 Found 13 hits of ki data for polymerid = 5642
Found 13 hits of ki data for polymerid = 5642 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM17290
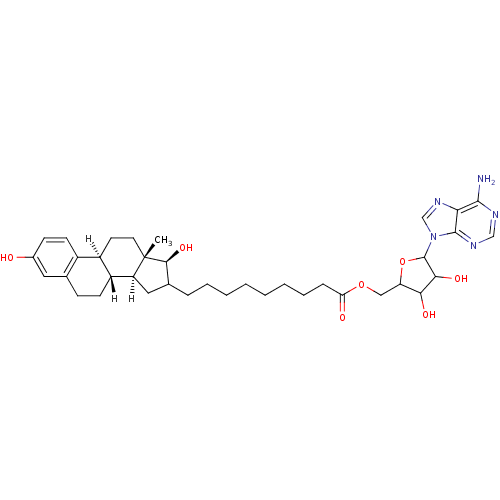
(E2-adenosine hybrid compound, 8 | EM-1745 | EM1745...)Show SMILES [H][C@@]12CC(CCCCCCCCC(=O)OCC3OC(C(O)C3O)n3cnc4c(N)ncnc34)[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C37H51N5O7/c1-37-15-14-25-24-13-11-23(43)16-21(24)10-12-26(25)27(37)17-22(33(37)47)8-6-4-2-3-5-7-9-29(44)48-18-28-31(45)32(46)36(49-28)42-20-41-30-34(38)39-19-40-35(30)42/h11,13,16,19-20,22,25-28,31-33,36,43,45-47H,2-10,12,14-15,17-18H2,1H3,(H2,38,39,40)/t22?,25-,26-,27+,28?,31?,32?,33+,36?,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | -12.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
CHUL
| Assay Description
For steady-state kinetic study of hybrid inhibitors, a Fluorolog 3 instrument was used to monitor the fluorescent signal of NADPH formed during estra... |
FASEB J 16: 1829-31 (2002)
Article DOI: 10.1096/fj.02-0026fje
BindingDB Entry DOI: 10.7270/Q23T9FGW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50179201
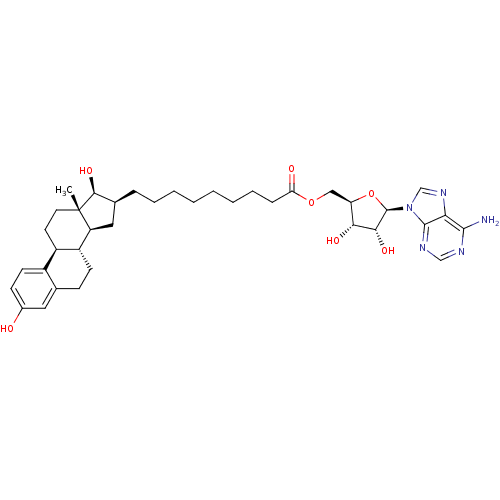
(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1C[C@H](CCCCCCCCC(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc3c(N)ncnc13)[C@@H]2O |r| Show InChI InChI=1S/C37H51N5O7/c1-37-15-14-25-24-13-11-23(43)16-21(24)10-12-26(25)27(37)17-22(33(37)47)8-6-4-2-3-5-7-9-29(44)48-18-28-31(45)32(46)36(49-28)42-20-41-30-34(38)39-19-40-35(30)42/h11,13,16,19-20,22,25-28,31-33,36,43,45-47H,2-10,12,14-15,17-18H2,1H3,(H2,38,39,40)/t22-,25+,26+,27-,28+,31+,32+,33-,36+,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol |
J Med Chem 51: 4188-99 (2008)
Article DOI: 10.1021/jm800054h
BindingDB Entry DOI: 10.7270/Q2Q81F0N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM17289

((1S,10R,11S,15S)-5-hydroxy-15-methyltetracyclo[8.7...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CHUQ - Pavillon CHUL and Université Laval
Curated by ChEMBL
| Assay Description
Inhibition of (unknown origin) 17beta-HSD1 assessed as conversion of [14C]estradiol to [14C]estrone using NADP+ |
Eur J Med Chem 43: 2298-306 (2008)
Article DOI: 10.1016/j.ejmech.2008.01.044
BindingDB Entry DOI: 10.7270/Q2P84BP6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50406414

(CHEMBL76061)Show InChI InChI=1S/C18H20O2/c1-3-17(20)18(2)9-8-16-13(11-18)5-4-12-10-14(19)6-7-15(12)16/h1,6-7,10,13,16,19H,4-5,8-9,11H2,2H3/t13?,16?,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Tested by protection experiments to demonstrate the inactivation of estradiol dehydrogenase and the kinetic parameter Ki app was reported at a concen... |
J Med Chem 33: 2319-21 (1990)
BindingDB Entry DOI: 10.7270/Q2765DB6 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50400841

(CHEMBL2205681)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1Cc1cn[nH]c21 |r| Show InChI InChI=1S/C19H22N2O/c1-19-7-6-15-14-5-3-13(22)8-11(14)2-4-16(15)17(19)9-12-10-20-21-18(12)19/h3,5,8,10,15-17,22H,2,4,6-7,9H2,1H3,(H,20,21)/t15-,16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human estradiol 17beta-dehydrogenase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM17289

