

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM17294 ((13S,14S,15S)-15-methyl-13-nonyltetracyclo[8.7.0.0...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
CHUL | Assay Description For steady-state kinetic study of hybrid inhibitors, a Fluorolog 3 instrument was used to monitor the fluorescent signal of NADPH formed during estra... | FASEB J 16: 1829-31 (2002) Article DOI: 10.1096/fj.02-0026fje BindingDB Entry DOI: 10.7270/Q23T9FGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM25859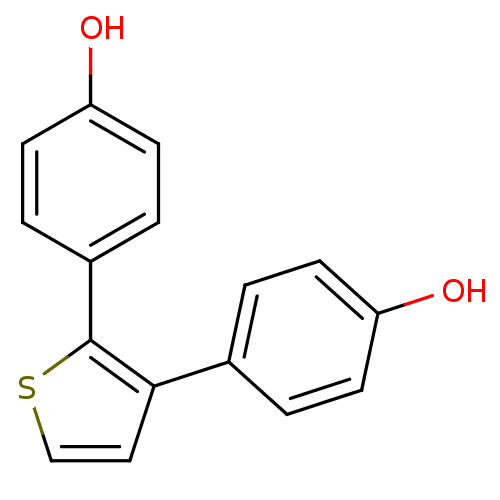 (4-[3-(4-hydroxyphenyl)thiophen-2-yl]phenol | hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description Tritiated E1 was incubated with 17beta-HSD1, cofactor, and inhibitor. The amount of labeled E2 formed was quantified by HPLC. Detection and quantific... | J Med Chem 51: 6725-39 (2008) Article DOI: 10.1021/jm8006917 BindingDB Entry DOI: 10.7270/Q2RB72XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM25834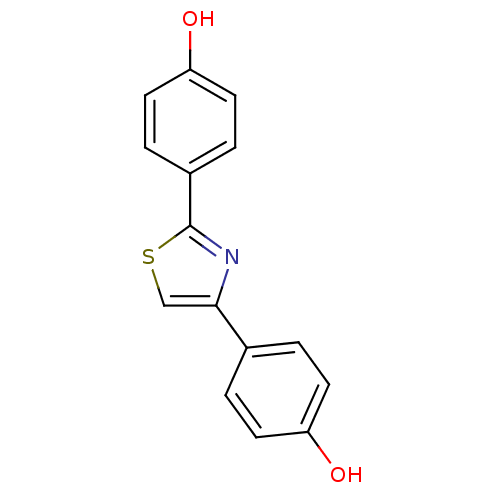 (4-[2-(4-hydroxyphenyl)-1,3-thiazol-4-yl]phenol | h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Saarland University | Assay Description Tritiated E1 was incubated with 17beta-HSD1, cofactor, and inhibitor. The amount of labeled E2 formed was quantified by HPLC. Detection and quantific... | J Med Chem 51: 6725-39 (2008) Article DOI: 10.1021/jm8006917 BindingDB Entry DOI: 10.7270/Q2RB72XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM25840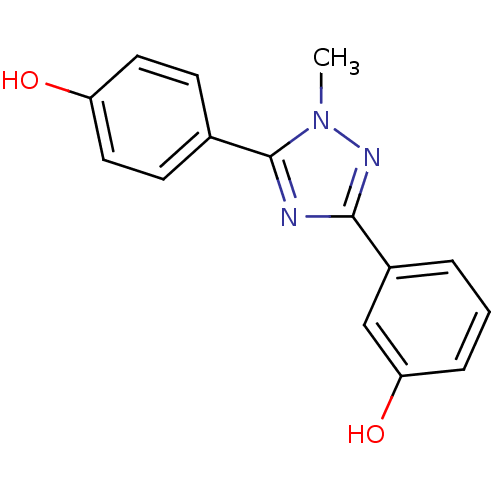 (3-[5-(4-hydroxyphenyl)-1-methyl-1H-1,2,4-triazol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Saarland University | Assay Description Tritiated E1 was incubated with 17beta-HSD1, cofactor, and inhibitor. The amount of labeled E2 formed was quantified by HPLC. Detection and quantific... | J Med Chem 51: 6725-39 (2008) Article