 Found 3142 hits of ic50 data for polymerid = 575
Found 3142 hits of ic50 data for polymerid = 575 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265746
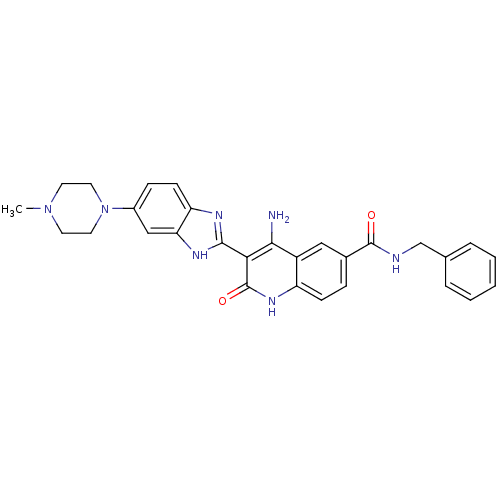
(CHEMBL526507 | {4-Amino-3-[6-(4-methylpiperazinyl)...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2cc(ccc2[nH]c1=O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C29H29N7O2/c1-35-11-13-36(14-12-35)20-8-10-23-24(16-20)33-27(32-23)25-26(30)21-15-19(7-9-22(21)34-29(25)38)28(37)31-17-18-5-3-2-4-6-18/h2-10,15-16H,11-14,17H2,1H3,(H,31,37)(H,32,33)(H3,30,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265774
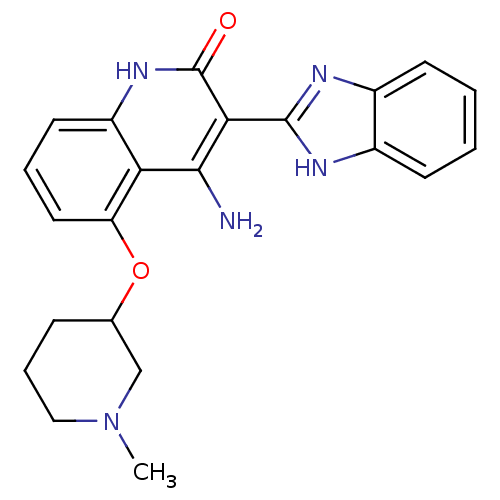
(4-Amino-3-benzimidazol-2-yl-5-(1-methyl(3-piperidy...)Show SMILES CN1CCCC(C1)Oc1cccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(N)c12 Show InChI InChI=1S/C22H23N5O2/c1-27-11-5-6-13(12-27)29-17-10-4-9-16-18(17)20(23)19(22(28)26-16)21-24-14-7-2-3-8-15(14)25-21/h2-4,7-10,13H,5-6,11-12H2,1H3,(H,24,25)(H3,23,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM581371

(US11505527, Compound 4j)Show SMILES Cc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C1C(C(=O)c2ccccc2)=C(N)NC2=C1C(=O)CC(C)(C)C2 |c:32,t:28| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
An in vitro kinase assay was performed to evaluate the kinase suppression activity of the most promising cytotoxic candidates 4b, 4j against four dif... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25B06BK |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50381945
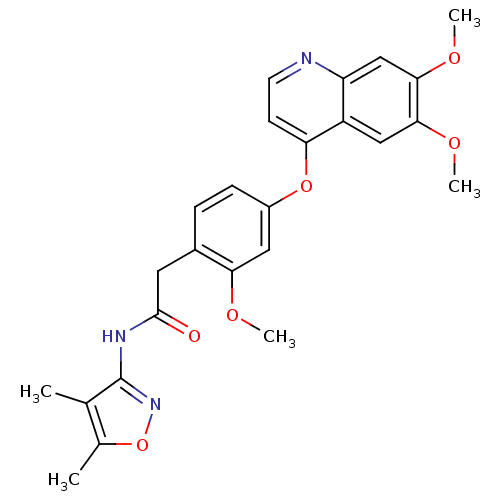
(CHEMBL2023477)Show SMILES COc1cc(Oc2ccnc3cc(OC)c(OC)cc23)ccc1CC(=O)Nc1noc(C)c1C Show InChI InChI=1S/C25H25N3O6/c1-14-15(2)34-28-25(14)27-24(29)10-16-6-7-17(11-21(16)30-3)33-20-8-9-26-19-13-23(32-5)22(31-4)12-18(19)20/h6-9,11-13H,10H2,1-5H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta |
