14.34,-17.41,;11.73,-16.84,;11.4,-18.35,;13.03,-7.6,;14.57,-7.59,;15.33,-6.25,;14.55,-4.92,;16.86,-6.24,;17.75,-4.98,;19.21,-5.45,;19.23,-6.98,;17.77,-7.47,;20.47,-7.87,;21.93,-7.39,;22.84,-8.62,;21.95,-9.87,;20.49,-9.4,;12.11,-6.35,;10.63,-6.84,;9.3,-6.07,)| Show InChI InChI=1S/C25H22FN5O3S/c1-2-22(32)30-8-7-25(13-30)10-16(11-25)31-18-4-3-15(26)9-17(18)28-24(31)29-23(33)21-6-5-20(35-21)19-12-27-14-34-19/h2-6,9,12,14,16H,1,7-8,10-11,13H2,(H,28,29,33)/t16-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
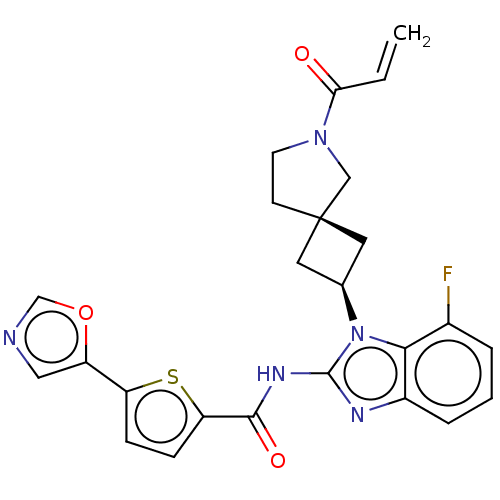
(Homo sapiens (Human)) | BDBM458164
(US10752615, Compound 56)Show SMILES Fc1cccc2nc(NC(=O)c3ccc(s3)-c3cnco3)n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c12 |r,wU:22.23,24.26,(-6.15,.94,;-6.15,2.48,;-7.48,3.25,;-7.48,4.79,;-6.15,5.56,;-4.81,4.79,;-3.35,5.26,;-2.44,4.02,;-.9,4.02,;-.13,5.35,;-.9,6.68,;1.41,5.35,;2.31,6.6,;3.78,6.12,;3.78,4.58,;2.31,4.11,;5.11,3.81,;6.58,4.29,;7.48,3.04,;6.58,1.8,;5.11,2.27,;-3.35,2.77,;-2.95,1.28,;-1.62,.51,;-2.39,-.82,;-3.72,-.05,;-.92,-1.3,;-.92,-2.84,;-2.39,-3.31,;-3.29,-2.07,;-2.78,-4.8,;-1.7,-5.89,;-4.27,-5.2,;-4.67,-6.68,;-4.81,3.25,)| Show InChI InChI=1S/C25H22FN5O3S/c1-2-21(32)30-9-8-25(13-30)10-15(11-25)31-22-16(26)4-3-5-17(22)28-24(31)29-23(33)20-7-6-19(35-20)18-12-27-14-34-18/h2-7,12,14-15H,1,8-11,13H2,(H,28,29,33)/t15-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50557485
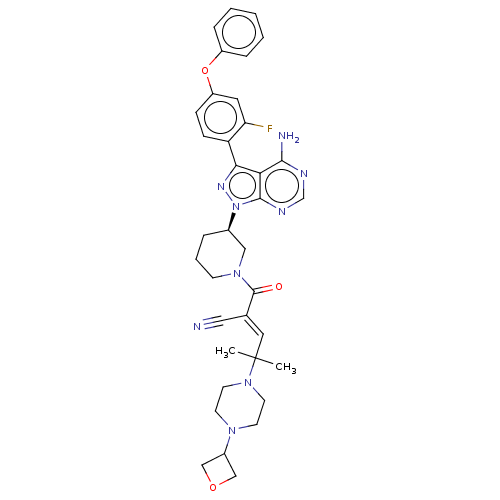
(Prn-1008 | Prn1008 | Rilzabrutinib)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCN(CC1)C1COC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458162
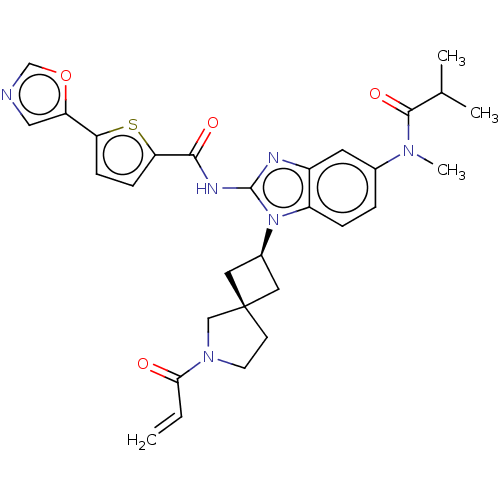