DOI: 10.1021/jm8006917 BindingDB Entry DOI: 10.7270/Q2RB72XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM25841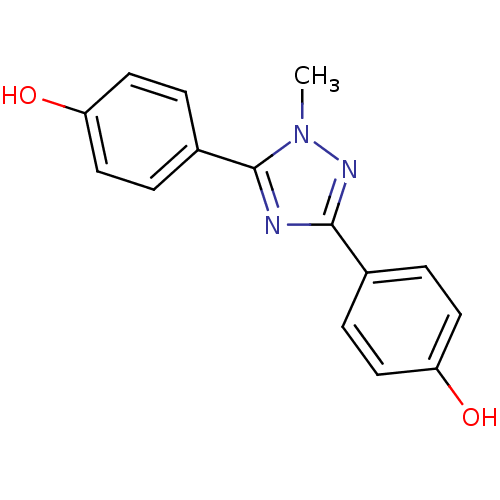 (4-[5-(4-hydroxyphenyl)-1-methyl-1H-1,2,4-triazol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Saarland University | Assay Description Tritiated E1 was incubated with 17beta-HSD1, cofactor, and inhibitor. The amount of labeled E2 formed was quantified by HPLC. Detection and quantific... | J Med Chem 51: 6725-39 (2008) Article DOI: 10.1021/jm8006917 BindingDB Entry DOI: 10.7270/Q2RB72XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM25842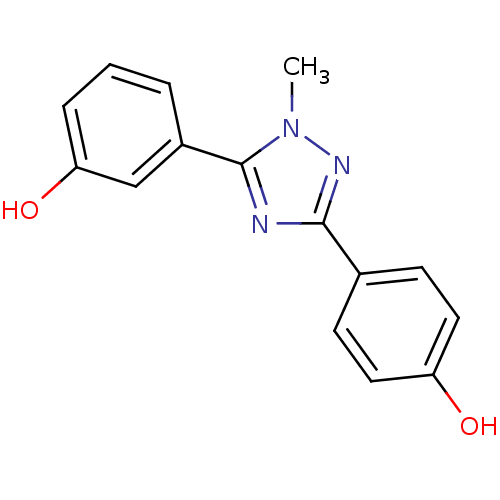 (4-[5-(3-hydroxyphenyl)-1-methyl-1H-1,2,4-triazol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Saarland University | Assay Description Tritiated E1 was incubated with 17beta-HSD1, cofactor, and inhibitor. The amount of labeled E2 formed was quantified by HPLC. Detection and quantific... | J Med Chem 51: 6725-39 (2008) Article DOI: 10.1021/jm8006917 BindingDB Entry DOI: 10.7270/Q2RB72XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM25843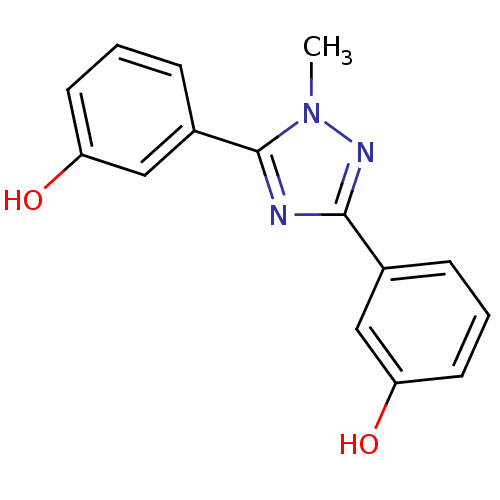 (3-[5-(3-hydroxyphenyl)-1-methyl-1H-1,2,4-triazol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Saarland University | Assay Description Tritiated E1 was incubated with 17beta-HSD1, cofactor, and inhibitor. The amount of labeled E2 formed was quantified by HPLC. Detection and quantific... | J Med Chem 51: 6725-39 (2008) Article DOI: 10.1021/jm8006917 BindingDB Entry DOI: 10.7270/Q2RB72XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM25845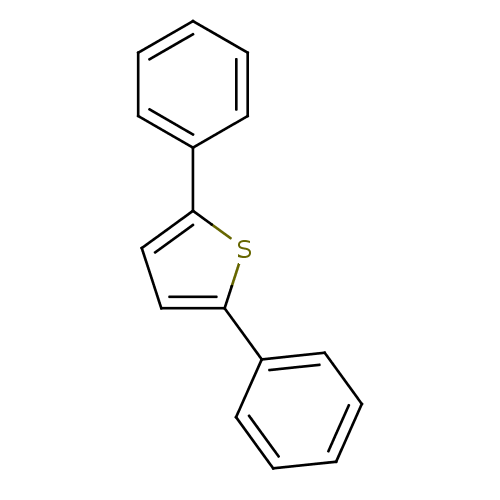 (2,5-diphenylthiophene | thiophene, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Saarland University | Assay Description Tritiated E1 was incubated with 17beta-HSD1, cofactor, and inhibitor. The amount