Bioorg Med Chem Lett 22: 3050-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.074
BindingDB Entry DOI: 10.7270/Q298881Z |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265574
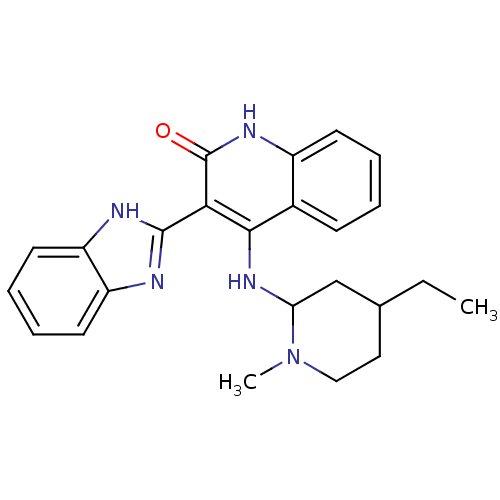
(3-(1H-benzo[d]imidazol-2-yl)-4-(4-ethyl-1-methylpi...)Show SMILES CCC1CCN(C)C(C1)Nc1c(-c2nc3ccccc3[nH]2)c(=O)[nH]c2ccccc12 Show InChI InChI=1S/C24H27N5O/c1-3-15-12-13-29(2)20(14-15)28-22-16-8-4-5-9-17(16)27-24(30)21(22)23-25-18-10-6-7-11-19(18)26-23/h4-11,15,20H,3,12-14H2,1-2H3,(H,25,26)(H2,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50381946
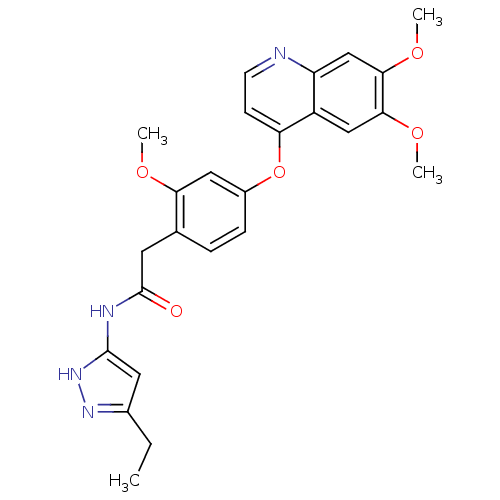
(CHEMBL2023485)Show SMILES CCc1cc(NC(=O)Cc2ccc(Oc3ccnc4cc(OC)c(OC)cc34)cc2OC)[nH]n1 Show InChI InChI=1S/C25H26N4O5/c1-5-16-11-24(29-28-16)27-25(30)10-15-6-7-17(12-21(15)31-2)34-20-8-9-26-19-14-23(33-4)22(32-3)13-18(19)20/h6-9,11-14H,5,10H2,1-4H3,(H2,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta |
Bioorg Med Chem Lett 22: 3050-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.074
BindingDB Entry DOI: 10.7270/Q298881Z |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
US Patent
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
An in vitro kinase assay was performed to evaluate the kinase suppression activity of the most promising cytotoxic candidates 4b, 4j against four dif... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25B06BK |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50362065

(CHEMBL1940109)Show SMILES COCCOc1cc2ncnc(Oc3cnn(CC(=O)Nc4cccc(OC)c4)c3)c2cc1OC Show InChI InChI=1S/C24H25N5O6/c1-31-7-8-34-22-11-20-19(10-21(22)33-3)24(26-15-25-20)35-18-12-27-29(13-18)14-23(30)28-16-5-4-6-17(9-16)32-2/h4-6,9-13,15H,7-8,14H2,1-3H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta phosphorylation by cell based assay |
Bioorg Med Chem Lett 22: 262-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.019