(US10752615, Compound 54)Show SMILES CC(C)C(=O)N(C)c1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:12.11,14.14,(-10.15,4.58,;-8.81,5.35,;-8.81,6.89,;-7.48,4.58,;-7.48,3.04,;-6.15,5.35,;-6.15,6.89,;-4.81,4.58,;-4.81,3.04,;-3.48,2.27,;-2.15,3.04,;-.68,2.57,;-.28,1.08,;1.05,.31,;.28,-1.03,;-1.05,-.26,;1.75,-1.5,;1.75,-3.04,;.28,-3.52,;-.62,-2.27,;-.12,-5.01,;.97,-6.09,;-1.6,-5.4,;-2,-6.89,;.22,3.81,;1.76,3.81,;2.53,5.14,;1.76,6.48,;4.07,5.14,;4.98,6.39,;6.44,5.91,;6.44,4.37,;4.98,3.9,;7.78,3.6,;9.24,4.08,;10.15,2.83,;9.24,1.59,;7.78,2.06,;-.68,5.06,;-2.15,4.58,;-3.48,5.35,)| Show InChI InChI=1S/C30H32N6O4S/c1-5-26(37)35-11-10-30(16-35)13-20(14-30)36-22-7-6-19(34(4)28(39)18(2)3)12-21(22)32-29(36)33-27(38)25-9-8-24(41-25)23-15-31-17-40-23/h5-9,12,15,17-18,20H,1,10-11,13-14,16H2,2-4H3,(H,32,33,38)/t20-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM165209
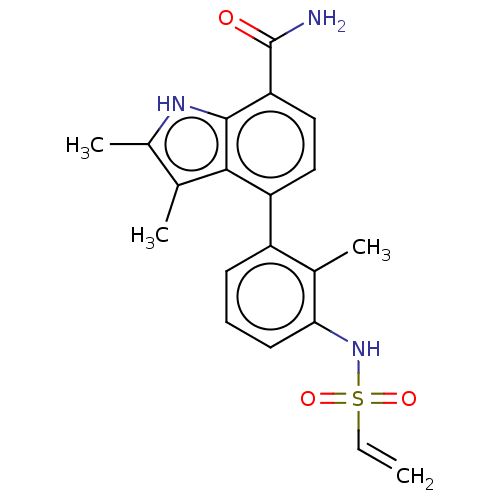
(US10604504, Example 34 | US11623921, Example 34 | ...)Show SMILES Cc1[nH]c2c(ccc(-c3cccc(NS(=O)(=O)C=C)c3C)c2c1C)C(N)=O Show InChI InChI=1S/C20H21N3O3S/c1-5-27(25,26)23-17-8-6-7-14(12(17)3)15-9-10-16(20(21)24)19-18(15)11(2)13(4)22-19/h5-10,22-23H,1H2,2-4H3,(H2,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) |
Bioorg Med Chem Lett 28: 3080-3084 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.041
BindingDB Entry DOI: 10.7270/Q2NZ8B9C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458593
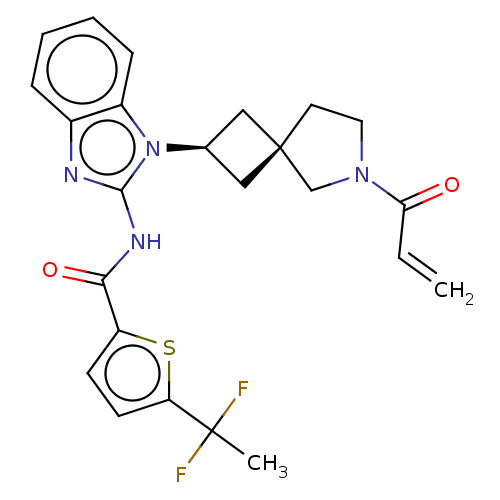
(CHEMBL4204572 | US10752615, Compound 30)Show SMILES CC(F)(F)c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:23.28,21.23,(21.07,-29.11,;21.21,-27.57,;21.97,-26.23,;22.75,-27.56,;19.96,-26.68,;19.95,-25.15,;18.49,-24.68,;17.6,-25.94,;18.51,-27.17,;16.07,-25.95,;15.29,-24.62,;15.3,-27.29,;13.76,-27.3,;12.84,-26.05,;11.37,-26.54,;10.04,-25.77,;8.71,-26.54,;8.71,-28.09,;10.04,-28.86,;11.38,-28.09,;12.86,-28.56,;13.34,-30.02,;14.72,-30.71,;14.02,-32.09,;12.65,-31.39,;15.52,-32.42,;15.68,-33.95,;14.27,-34.57,;13.24,-33.42,;13.94,-36.08,;15.08,-37.11,;12.47,-36.54,;12.14,-38.05,)| Show