of labeled E2 formed was quantified by HPLC. Detection and quantific... | J Med Chem 51: 6725-39 (2008) Article DOI: 10.1021/jm8006917 BindingDB Entry DOI: 10.7270/Q2RB72XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM25846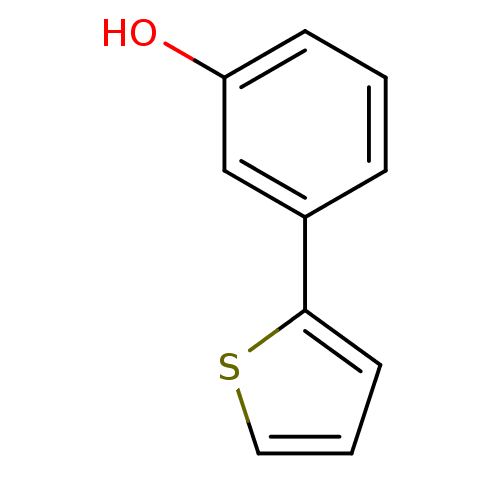 (3-(thiophen-2-yl)phenol | hydroxyphenyl substitute...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Saarland University | Assay Description Tritiated E1 was incubated with 17beta-HSD1, cofactor, and inhibitor. The amount of labeled E2 formed was quantified by HPLC. Detection and quantific... | J Med Chem 51: 6725-39 (2008) Article DOI: 10.1021/jm8006917 BindingDB Entry DOI: 10.7270/Q2RB72XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM25848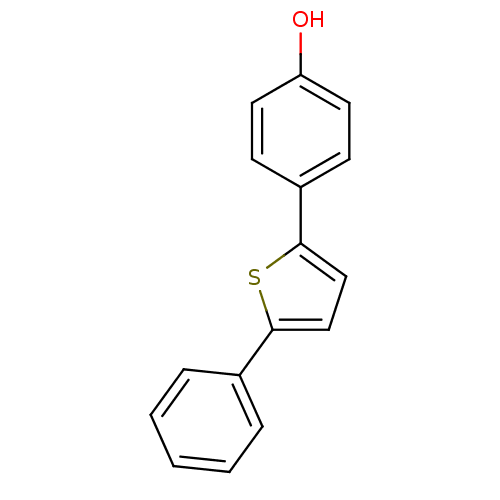 (4-(5-phenylthiophen-2-yl)phenol | hydroxyphenyl su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Saarland University | Assay Description Tritiated E1 was incubated with 17beta-HSD1, cofactor, and inhibitor. The amount of labeled E2 formed was quantified by HPLC. Detection and quantific... | J Med Chem 51: 6725-39 (2008) Article DOI: 10.1021/jm8006917 BindingDB Entry DOI: 10.7270/Q2RB72XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM25849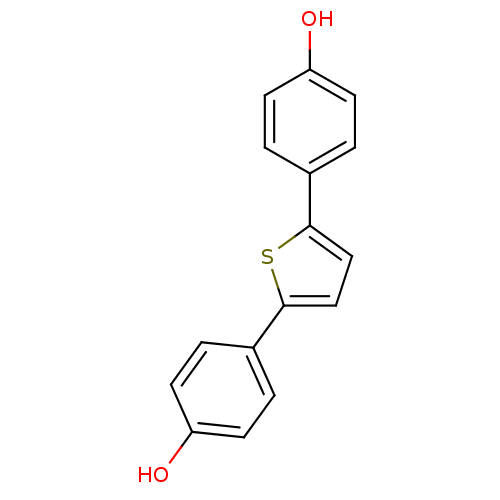 (4-[5-(4-hydroxyphenyl)thiophen-2-yl]phenol | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Saarland University | Assay Description Tritiated E1 was incubated with 17beta-HSD1, cofactor, and inhibitor. The amount of labeled E2 formed was quantified by HPLC. Detection and quantific... | J Med Chem 51: 6725-39 (2008) Article DOI: 10.1021/jm8006917 BindingDB Entry DOI: 10.7270/Q2RB72XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM25854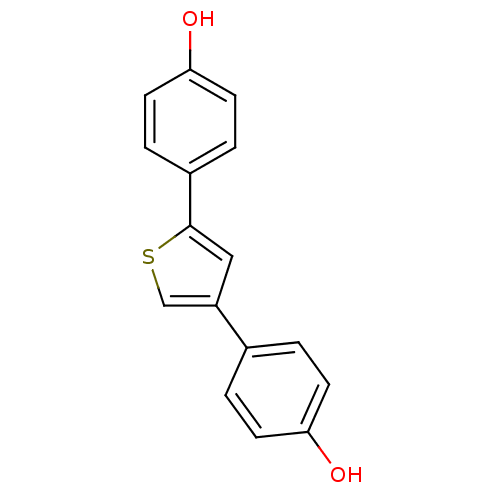 (4-[4-(4-hydroxyphenyl)thiophen-2-yl]phenol | hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Saarland