BindingDB Entry DOI: 10.7270/Q24F1R6H |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265773
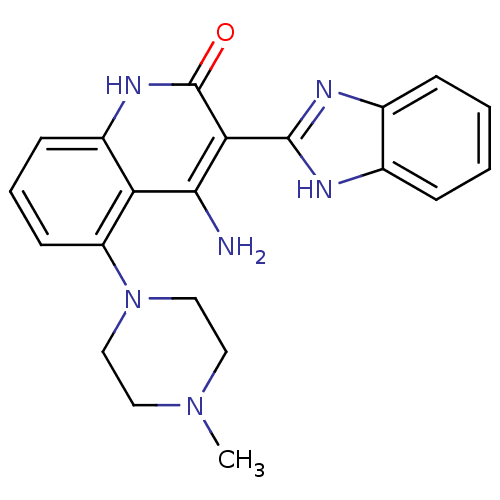
(4-Amino-3-benzimidazol-2-yl-5-(4-methylpiperazinyl...)Show SMILES CN1CCN(CC1)c1cccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(N)c12 Show InChI InChI=1S/C21H22N6O/c1-26-9-11-27(12-10-26)16-8-4-7-15-17(16)19(22)18(21(28)25-15)20-23-13-5-2-3-6-14(13)24-20/h2-8H,9-12H2,1H3,(H,23,24)(H3,22,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50381931
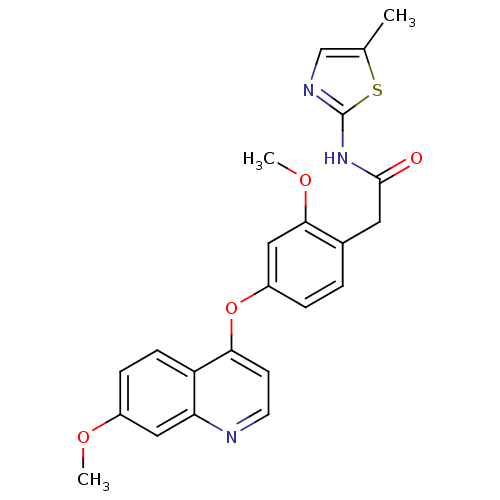
(CHEMBL2023476)Show SMILES COc1ccc2c(Oc3ccc(CC(=O)Nc4ncc(C)s4)c(OC)c3)ccnc2c1 Show InChI InChI=1S/C23H21N3O4S/c1-14-13-25-23(31-14)26-22(27)10-15-4-5-17(12-21(15)29-3)30-20-8-9-24-19-11-16(28-2)6-7-18(19)20/h4-9,11-13H,10H2,1-3H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta |
Bioorg Med Chem Lett 22: 3050-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.074
BindingDB Entry DOI: 10.7270/Q298881Z |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265678
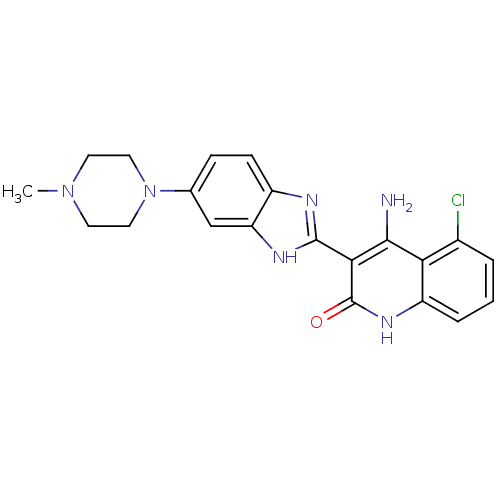
(4-Amino-5-chloro-3-[6-(4-methylpiperazinyl)benzimi...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2c(Cl)cccc2[nH]c1=O Show InChI InChI=1S/C21H21ClN6O/c1-27-7-9-28(10-8-27)12-5-6-14-16(11-12)25-20(24-14)18-19(23)17-13(22)3-2-4-15(17)26-21(18)29/h2-6,11H,7-10H2,1H3,(H,24,25)(H3,23,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50381947
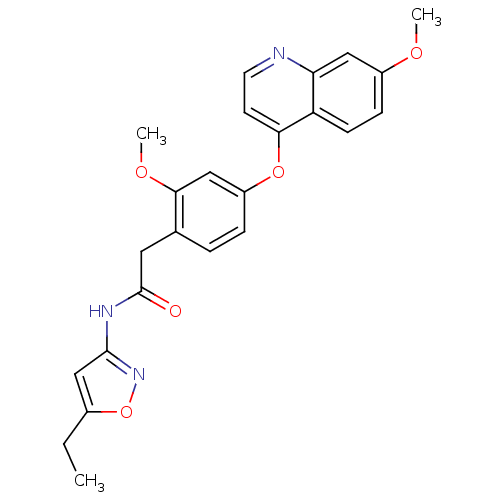
(CHEMBL2023482)Show SMILES CCc1cc(NC(=O)Cc2ccc(Oc3ccnc4cc(OC)ccc34)cc2OC)no1 Show InChI InChI=1S/C24H23N3O5/c1-4-16-14-23(27-32-16)26-24(28)11-15-5-6-18(13-22(15)30-3)31-21-9-10-25-20-12-17(29-2)7-8-19(20)21/h5-10,12-14H,4,11H2,1-3H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta |
Bioorg Med Chem Lett 22: 3050-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.074
