 Found 2774 hits of ki for UniProtKB: P20309
Found 2774 hits of ki for UniProtKB: P20309 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M3 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50066861

(BA-679-BR | Spiriva | Tiotropium Bromide | Tiotrop...)Show SMILES O.[Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@@]([H])(C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r,TLB:4:3:15:10.12.9,4:5:15:10.12.9,18:10:15:3.5| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13+,16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Binding affinity to human M3 K523A mutant expressed in HEK293T cells up to 24 hrs by radioligand displacement assay |
J Med Chem 56: 8746-56 (2013)
Article DOI: 10.1021/jm401219y
BindingDB Entry DOI: 10.7270/Q28055J7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50066861

(BA-679-BR | Spiriva | Tiotropium Bromide | Tiotrop...)Show SMILES O.[Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@@]([H])(C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r,TLB:4:3:15:10.12.9,4:5:15:10.12.9,18:10:15:3.5| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13+,16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Binding affinity to human M3 D518A mutant expressed in HEK293T cells up to 24 hrs by radioligand displacement assay |
J Med Chem 56: 8746-56 (2013)
Article DOI: 10.1021/jm401219y
BindingDB Entry DOI: 10.7270/Q28055J7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50066861

(BA-679-BR | Spiriva | Tiotropium Bromide | Tiotrop...)Show SMILES O.[Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@@]([H])(C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r,TLB:4:3:15:10.12.9,4:5:15:10.12.9,18:10:15:3.5| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13+,16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Binding affinity to human M3 K213A mutant expressed in HEK293T cells up to 24 hrs by radioligand displacement assay |
J Med Chem 56: 8746-56 (2013)
Article DOI: 10.1021/jm401219y
BindingDB Entry DOI: 10.7270/Q28055J7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50066861

(BA-679-BR | Spiriva | Tiotropium Bromide | Tiotrop...)Show SMILES O.[Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@@]([H])(C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r,TLB:4:3:15:10.12.9,4:5:15:10.12.9,18:10:15:3.5| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13+,16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Antagonist activity at human M3 receptor expressed in CHO cells assessed as inhibition of carbachol-induced response after 30 mins by AP-1-driven luc... |
J Med Chem 56: 8746-56 (2013)
Article DOI: 10.1021/jm401219y
BindingDB Entry DOI: 10.7270/Q28055J7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50337878
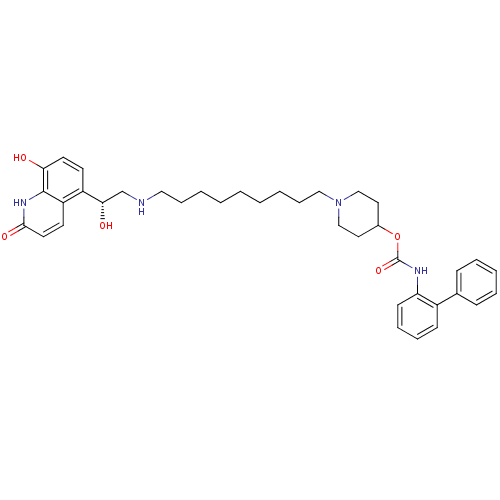
((R)-1-(9-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...)Show SMILES O[C@@H](CNCCCCCCCCCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C38H48N4O5/c43-34-19-17-31(32-18-20-36(45)41-37(32)34)35(44)27-39-23-11-4-2-1-3-5-12-24-42-25-21-29(22-26-42)47-38(46)40-33-16-10-9-15-30(33)28-13-7-6-8-14-28/h6-10,13-20,29,35,39,43-44H,1-5,11-12,21-27H2,(H,40,46)(H,41,45)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay |
Bioorg Med Chem Lett 21: 1354-8 (2011)
Checked by Author
Article DOI: 10.1016/j.bmcl.2011.01.043
BindingDB Entry DOI: 10.7270/Q2NC61HQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50066861

(BA-679-BR | Spiriva | Tiotropium Bromide | Tiotrop...)Show SMILES O.[Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@@]([H])(C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r,TLB:4:3:15:10.12.9,4:5:15:10.12.9,18:10:15:3.5| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13+,16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Binding affinity to human M3 R133A mutant expressed in HEK293T cells up to 24 hrs by radioligand displacement assay |
J Med Chem 56: 8746-56 (2013)
Article DOI: 10.1021/jm401219y
BindingDB Entry DOI: 10.7270/Q28055J7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50066861

(BA-679-BR | Spiriva | Tiotropium Bromide | Tiotrop...)Show SMILES O.[Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@@]([H])(C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r,TLB:4:3:15:10.12.9,4:5:15:10.12.9,18:10:15:3.5| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13+,16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Binding affinity to human M3 F222A mutant expressed in HEK293T cells up to 24 hrs by radioligand displacement assay |
J Med Chem 56: 8746-56 (2013)
Article DOI: 10.1021/jm401219y
BindingDB Entry DOI: 10.7270/Q28055J7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50066861

(BA-679-BR | Spiriva | Tiotropium Bromide | Tiotrop...)Show SMILES O.[Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@@]([H])(C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r,TLB:4:3:15:10.12.9,4:5:15:10.12.9,18:10:15:3.5| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13+,16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Binding affinity to human M3 E220A mutant expressed in HEK293T cells up to 24 hrs by radioligand displacement assay |
J Med Chem 56: 8746-56 (2013)
Article DOI: 10.1021/jm401219y
BindingDB Entry DOI: 10.7270/Q28055J7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50066861

(BA-679-BR | Spiriva | Tiotropium Bromide | Tiotrop...)Show SMILES O.[Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@@]([H])(C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r,TLB:4:3:15:10.12.9,4:5:15:10.12.9,18:10:15:3.5| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13+,16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Binding affinity to human M3 F225A mutant expressed in HEK293T cells up to 24 hrs by radioligand displacement assay |
J Med Chem 56: 8746-56 (2013)
Article DOI: 10.1021/jm401219y
BindingDB Entry DOI: 10.7270/Q28055J7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50066861

(BA-679-BR | Spiriva | Tiotropium Bromide | Tiotrop...)Show SMILES O.[Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@@]([H])(C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r,TLB:4:3:15:10.12.9,4:5:15:10.12.9,18:10:15:3.5| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13+,16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Binding affinity to wild type human M3 receptor expressed in HEK293T cells up to 24 hrs by radioligand displacement assay |
J Med Chem 56: 8746-56 (2013)
Article DOI: 10.1021/jm401219y
BindingDB Entry DOI: 10.7270/Q28055J7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50066861