University | Assay Description Tritiated E1 was incubated with 17beta-HSD1, cofactor, and inhibitor. The amount of labeled E2 formed was quantified by HPLC. Detection and quantific... | J Med Chem 51: 6725-39 (2008) Article DOI: 10.1021/jm8006917 BindingDB Entry DOI: 10.7270/Q2RB72XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM25857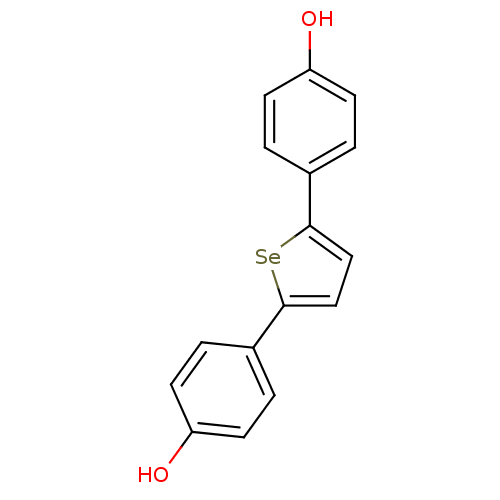 (4-[5-(4-hydroxyphenyl)selenophen-2-yl]phenol | hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Saarland University | Assay Description Tritiated E1 was incubated with 17beta-HSD1, cofactor, and inhibitor. The amount of labeled E2 formed was quantified by HPLC. Detection and quantific... | J Med Chem 51: 6725-39 (2008) Article DOI: 10.1021/jm8006917 BindingDB Entry DOI: 10.7270/Q2RB72XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM25858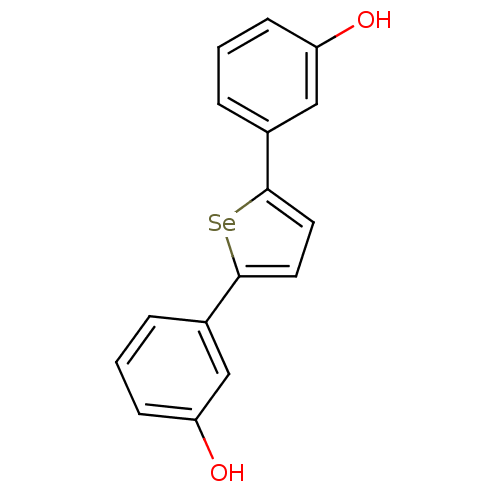 (3-[5-(3-hydroxyphenyl)selenophen-2-yl]phenol | hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Saarland University | Assay Description Tritiated E1 was incubated with 17beta-HSD1, cofactor, and inhibitor. The amount of labeled E2 formed was quantified by HPLC. Detection and quantific... | J Med Chem 51: 6725-39 (2008) Article DOI: 10.1021/jm8006917 BindingDB Entry DOI: 10.7270/Q2RB72XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM25830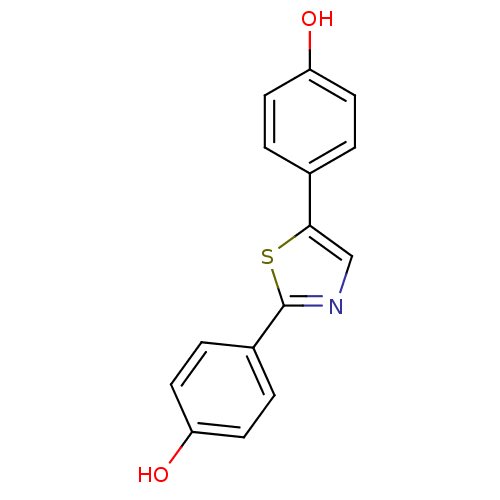 (4-[2-(4-hydroxyphenyl)-1,3-thiazol-5-yl]phenol | h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Saarland University | Assay Description Tritiated E1 was incubated with 17beta-HSD1, cofactor, and inhibitor. The amount of labeled E2 formed was quantified by HPLC. Detection and quantific... | J Med Chem 51: 6725-39 (2008) Article DOI: 10.1021/jm8006917 BindingDB Entry DOI: 10.7270/Q2RB72XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||