InChI InChI=1S/C24H24F2N4O2S/c1-3-20(31)29-11-10-24(14-29)12-15(13-24)30-17-7-5-4-6-16(17)27-22(30)28-21(32)18-8-9-19(33-18)23(2,25)26/h3-9,15H,1,10-14H2,2H3,(H,27,28,32)/t15-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) using fluorescently labeled peptide as substrate after 3 hrs by microfluidic mobility shift assay |
ACS Med Chem Lett 9: 587-589 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00178
BindingDB Entry DOI: 10.7270/Q22V2JQN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50458593

(CHEMBL4204572 | US10752615, Compound 30)Show SMILES CC(F)(F)c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:23.28,21.23,(21.07,-29.11,;21.21,-27.57,;21.97,-26.23,;22.75,-27.56,;19.96,-26.68,;19.95,-25.15,;18.49,-24.68,;17.6,-25.94,;18.51,-27.17,;16.07,-25.95,;15.29,-24.62,;15.3,-27.29,;13.76,-27.3,;12.84,-26.05,;11.37,-26.54,;10.04,-25.77,;8.71,-26.54,;8.71,-28.09,;10.04,-28.86,;11.38,-28.09,;12.86,-28.56,;13.34,-30.02,;14.72,-30.71,;14.02,-32.09,;12.65,-31.39,;15.52,-32.42,;15.68,-33.95,;14.27,-34.57,;13.24,-33.42,;13.94,-36.08,;15.08,-37.11,;12.47,-36.54,;12.14,-38.05,)| Show InChI InChI=1S/C24H24F2N4O2S/c1-3-20(31)29-11-10-24(14-29)12-15(13-24)30-17-7-5-4-6-16(17)27-22(30)28-21(32)18-8-9-19(33-18)23(2,25)26/h3-9,15H,1,10-14H2,2H3,(H,27,28,32)/t15-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458166
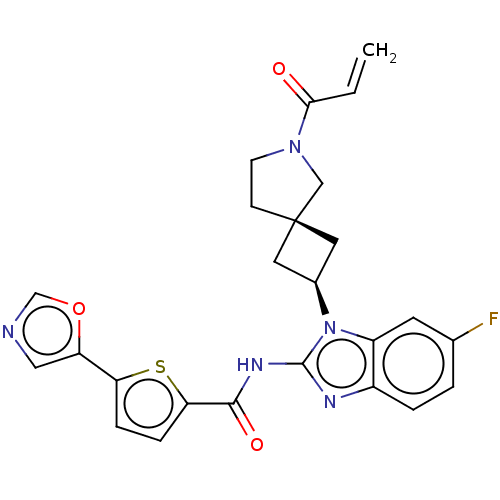