((1S,10R,11S,15S)-5-hydroxy-15-methyltetracyclo[8.7...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human estradiol 17beta-dehydrogenase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50400840

(CHEMBL2205682)Show SMILES COc1ccc2[C@H]3CC[C@@]4(C)[C@@H](Cc5cn[nH]c45)[C@@H]3CCc2c1 |r| Show InChI InChI=1S/C20H24N2O/c1-20-8-7-16-15-6-4-14(23-2)9-12(15)3-5-17(16)18(20)10-13-11-21-22-19(13)20/h4,6,9,11,16-18H,3,5,7-8,10H2,1-2H3,(H,21,22)/t16-,17-,18+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human estradiol 17beta-dehydrogenase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM48630

((8R,9S,13S,14S)-3-methoxy-13-methyl-7,8,9,11,12,14...)Show SMILES COc1ccc2[C@H]3CC[C@@]4(C)[C@@H](CCC4=O)[C@@H]3CCc2c1 Show InChI InChI=1S/C19H24O2/c1-19-10-9-15-14-6-4-13(21-2)11-12(14)3-5-16(15)17(19)7-8-18(19)20/h4,6,11,15-17H,3,5,7-10H2,1-2H3/t15-,16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human estradiol 17beta-dehydrogenase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50015834

(CHEMBL307621 | Diastereomer-7-Methyl-7-(4,4,4-trif...)Show SMILES C[C@@]1(CCC2C(CCc3cc(O)ccc23)C1)C(O)C#CC(F)(F)F Show InChI InChI=1S/C19H21F3O2/c1-18(17(24)7-9-19(20,21)22)8-6-16-13(11-18)3-2-12-10-14(23)4-5-15(12)16/h4-5,10,13,16-17,23-24H,2-3,6,8,11H2,1H3/t13?,16?,17?,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Tested for time-dependent inactivation of the enzyme that followed pseudo- first- order kinetics in the absence of NAD+ and the Ki values were report... |
J Med Chem 33: 2319-21 (1990)
BindingDB Entry DOI: 10.7270/Q2765DB6 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50015835

(CHEMBL75893 | Diastereomer-7-Methyl-7-(4,4,4-trifl...)Show SMILES C[C@]1(CCC2C(CCc3cc(O)ccc23)C1)C(O)C#CC(F)(F)F Show InChI InChI=1S/C19H21F3O2/c1-18(17(24)7-9-19(20,21)22)8-6-16-13(11-18)3-2-12-10-14(23)4-5-15(12)16/h4-5,10,13,16-17,23-24H,2-3,6,8,11H2,1H3/t13?,16?,17?,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Tested for time-dependent inactivation of the enzyme that followed pseudo- first- order kinetics in the absence of NAD+ and the Ki values were report... |
J Med Chem 33: 2319-21 (1990)
BindingDB Entry DOI: 10.7270/Q2765DB6 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50400839

(CHEMBL2205683)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1Cc1cnoc21 |r| Show InChI InChI=1S/C19H21NO2/c1-19-7-6-15-14-5-3-13(21)8-11(14)2-4-16(15)17(19)9-12-10-20-22-18(12)19/h3,5,8,10,15-17,21H,2,4,6-7,9H2,1H3/t15-,16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human estradiol 17beta-dehydrogenase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM17293
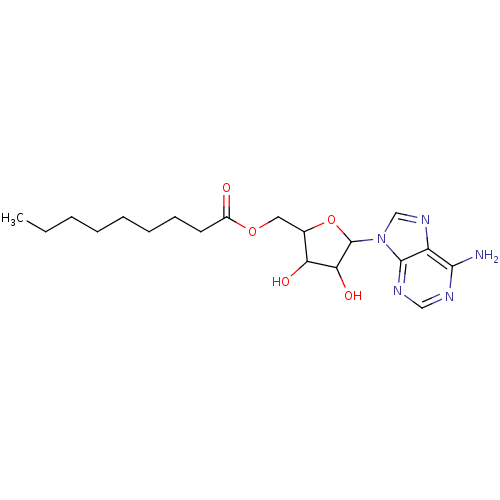
(Compound 10 | MB-329-131A2 | [5-(6-amino-9H-purin-...)Show SMILES CCCCCCCCC(=O)OCC1OC(C(O)C1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C19H29N5O5/c1-2-3-4-5-6-7-8-13(25)28-9-12-15(26)16(27)19(29-12)24-11-23-14-17(20)21-10-22-18(14)24/h10-12,15-16,19,26-27H,2-9H2,1H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+5 | -5.11 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
CHUL
| Assay Description
For steady-state kinetic study of hybrid inhibitors, a Fluorolog 3 instrument was used to monitor the fluorescent signal of NADPH formed during estra... |
FASEB J 16: 1829-31 (2002)
Article DOI: 10.1096/fj.02-0026fje
BindingDB Entry DOI: 10.7270/Q23T9FGW |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50400838

(CHEMBL2205684)Show SMILES COc1ccc2[C@H]3CC[C@@]4(C)[C@@H](Cc5cnoc45)[C@@H]3CCc2c1 |r| Show InChI InChI=1S/C20H23NO2/c1-20-8-7-16-15-6-4-14(22-2)9-12(15)3-5-17(16)18(20)10-13-11-21-23-19(13)20/h4,6,9,11,16-18H,3,5,7-8,10H2,1-2H3/t16-,17-,18+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human estradiol 17beta-dehydrogenase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data