BindingDB Entry DOI: 10.7270/Q298881Z |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265750
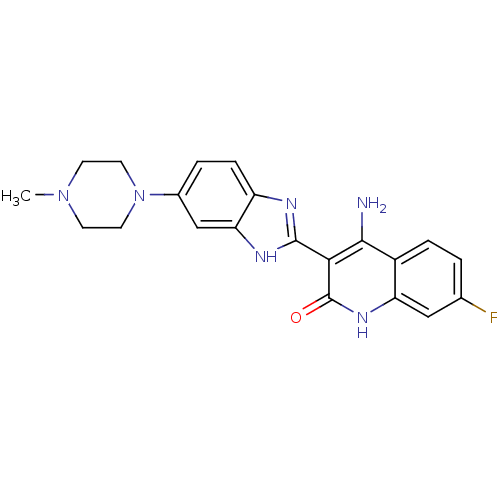
(4-Amino-7-fluoro-3-[6-(4-methylpiperazinyl)benzimi...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2ccc(F)cc2[nH]c1=O Show InChI InChI=1S/C21H21FN6O/c1-27-6-8-28(9-7-27)13-3-5-15-17(11-13)25-20(24-15)18-19(23)14-4-2-12(22)10-16(14)26-21(18)29/h2-5,10-11H,6-9H2,1H3,(H,24,25)(H3,23,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50117342
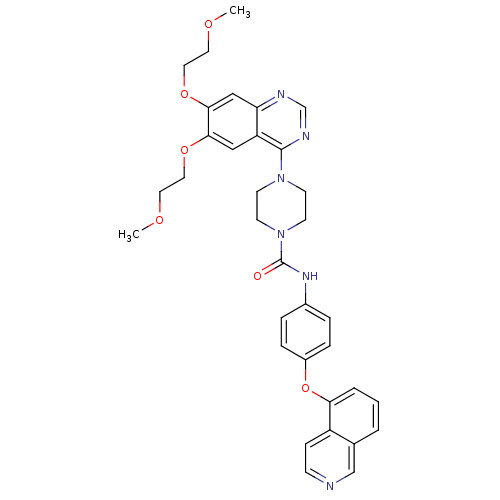
(4-[6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-pip...)Show SMILES COCCOc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Oc4cccc5cnccc45)cc3)c2cc1OCCOC Show InChI InChI=1S/C34H36N6O6/c1-42-16-18-44-31-20-28-29(21-32(31)45-19-17-43-2)36-23-37-33(28)39-12-14-40(15-13-39)34(41)38-25-6-8-26(9-7-25)46-30-5-3-4-24-22-35-11-10-27(24)30/h3-11,20-23H,12-19H2,1-2H3,(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma |
J Med Chem 45: 3772-93 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7JD9 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50117334
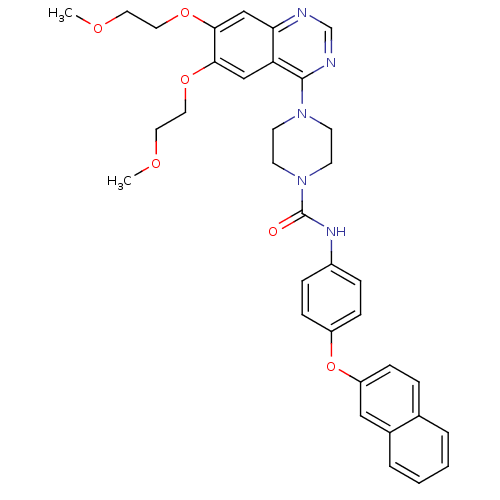
(4-[6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-pip...)Show SMILES COCCOc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Oc4ccc5ccccc5c4)cc3)c2cc1OCCOC Show InChI InChI=1S/C35H37N5O6/c1-42-17-19-44-32-22-30-31(23-33(32)45-20-18-43-2)36-24-37-34(30)39-13-15-40(16-14-39)35(41)38-27-8-11-28(12-9-27)46-29-10-7-25-5-3-4-6-26(25)21-29/h3-12,21-24H,13-20H2,1-2H3,(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma |
J Med Chem 45: 3772-93 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7JD9 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50362089