(US10752615, Compound 58)Show SMILES Fc1ccc2nc(NC(=O)c3ccc(s3)-c3cnco3)n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c2c1 |r,wU:21.22,23.25,(-8.15,2.48,;-6.81,3.25,;-6.81,4.79,;-5.48,5.56,;-4.15,4.79,;-2.68,5.26,;-1.78,4.02,;-.24,4.02,;.53,5.35,;-.24,6.68,;2.07,5.35,;2.98,6.6,;4.44,6.12,;4.44,4.58,;2.98,4.11,;5.78,3.81,;7.24,4.29,;8.15,3.04,;7.24,1.8,;5.78,2.27,;-2.68,2.77,;-2.28,1.28,;-.95,.51,;-1.72,-.82,;-3.05,-.05,;-.25,-1.3,;-.25,-2.84,;-1.72,-3.31,;-2.62,-2.07,;-2.12,-4.8,;-1.03,-5.89,;-3.61,-5.2,;-4,-6.68,;-4.15,3.25,;-5.48,2.48,)| Show InChI InChI=1S/C25H22FN5O3S/c1-2-22(32)30-8-7-25(13-30)10-16(11-25)31-18-9-15(26)3-4-17(18)28-24(31)29-23(33)21-6-5-20(35-21)19-12-27-14-34-19/h2-6,9,12,14,16H,1,7-8,10-11,13H2,(H,28,29,33)/t16-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
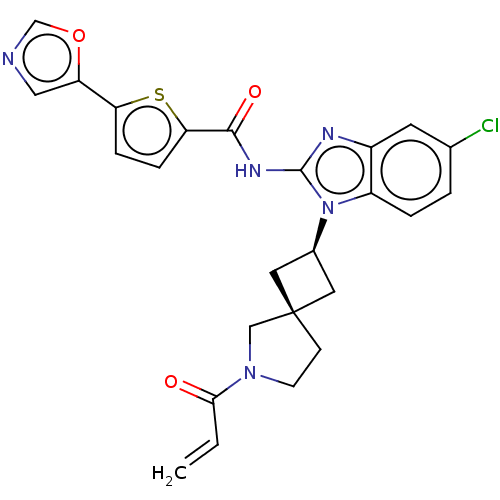
(Homo sapiens (Human)) | BDBM458171
(US10752615, Compound 63)Show SMILES Clc1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:6.5,8.8,(-8.15,5.56,;-6.81,4.79,;-6.81,3.25,;-5.48,2.48,;-4.15,3.25,;-2.68,2.77,;-2.28,1.28,;-.95,.51,;-1.72,-.82,;-3.05,-.05,;-.25,-1.3,;-.25,-2.84,;-1.72,-3.31,;-2.62,-2.07,;-2.12,-4.8,;-1.03,-5.89,;-3.61,-5.2,;-4,-6.68,;-1.78,4.02,;-.24,4.02,;.53,5.35,;-.24,6.68,;2.07,5.35,;2.98,6.6,;4.44,6.12,;4.44,4.58,;2.98,4.11,;5.78,3.81,;7.24,4.29,;8.15,3.04,;7.24,1.8,;5.78,2.27,;-2.68,5.26,;-4.15,4.79,;-5.48,5.56,)| Show InChI InChI=1S/C25H22ClN5O3S/c1-2-22(32)30-8-7-25(13-30)10-16(11-25)31-18-4-3-15(26)9-17(18)28-24(31)29-23(33)21-6-5-20(35-21)19-12-27-14-34-19/h2-6,9,12,14,16H,1,7-8,10-11,13H2,(H,28,29,33)/t16-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
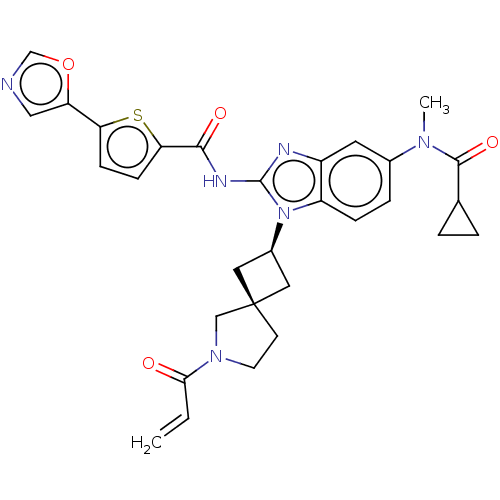
(Homo sapiens (Human)) | BDBM458160
(US10752615, Compound 52)Show SMILES CN(C(=O)C1CC1)c1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:12.12,14.15,(-6.04,6.89,;-6.04,5.35,;-7.38,4.58,;-7.38,3.04,;-8.71,5.35,;-9.48,6.68,;-10.25,5.35,;-4.71,4.58,;-4.71,3.04,;-3.38,2.27,;-2.04,3.04,;-.58,2.57,;-.18,1.08,;1.15,.31,;.38,-1.03,;-.95,-.26