(BA-679-BR | Spiriva | Tiotropium Bromide | Tiotrop...)Show SMILES O.[Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@@]([H])(C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r,TLB:4:3:15:10.12.9,4:5:15:10.12.9,18:10:15:3.5| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13+,16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Binding affinity to human M3 E228A mutant expressed in HEK293T cells up to 24 hrs by radioligand displacement assay |
J Med Chem 56: 8746-56 (2013)
Article DOI: 10.1021/jm401219y
BindingDB Entry DOI: 10.7270/Q28055J7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50066861

(BA-679-BR | Spiriva | Tiotropium Bromide | Tiotrop...)Show SMILES O.[Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@@]([H])(C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r,TLB:4:3:15:10.12.9,4:5:15:10.12.9,18:10:15:3.5| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13+,16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Binding affinity to human M3 Q224A mutant expressed in HEK293T cells up to 24 hrs by radioligand displacement assay |
J Med Chem 56: 8746-56 (2013)
Article DOI: 10.1021/jm401219y
BindingDB Entry DOI: 10.7270/Q28055J7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50412728
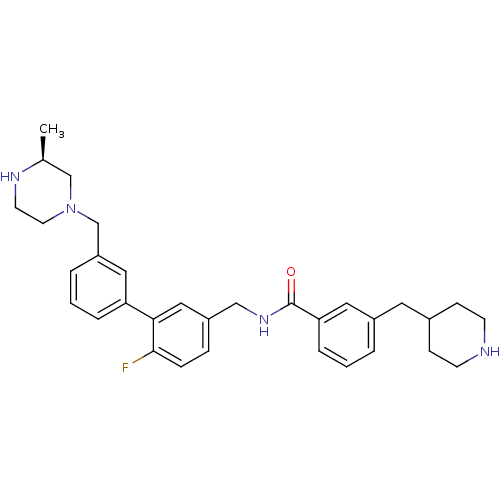
(CHEMBL521523)Show SMILES C[C@H]1CN(Cc2cccc(c2)-c2cc(CNC(=O)c3cccc(CC4CCNCC4)c3)ccc2F)CCN1 |r| Show InChI InChI=1S/C32H39FN4O/c1-23-21-37(15-14-35-23)22-27-5-3-6-28(18-27)30-19-26(8-9-31(30)33)20-36-32(38)29-7-2-4-25(17-29)16-24-10-12-34-13-11-24/h2-9,17-19,23-24,34-35H,10-16,20-22H2,1H3,(H,36,38)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human cloned muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
J Med Chem 51: 5915-8 (2008)
Article DOI: 10.1021/jm800935u
BindingDB Entry DOI: 10.7270/Q21G0NHB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay |
Bioorg Med Chem Lett 21: 1354-8 (2011)
Checked by Author
Article DOI: 10.1016/j.bmcl.2011.01.043
BindingDB Entry DOI: 10.7270/Q2NC61HQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50010096
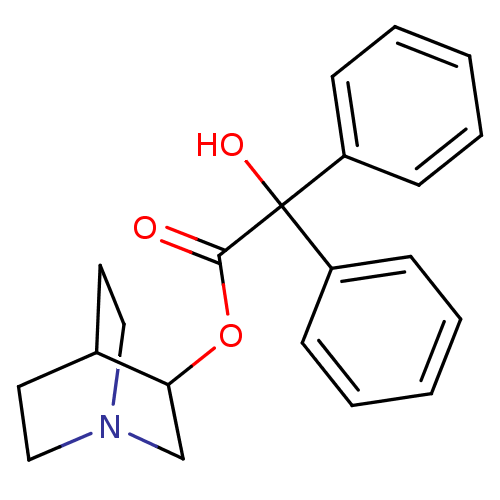
(CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...)Show SMILES OC(C(=O)OC1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(5.41,-4.43,;4.65,-5.78,;6.2,-5.78,;6.97,-7.12,;6.97,-4.45,;8.52,-4.1,;8.52,-2.56,;9.86,-1.78,;11.19,-2.56,;11.19,-4.1,;9.86,-4.88,;9.09,-3.78,;9.09,-2.9,;3.1,-5.81,;2.36,-7.19,;.82,-7.2,;.01,-5.88,;.78,-4.52,;2.32,-4.49,;4.65,-7.23,;3.39,-7.96,;3.38,-9.41,;4.64,-10.15,;5.91,-9.42,;5.91,-7.97,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Ability of compound to displace (-)-[3H]3-Quinuclidinyl benzilate (-)-[3H]-QNB from Muscarinic acetylcholine receptors in the heart from Guinea Pig. |
J Med Chem 40: 3804-19 (1997)
Article DOI: 10.1021/jm970346t
BindingDB Entry DOI: 10.7270/Q2XG9TVC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50355612
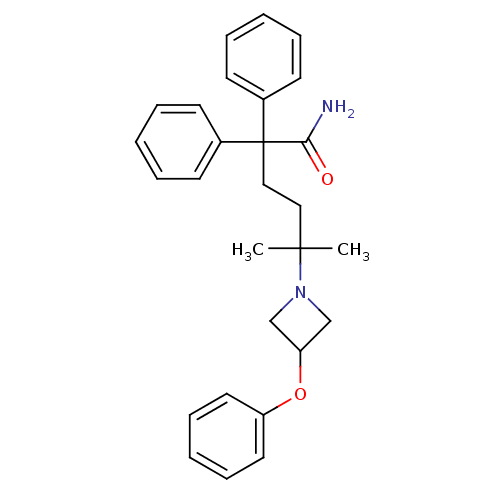
(CHEMBL1910848)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC(C1)Oc1ccccc1 Show InChI InChI=1S/C28H32N2O2/c1-27(2,30-20-25(21-30)32-24-16-10-5-11-17-24)18-19-28(26(29)31,22-12-6-3-7-13-22)23-14-8-4-9-15-23/h3-17,25H,18-21H2,1-2H3,(H2,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant M3 receptor expressed in CHO-K1 cells assessed as inhibition of carbamoyl choline-induced calcium currents a... |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176723
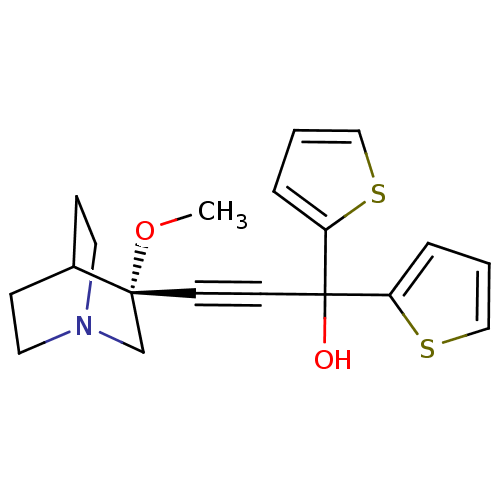
((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1cccs1)c1cccs1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(-4.55,6.62,;-3.06,6.24,;-2.65,4.75,;-2.46,3.37,;-.93,4.03,;.43,3.4,;.16,4.79,;-1.19,5.39,;-1.12,7.02,;-.67,5.92,;-4.15,4.35,;-5.64,3.96,;-7.13,3.56,;-7.52,5.04,;-8.62,3.16,;-9.16,1.72,;-10.68,1.79,;-11.09,3.3,;-9.81,4.14,;-6.73,2.08,;-5.29,1.53,;-5.36,.02,;-6.86,-.39,;-7.7,.89,)| Show InChI InChI=1S/C19H21NO2S2/c1-22-18(14-20-10-6-15(18)7-11-20)8-9-19(21,16-4-2-12-23-16)17-5-3-13-24-17/h2-5,12-13,15,21H,6-7,10-11,14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50494453
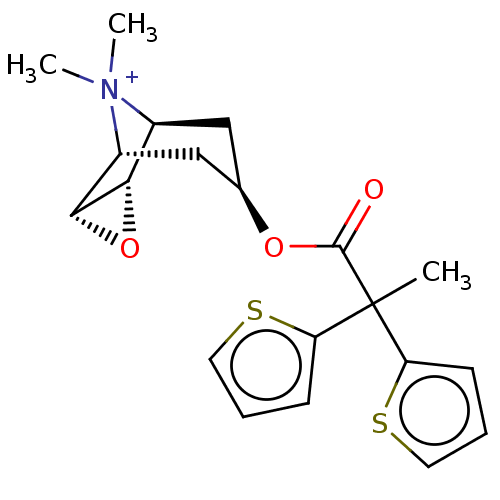
(CHEMBL3091665)Show SMILES [Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@H](C[C@@]2([H])[N+]1(C)C)OC(=O)C(C)(c1cccs1)c1cccs1 |r| Show InChI InChI=1S/C20H24NO3S2.BrH/c1-20(15-6-4-8-25-15,16-7-5-9-26-16)19(22)23-12-10-13-17-18(24-17)14(11-12)21(13,2)3;/h4-9,12-14,17-18H,10-11H2,1-3H3;1H/q+1;/p-1/t12-,13+,14-,17+,18-; | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Binding affinity to wild type human M3 receptor expressed in HEK293T cells up to 24 hrs by radioligand displacement assay |
J Med Chem 56: 8746-56 (2013)
Article DOI: 10.1021/jm401219y
BindingDB Entry DOI: 10.7270/Q28055J7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50084436
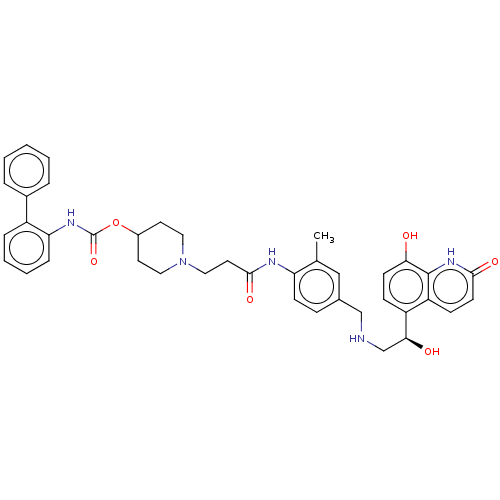
(CHEMBL3426693)Show SMILES Cc1cc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)ccc1NC(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C40H43N5O6/c1-26-23-27(24-41-25-36(47)31-12-15-35(46)39-32(31)13-16-37(48)44-39)11-14-33(26)42-38(49)19-22-45-20-17-29(18-21-45)51-40(50)43-34-10-6-5-9-30(34)28-7-3-2-4-8-28/h2-16,23,29,36,41,46-47H,17-22,24-25H2,1H3,(H,42,49)(H,43,50)(H,44,48)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50010096

(CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...)Show SMILES OC(C(=O)OC1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(5.41,-4.43,;4.65,-5.78,;6.2,-5.78,;6.97,-7.12,;6.97,-4.45,;8.52,-4.1,;8.52,-2.56,;9.86,-1.78,;11.19,-2.56,;11.19,-4.1,;9.86,-4.88,;9.09,-3.78,;9.09,-2.9,;3.1,-5.81,;2.36,-7.19,;.82,-7.2,;.01,-5.88,;.78,-4.52,;2.32,-4.49,;4.65,-7.23,;3.39,-7.96,;3.38,-9.41,;4.64,-10.15,;5.91,-9.42,;5.91,-7.97,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Ability of compound to displace (-)-[3H]3-Quinuclidinyl benzilate (-)-[3H]-QNB from Muscarinic acetylcholine receptors in cerebral cortex from Guinea... |
J Med Chem 40: 3804-19 (1997)
Article DOI: 10.1021/jm970346t
BindingDB Entry DOI: 10.7270/Q2XG9TVC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant M3 receptor expressed in CHO-K1 cells assessed as inhibition of carbamoyl choline-induced calcium currents a... |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50011851
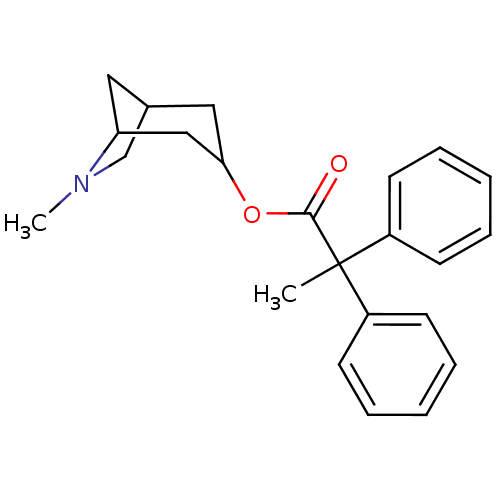
(2,2-Diphenyl-propionic acid 6-methyl-6-aza-bicyclo...)Show SMILES CN1CC2CC1CC(C2)OC(=O)C(C)(c1ccccc1)c1ccccc1 |TLB:9:7:1.2:4,THB:0:1:4:6.7.8| Show InChI InChI=1S/C23H27NO2/c1-23(18-9-5-3-6-10-18,19-11-7-4-8-12-19)22(25)26-21-14-17-13-20(15-21)24(2)16-17/h3-12,17,20-21H,13-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]QNB binding against muscarinic acetylcholine receptor in guinea pig ileum |
J Med Chem 34: 3164-71 (1991)
BindingDB Entry DOI: 10.7270/Q2V988Q7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50345693
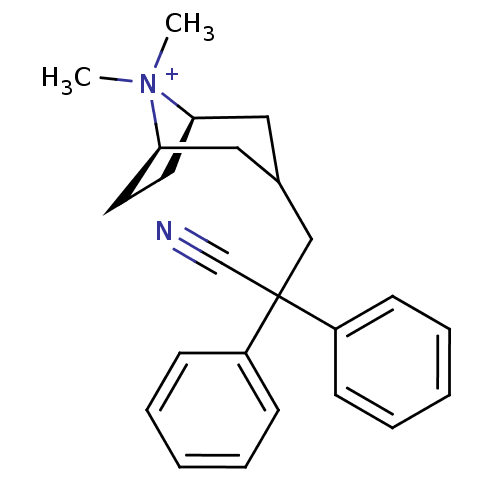
((3-endo)-3-(2-Cyano-2,2-diphenylethyl)-8,8-dimethy...)Show SMILES C[N+]1(C)[C@@H]2CC[C@@H]1CC(CC(C#N)(c1ccccc1)c1ccccc1)C2 |r,THB:9:8:1:4.5| Show InChI InChI=1S/C24H29N2/c1-26(2)22-13-14-23(26)16-19(15-22)17-24(18-25,20-9-5-3-6-10-20)21-11-7-4-8-12-21/h3-12,19,22-23H,13-17H2,1-2H3/q+1/t22-,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
J Med Chem 52: 5241-52 (2010)
Article DOI: 10.1021/jm900736e
BindingDB Entry DOI: 10.7270/Q2PK0H54 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50267614
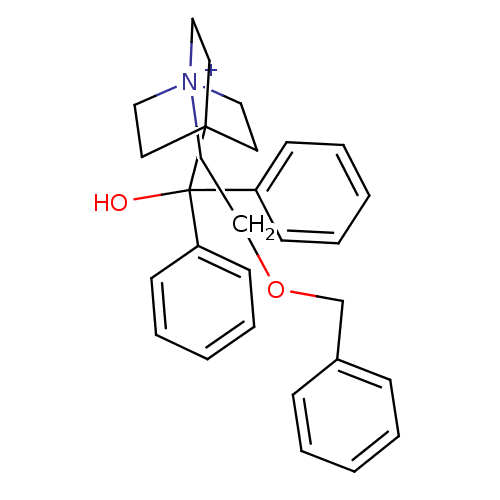
(4-[Hydroxy(diphenyl)methyl]-1-{2-[(phenylmethyl)ox...)Show SMILES OC(c1ccccc1)(c1ccccc1)C12CC[N+](CCOCc3ccccc3)(CC1)CC2 Show InChI InChI=1S/C29H34NO2/c31-29(26-12-6-2-7-13-26,27-14-8-3-9-15-27)28-16-19-30(20-17-28,21-18-28)22-23-32-24-25-10-4-1-5-11-25/h1-15,31H,16-24H2/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopalamine from human cloned muscarinic M3 receptor expressed in CHO cells coexpressing Gqi5 by scintillation proximity... |
J Med Chem 52: 2493-505 (2009)
Article DOI: 10.1021/jm801601v
BindingDB Entry DOI: 10.7270/Q2H131X8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595424
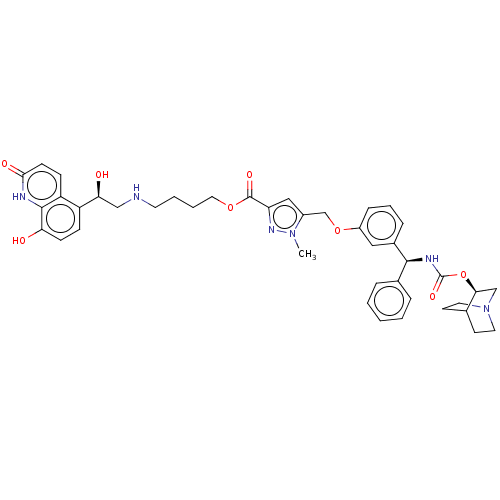
(CHEMBL5172865)Show SMILES Cn1nc(cc1COc1cccc(c1)[C@@H](NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)C(=O)OCCCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:42.47,wD:14.16,19.20,(.73,4.28,;.18,2.84,;-1.31,2.44,;-1.39,.91,;.04,.35,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;-2.69,.07,;-2.61,-1.47,;-4.06,.77,;-5.35,-.07,;-6.72,.63,;-8.01,-.2,;-9.38,.5,;-10.68,-.34,;-12.05,.36,;-13.34,-.48,;-14.71,.22,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595423
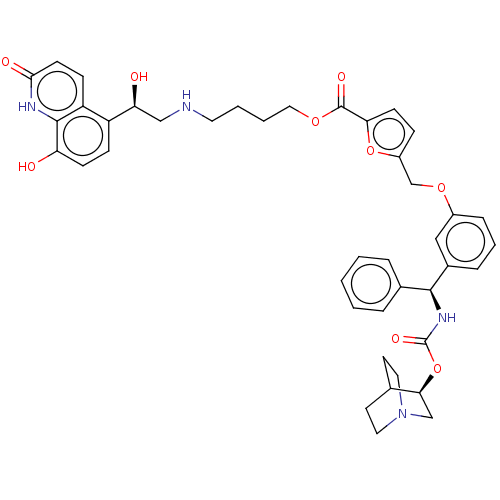
(CHEMBL5190387)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)o1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.71,.22,;-13.34,-.48,;-12.05,.36,;-10.68,-.34,;-9.38,.5,;-8.01,-.2,;-6.72,.63,;-5.35,-.07,;-4.06,.77,;-2.69,.07,;-2.61,-1.47,;-1.39,.91,;-1.31,2.44,;.18,2.84,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;.04,.35,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50494455

(CHEMBL3091664)Show SMILES [Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@H](C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C23H26NO4.BrH/c1-24(2)18-13-17(14-19(24)21-20(18)28-21)27-22(25)23(26,15-9-5-3-6-10-15)16-11-7-4-8-12-16;/h3-12,17-21,26H,13-14H2,1-2H3;1H/q+1;/p-1/t17-,18+,19-,20+,21-; | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Binding affinity to wild type human M3 receptor expressed in HEK293T cells up to 24 hrs by radioligand displacement assay |
J Med Chem 56: 8746-56 (2013)
Article DOI: 10.1021/jm401219y
BindingDB Entry DOI: 10.7270/Q28055J7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538205
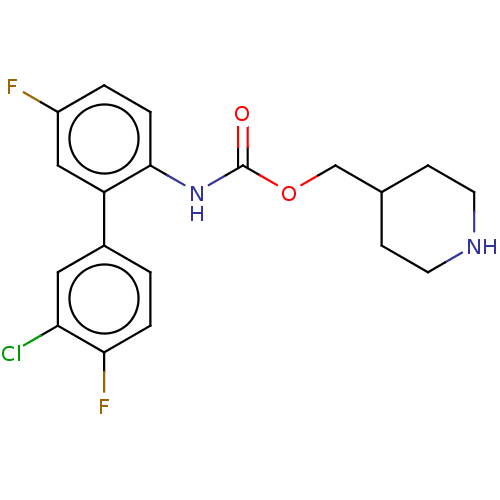
(CHEMBL4636528)Show SMILES Fc1ccc(NC(=O)OCC2CCNCC2)c(c1)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C19H19ClF2N2O2/c20-16-9-13(1-3-17(16)22)15-10-14(21)2-4-18(15)24-19(25)26-11-12-5-7-23-8-6-12/h1-4,9-10,12,23H,5-8,11H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from human muscarinic M3 receptor transiently expressed in HEK293T cell membranes incubated for 1 hr by scint... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296331
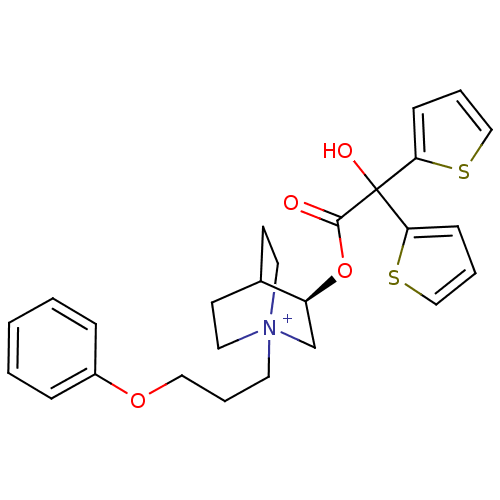
((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(-4.87,-33.87,;-5.96,-34.96,;-4.62,-35.72,;-4.61,-37.26,;-3.29,-34.95,;-1.95,-35.72,;-1.95,-37.26,;-.62,-38.02,;-.64,-39.56,;.69,-40.34,;2.03,-39.58,;3.36,-40.37,;4.7,-39.61,;6.02,-40.39,;7.36,-39.64,;7.37,-38.1,;6.04,-37.31,;4.7,-38.07,;.71,-37.26,;.71,-35.72,;-.62,-34.94,;-.2,-36.18,;-1.25,-36.53,;-5.96,-33.42,;-7.21,-32.51,;-6.74,-31.04,;-5.2,-31.04,;-4.72,-32.5,;-7.29,-35.73,;-8.7,-35.11,;-9.73,-36.25,;-8.96,-37.59,;-7.45,-37.26,)| Show InChI InChI=1S/C26H30NO4S2/c28-25(26(29,23-9-4-17-32-23)24-10-5-18-33-24)31-22-19-27(14-11-20(22)12-15-27)13-6-16-30-21-7-2-1-3-8-21/h1-5,7-10,17-18,20,22,29H,6,11-16,19H2/q+1/t20?,22-,27?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538202