,;1.85,-1.5,;1.85,-3.04,;.38,-3.52,;-.52,-2.27,;-.01,-5.01,;1.07,-6.09,;-1.5,-5.4,;-1.9,-6.89,;.33,3.81,;1.87,3.81,;2.64,5.14,;1.87,6.48,;4.18,5.14,;5.08,6.39,;6.55,5.91,;6.55,4.37,;5.08,3.9,;7.88,3.6,;9.35,4.08,;10.25,2.83,;9.35,1.59,;7.88,2.06,;-.58,5.06,;-2.04,4.58,;-3.38,5.35,)| Show InChI InChI=1S/C30H30N6O4S/c1-3-26(37)35-11-10-30(16-35)13-20(14-30)36-22-7-6-19(34(2)28(39)18-4-5-18)12-21(22)32-29(36)33-27(38)25-9-8-24(41-25)23-15-31-17-40-23/h3,6-9,12,15,17-18,20H,1,4-5,10-11,13-14,16H2,2H3,(H,32,33,38)/t20-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
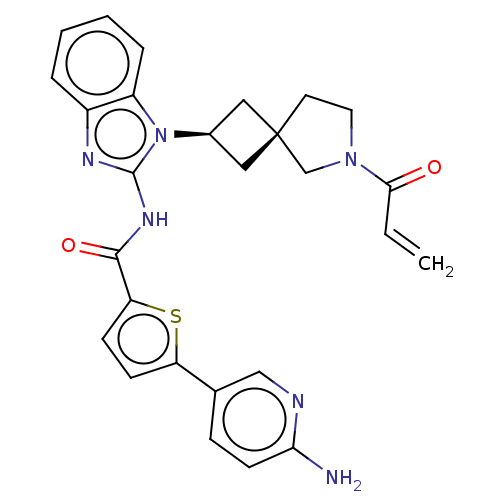
(Homo sapiens (Human)) | BDBM458139
(US10752615, Compound 31)Show SMILES Nc1ccc(cn1)-c1ccc(s1)C(=O)Nc1nc2ccccc2n1[C@H]1C[C@]2(C1)CCN(C2)C(=O)C=C |r,wU:24.27,26.30,(8.3,1.5,;6.96,2.27,;5.63,1.5,;4.29,2.27,;4.29,3.81,;5.63,4.58,;6.96,3.81,;2.96,4.58,;2.96,6.12,;1.5,6.6,;.59,5.35,;1.5,4.11,;-.95,5.35,;-1.72,6.68,;-1.72,4.02,;-3.26,4.02,;-4.16,5.26,;-5.63,4.79,;-6.96,5.56,;-8.3,4.79,;-8.3,3.25,;-6.96,2.48,;-5.63,3.25,;-4.16,2.77,;-3.77,1.28,;-2.43,.51,;-3.2,-.82,;-4.54,-.05,;-1.74,-1.3,;-1.74,-2.84,;-3.2,-3.31,;-4.11,-2.07,;-3.6,-4.8,;-2.51,-5.89,;-5.09,-5.2,;-5.49,-6.68,)| Show InChI InChI=1S/C27H26N6O2S/c1-2-24(34)32-12-11-27(16-32)13-18(14-27)33-20-6-4-3-5-19(20)30-26(33)31-25(35)22-9-8-21(36-22)17-7-10-23(28)29-15-17/h2-10,15,18H,1,11-14,16H2,(H2,28,29)(H,30,31,35)/t18-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
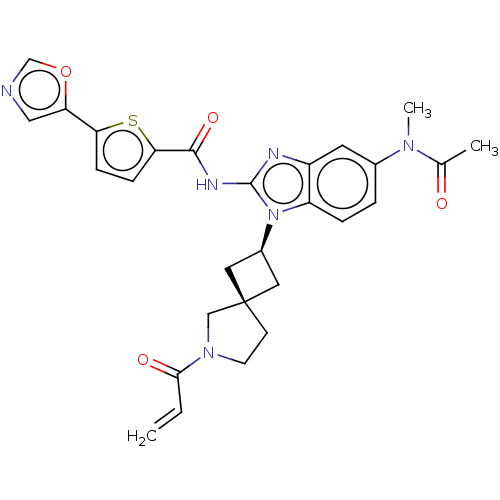
(Homo sapiens (Human)) | BDBM458161