(CHEMBL4633816)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)Nc1ccc(F)cc1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C21H21ClF2N2O2.CH2O2/c1-26-8-6-13(7-9-26)20(12-26)28-21(27)25-19-5-3-15(23)11-16(19)14-2-4-18(24)17(22)10-14;2-1-3/h2-5,10-11,13,20H,6-9,12H2,1H3;1H,(H,2,3)/t13?,20-,26?;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from human muscarinic M3 receptor transiently expressed in HEK293T cell membranes incubated for 1 hr by scint... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296336
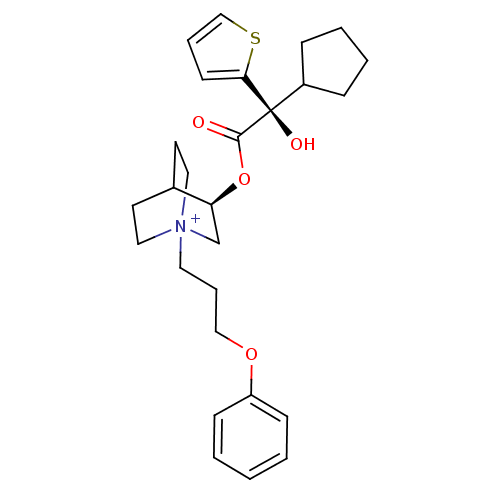
((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...)Show SMILES O[C@@](C1CCCC1)(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)c1cccs1 |r,wU:1.31,wD:10.10,1.0,(16.18,-14.66,;15.09,-15.75,;13.76,-16.53,;12.35,-15.91,;11.32,-17.05,;12.1,-18.39,;13.6,-18.06,;16.43,-16.52,;16.43,-18.06,;17.76,-15.75,;19.09,-16.51,;19.09,-18.05,;20.42,-18.81,;20.41,-20.35,;21.74,-21.14,;23.08,-20.38,;24.4,-21.16,;25.74,-20.41,;27.07,-21.19,;28.41,-20.43,;28.42,-18.89,;27.09,-18.11,;25.75,-18.87,;21.75,-18.05,;21.75,-16.51,;20.42,-15.73,;20.84,-16.98,;19.79,-17.33,;15.09,-14.21,;13.83,-13.31,;14.3,-11.85,;15.84,-11.84,;16.32,-13.31,)| Show InChI InChI=1S/C27H36NO4S/c29-26(27(30,22-8-4-5-9-22)25-12-6-19-33-25)32-24-20-28(16-13-21(24)14-17-28)15-7-18-31-23-10-2-1-3-11-23/h1-3,6,10-12,19,21-22,24,30H,4-5,7-9,13-18,20H2/q+1/t21?,24-,27+,28?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538203
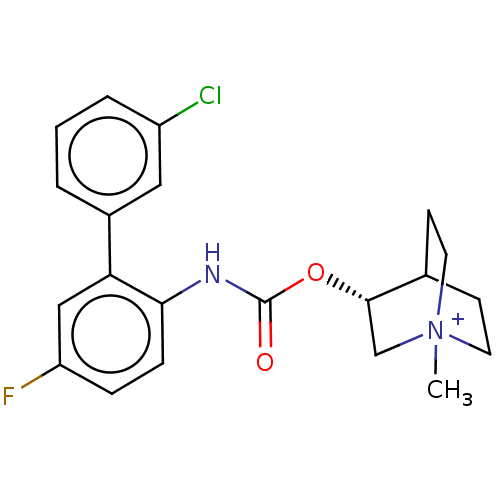
(CHEMBL4647437)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)Nc1ccc(F)cc1-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C21H22ClFN2O2.CH2O2/c1-25-9-7-14(8-10-25)20(13-25)27-21(26)24-19-6-5-17(23)12-18(19)15-3-2-4-16(22)11-15;2-1-3/h2-6,11-12,14,20H,7-10,13H2,1H3;1H,(H,2,3)/t14?,20-,25?;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from human muscarinic M3 receptor transiently expressed in HEK293T cell membranes incubated for 1 hr by scint... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M3 receptor expressed in CHO cells after 2 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595426
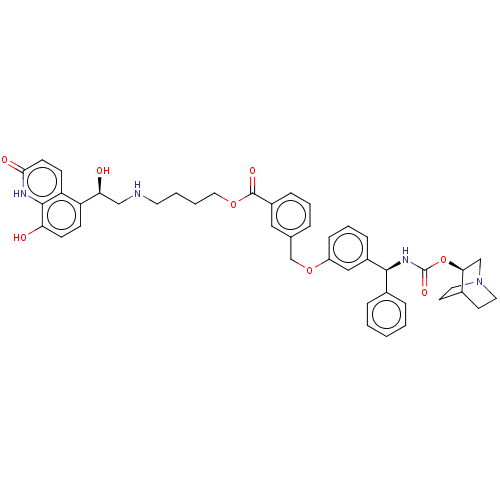
(CHEMBL5193853)Show SMILES O[C@@H](CNCCCCOC(=O)c1cccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)c1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:24.25,29.29,(-13.81,.82,;-12.39,.23,;-11.16,1.16,;-9.74,.56,;-8.52,1.49,;-7.1,.89,;-5.87,1.82,;-4.45,1.23,;-3.22,2.16,;-1.8,1.56,;-1.61,.03,;-.58,2.49,;-.77,4.02,;.46,4.95,;1.88,4.35,;2.07,2.82,;3.49,2.22,;3.68,.7,;5.1,.1,;5.29,-1.43,;6.71,-2.03,;7.94,-1.1,;7.75,.43,;6.33,1.03,;8.98,1.36,;10.4,.76,;10.59,-.76,;9.36,-1.69,;12.01,-1.36,;12.2,-2.89,;10.97,-3.82,;11.16,-5.35,;11.72,-4.04,;13.07,-4.8,;13.62,-3.49,;13.81,-5.02,;12.58,-5.95,;8.78,2.89,;7.36,3.49,;7.17,5.02,;8.4,5.95,;9.82,5.35,;10.01,3.82,;.84,1.89,;-12.39,-1.31,;-13.73,-2.09,;-13.72,-3.63,;-12.39,-4.39,;-12.39,-5.93,;-11.06,-3.62,;-9.72,-4.39,;-8.4,-3.62,;-7.06,-4.39,;-8.4,-2.09,;-9.73,-1.32,;-11.06,-2.08,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
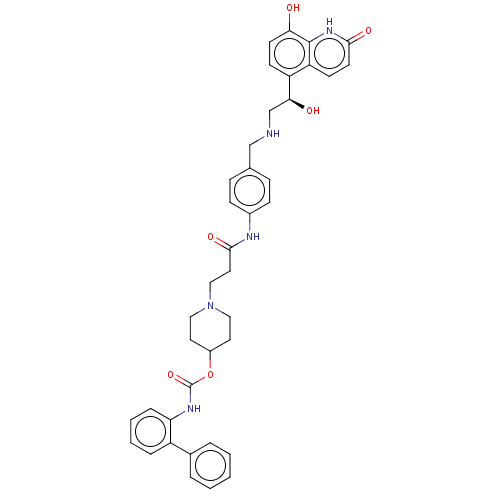
(Homo sapiens (Human)) | BDBM50084443
(CHEMBL3426687)Show SMILES O[C@@H](CNCc1ccc(NC(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C39H41N5O6/c45-34-16-14-31(32-15-17-36(47)43-38(32)34)35(46)25-40-24-26-10-12-28(13-11-26)41-37(48)20-23-44-21-18-29(19-22-44)50-39(49)42-33-9-5-4-8-30(33)27-6-2-1-3-7-27/h1-17,29,35,40,45-46H,18-25H2,(H,41,48)(H,42,49)(H,43,47)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
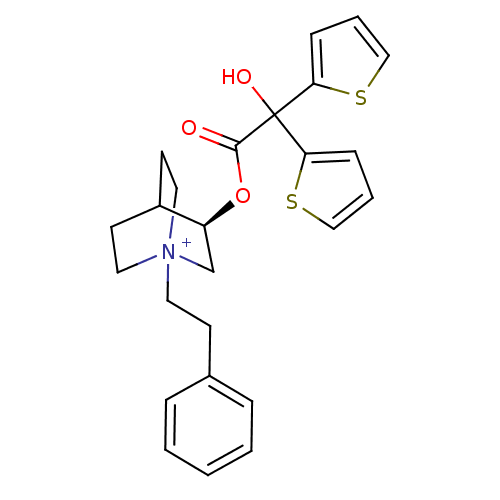
(Homo sapiens (Human)) | BDBM50296329
((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(12.26,-17.08,;11.17,-18.17,;12.51,-18.94,;12.51,-20.48,;13.84,-18.17,;15.17,-18.93,;15.17,-20.47,;16.5,-21.23,;16.49,-22.77,;17.82,-23.56,;19.16,-22.8,;20.48,-23.59,;21.82,-22.83,;21.84,-21.29,;20.5,-20.51,;19.17,-21.27,;17.83,-20.47,;17.83,-18.93,;16.5,-18.15,;16.92,-19.4,;15.87,-19.75,;11.17,-16.63,;9.92,-15.73,;10.39,-14.26,;11.93,-14.26,;12.41,-15.72,;9.84,-18.95,;8.42,-18.32,;7.4,-19.47,;8.17,-20.8,;9.68,-20.48,)| Show InChI InChI=1S/C25H28NO3S2/c27-24(25(28,22-8-4-16-30-22)23-9-5-17-31-23)29-21-18-26(14-11-20(21)12-15-26)13-10-19-6-2-1-3-7-19/h1-9,16-17,20-21,28H,10-15,18H2/q+1/t20?,21-,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM81768

(BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...)Show InChI InChI=1S/C14H12O3/c15-13(16)14(17,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,17H,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation
Curated by PDSP Ki Database
| |
Biochem Pharmacol 45: 2352-4 (1993)
Article DOI: 10.1016/0006-2952(93)90211-e
BindingDB Entry DOI: 10.7270/Q2F76B2C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176735
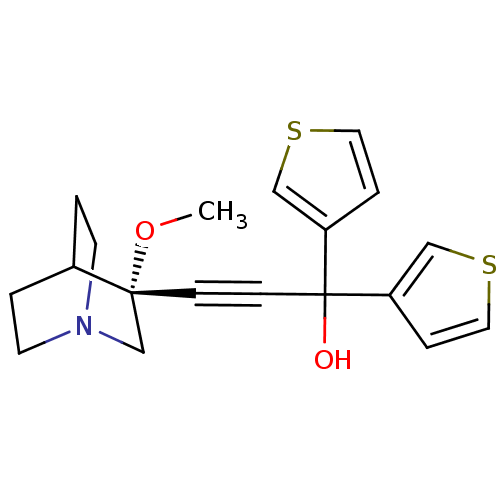
((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1ccsc1)c1ccsc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(9.1,6.91,;10.59,6.52,;11,5.02,;11.19,3.64,;12.73,4.3,;14.09,3.67,;13.82,5.06,;12.47,5.67,;12.54,7.31,;12.98,6.2,;9.5,4.63,;8,4.23,;6.51,3.83,;6.11,5.32,;5.02,3.44,;4.48,2,;2.91,2.09,;2.52,3.58,;3.83,4.41,;6.91,2.35,;8.33,1.8,;8.24,.24,;6.76,-.15,;5.94,1.16,)| Show InChI InChI=1S/C19H21NO2S2/c1-22-18(14-20-8-2-15(18)3-9-20)6-7-19(21,16-4-10-23-12-16)17-5-11-24-13-17/h4-5,10-13,15,21H,2-3,8-9,14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50538206
(CHEMBL4640703)Show InChI InChI=1S/C19H20ClFN2O2/c20-15-3-1-2-14(10-15)17-11-16(21)4-5-18(17)23-19(24)25-12-13-6-8-22-9-7-13/h1-5,10-11,13,22H,6-9,12H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from human muscarinic M3 receptor transiently expressed in HEK293T cell membranes incubated for 1 hr by scint... |
J Med Chem 63: 4349-4369 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00297
BindingDB Entry DOI: 10.7270/Q2CR5XWB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM81768

(BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...)Show InChI InChI=1S/C14H12O3/c15-13(16)14(17,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,17H,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 576-80 (1992)
BindingDB Entry DOI: 10.7270/Q28P5Z0G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
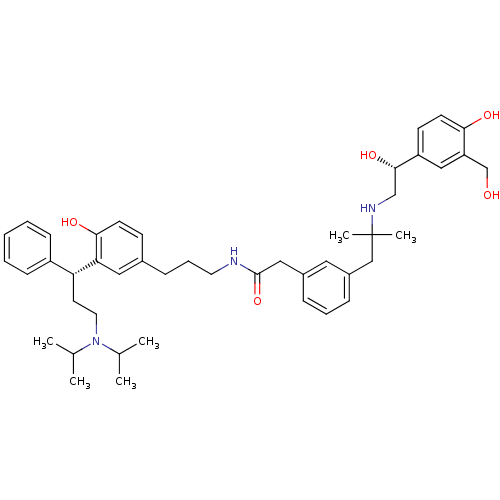
(Homo sapiens (Human)) | BDBM50343152
(CHEMBL1773196 | N-(3-(3-((R)-3-(diisopropylamino)-...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)Cc2cccc(CC(C)(C)NC[C@H](O)c3ccc(O)c(CO)c3)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C45H61N3O5/c1-31(2)48(32(3)4)23-21-39(36-15-8-7-9-16-36)40-25-33(17-19-42(40)51)14-11-22-46-44(53)26-34-12-10-13-35(24-34)28-45(5,6)47-29-43(52)37-18-20-41(50)38(27-37)30-49/h7-10,12-13,15-20,24-25,27,31-32,39,43,47,49-52H,11,14,21-23,26,28-30H2,1-6H3,(H,46,53)/t39-,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM6387
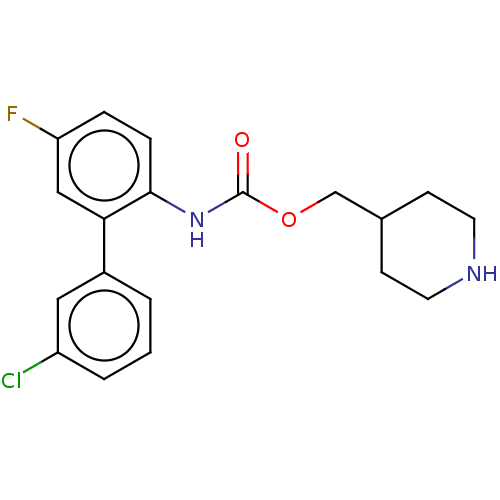
(US8816088, 18 | US9394275, I-18 | US9572802, Compo...)Show SMILES CN(CCCCC(=O)Nc1cc(C)c(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1C)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C47H56N6O7/c1-31-28-40(32(2)27-34(31)29-48-30-42(55)37-16-18-41(54)46-38(37)17-19-44(57)51-46)49-43(56)15-9-10-23-52(3)45(58)22-26-53-24-20-35(21-25-53)60-47(59)50-39-14-8-7-13-36(39)33-11-5-4-6-12-33/h4-8,11-14,16-19,27-28,35,42,48,54-55H,9-10,15,20-26,29-30H2,1-3H3,(H,49,56)(H,50,59)(H,51,57)/t42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Respiratory Company, LLC
US Patent
| Assay Description
Radioligand binding assays for cloned muscarinic receptors were performed in 96-well microtiter plates in a total assay volume of 100 uL. CHO cell me... |
US Patent US8816088 (2014)
BindingDB Entry DOI: 10.7270/Q2TQ6068 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
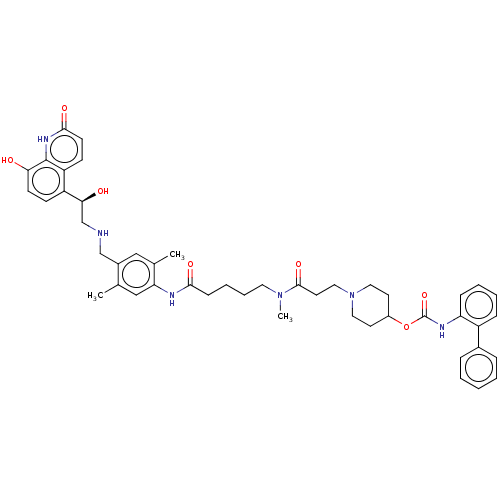
(Homo sapiens (Human)) | BDBM6427
(US8551978, I-25 | US8816088, 25 | US9572802, Compo...)Show SMILES CN(C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1)c1cccc(c1)C(=O)Nc1ccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1 |r| Show InChI InChI=1S/C47H48N6O7/c1-52(44(57)24-27-53-25-22-36(23-26-53)60-47(59)50-40-13-6-5-12-37(40)32-8-3-2-4-9-32)35-11-7-10-33(28-35)46(58)49-34-16-14-31(15-17-34)29-48-30-42(55)38-18-20-41(54)45-39(38)19-21-43(56)51-45/h2-21,28,36,42,48,54-55H,22-27,29-30H2,1H3,(H,49,58)(H,50,59)(H,51,56)/t42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Respiratory Company, LLC
US Patent
| Assay Description
Radioligand binding assays for cloned muscarinic receptors were performed in 96-well microtiter plates in a total assay volume of 100 uL. CHO cell me... |