(US10752615, Compound 53)Show SMILES CN(C(C)=O)c1ccc2n([C@H]3C[C@]4(C3)CCN(C4)C(=O)C=C)c(NC(=O)c3ccc(s3)-c3cnco3)nc2c1 |r,wU:10.9,12.12,(-6.81,6.89,;-6.81,5.35,;-8.15,4.58,;-9.48,5.35,;-8.15,3.04,;-5.48,4.58,;-5.48,3.04,;-4.15,2.27,;-2.81,3.04,;-1.35,2.57,;-.95,1.08,;.38,.31,;-.39,-1.03,;-1.72,-.26,;1.08,-1.5,;1.08,-3.04,;-.39,-3.52,;-1.29,-2.27,;-.78,-5.01,;.3,-6.09,;-2.27,-5.4,;-2.67,-6.89,;-.44,3.81,;1.1,3.81,;1.87,5.14,;1.1,6.48,;3.41,5.14,;4.31,6.39,;5.78,5.91,;5.78,4.37,;4.31,3.9,;7.11,3.6,;8.58,4.08,;9.48,2.83,;8.58,1.59,;7.11,2.06,;-1.35,5.06,;-2.81,4.58,;-4.15,5.35,)| Show InChI InChI=1S/C28H28N6O4S/c1-4-25(36)33-10-9-28(15-33)12-19(13-28)34-21-6-5-18(32(3)17(2)35)11-20(21)30-27(34)31-26(37)24-8-7-23(39-24)22-14-29-16-38-22/h4-8,11,14,16,19H,1,9-10,12-13,15H2,2-3H3,(H,30,31,37)/t19-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM458203
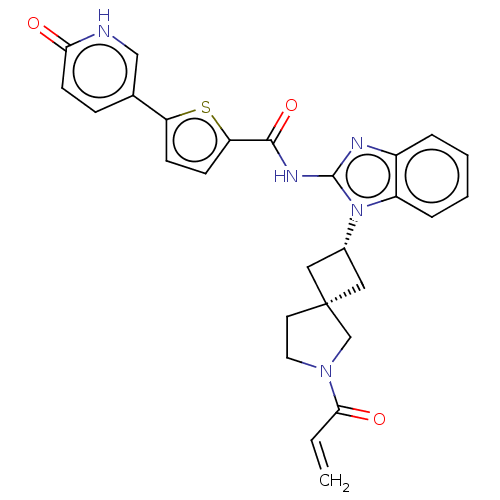
(US10752615, Compound 99)Show SMILES C=CC(=O)N1CC[C@@]2(C[C@@H](C2)n2c(NC(=O)c3ccc(s3)-c3ccc(=O)[nH]c3)nc3ccccc23)C1 |r,wU:9.11,7.10,(-5.49,-6.68,;-5.09,-5.2,;-3.6,-4.8,;-2.51,-5.89,;-3.2,-3.31,;-1.74,-2.84,;-1.74,-1.3,;-3.2,-.82,;-2.43,.51,;-3.77,1.28,;-4.54,-.05,;-4.16,2.77,;-3.26,4.02,;-1.72,4.02,;-.95,5.35,;-1.72,6.68,;.59,5.35,;1.5,6.6,;2.96,6.12,;2.96,4.58,;1.5,4.11,;4.29,3.81,;5.63,4.58,;6.96,3.81,;6.96,2.27,;8.3,1.5,;5.63,1.5,;4.29,2.27,;-4.16,5.26,;-5.63,4.79,;-6.96,5.56,;-8.3,4.79,;-8.3,3.25,;-6.96,2.48,;-5.63,3.25,;-4.11,-2.07,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.27 | n/a | n/a | n/a | n/a | n/a | n/a |
GB005, Inc.
US Patent
| Assay Description
The in vitro kinase assays were performed at Nanosyn (Santa Clara, Calif.) utilizing microfluidic detection technology. The test compounds were seria... |
US Patent US10752615 (2020)
BindingDB Entry DOI: 10.7270/Q2G163WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TEC (359 - 631 residues) expressed in baculovirus expression system by mobility shift assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