US Patent US8816088 (2014)
BindingDB Entry DOI: 10.7270/Q2TQ6068 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
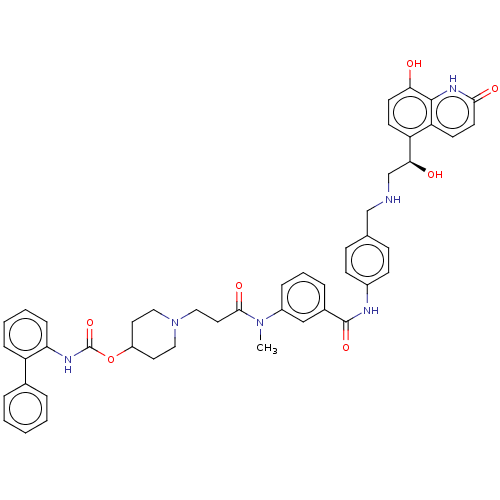
(Homo sapiens (Human)) | BDBM6449
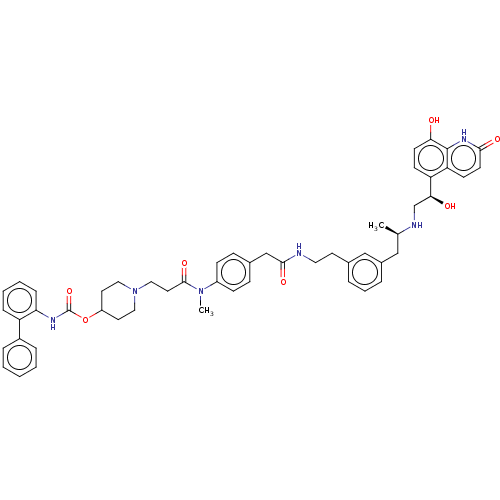
(US8551978, I-87 | US8816088, 87 | US9394275, I-7 |...)Show SMILES C[C@H](Cc1cccc(CCNC(=O)Cc2ccc(cc2)N(C)C(=O)CCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C52H58N6O7/c1-35(54-34-47(60)43-19-21-46(59)51-44(43)20-22-48(61)56-51)31-38-10-8-9-36(32-38)23-27-53-49(62)33-37-15-17-40(18-16-37)57(2)50(63)26-30-58-28-24-41(25-29-58)65-52(64)55-45-14-7-6-13-42(45)39-11-4-3-5-12-39/h3-22,32,35,41,47,54,59-60H,23-31,33-34H2,1-2H3,(H,53,62)(H,55,64)(H,56,61)/t35-,47+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Respiratory Company, LLC
US Patent
| Assay Description
Radioligand binding assays for cloned muscarinic receptors were performed in 96-well microtiter plates in a total assay volume of 100 uL. CHO cell me... |
US Patent US8816088 (2014)
BindingDB Entry DOI: 10.7270/Q2TQ6068 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Prog Neuropsychopharmacol Biol Psychiatry 27: 1125-43 (2003)
Article DOI: 10.1016/j.pnpbp.2003.09.008
BindingDB Entry DOI: 10.7270/Q2M61HTM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM6354
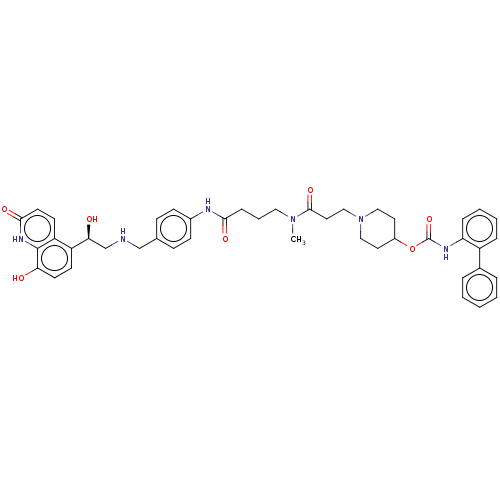
(US8551978, I-1 | US8816088, 1 | US9394275, I-1 | U...)Show SMILES CN(CCCC(=O)Nc1ccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C44H50N6O7/c1-49(42(55)23-27-50-25-21-33(22-26-50)57-44(56)47-37-11-6-5-10-34(37)31-8-3-2-4-9-31)24-7-12-40(53)46-32-15-13-30(14-16-32)28-45-29-39(52)35-17-19-38(51)43-36(35)18-20-41(54)48-43/h2-6,8-11,13-20,33,39,45,51-52H,7,12,21-29H2,1H3,(H,46,53)(H,47,56)(H,48,54)/t39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM38242
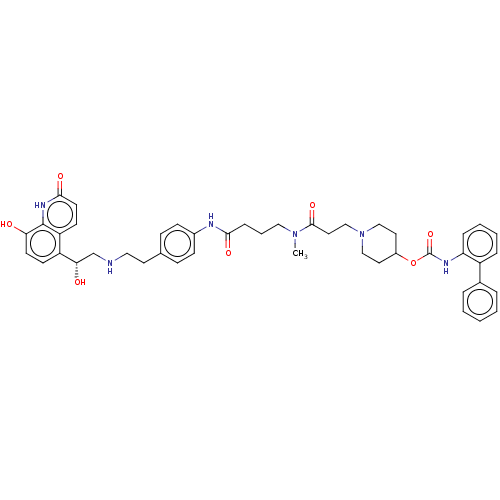
(US8551978, I-7 | US8551978, I-8 | US9394275, I-8 |...)Show SMILES CN(CCCC(=O)Nc1ccc(CCNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1)C(=O)CCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C45H52N6O7/c1-50(43(56)24-29-51-27-22-34(23-28-51)58-45(57)48-38-11-6-5-10-35(38)32-8-3-2-4-9-32)26-7-12-41(54)47-33-15-13-31(14-16-33)21-25-46-30-40(53)36-17-19-39(52)44-37(36)18-20-42(55)49-44/h2-6,8-11,13-20,34,40,46,52-53H,7,12,21-30H2,1H3,(H,47,54)(H,48,57)(H,49,55)/t40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
US Patent
| Assay Description
Radioligand binding assay using muscarinic receptors. |
US Patent US8551978 (2013)
BindingDB Entry DOI: 10.7270/Q2Q81BPC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data