(CHEMBL1940273)Show SMILES CCn1cc(NC(=O)Cc2ccc(Oc3ccnc4cc(OC)c(OC)cc34)cc2)cn1 Show InChI InChI=1S/C24H24N4O4/c1-4-28-15-17(14-26-28)27-24(29)11-16-5-7-18(8-6-16)32-21-9-10-25-20-13-23(31-3)22(30-2)12-19(20)21/h5-10,12-15H,4,11H2,1-3H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta phosphorylation by cell based assay |
Bioorg Med Chem Lett 22: 262-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.019
BindingDB Entry DOI: 10.7270/Q24F1R6H |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50431812
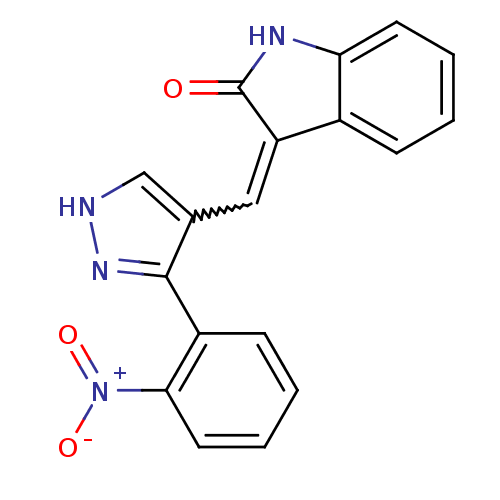
(CHEMBL2347053)Show SMILES [O-][N+](=O)c1ccccc1-c1n[nH]cc1C=C1C(=O)Nc2ccccc12 |w:14.15| Show InChI InChI=1S/C18H12N4O3/c23-18-14(12-5-1-3-7-15(12)20-18)9-11-10-19-21-17(11)13-6-2-4-8-16(13)22(24)25/h1-10H,(H,19,21)(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta (unknown origin) after 10 mins by mobility shift assay |
Bioorg Med Chem 21: 1724-34 (2013)
Article DOI: 10.1016/j.bmc.2013.01.047
BindingDB Entry DOI: 10.7270/Q2GQ704N |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM581370

(US11505527, Compound 4b)Show SMILES CCOC(=O)C1=C(NC2=C(C1c1ccc(cc1)S(=O)(=O)c1ccccc1)C(=O)CC(C)(C)C2)C(F)(F)F |c:8,t:5| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
An in vitro kinase assay was performed to evaluate the kinase suppression activity of the most promising cytotoxic candidates 4b, 4j against four dif... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25B06BK |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227812
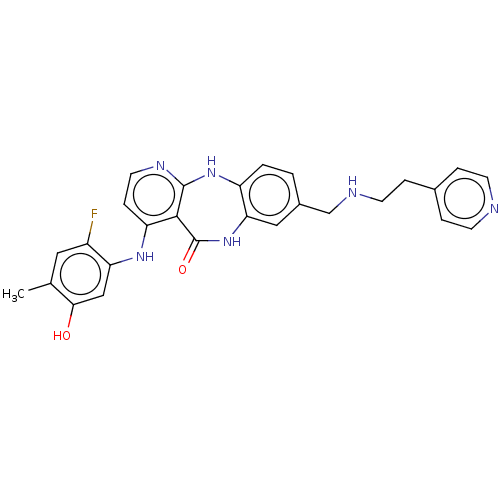
(4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-({[...)Show SMILES Cc1cc(F)c(Nc2ccnc3Nc4ccc(CNCCc5ccncc5)cc4NC(=O)c23)cc1O Show InChI InChI=1S/C27H25FN6O2/c1-16-12-19(28)22(14-24(16)35)32-21-7-11-31-26-25(21)27(36)34-23-13-18(2-3-20(23)33-26)15-30-10-6-17-4-8-29-9-5-17/h2-5,7-9,11-14,30,35H,6,10,15H2,1H3,(H,34,36)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.475 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227786
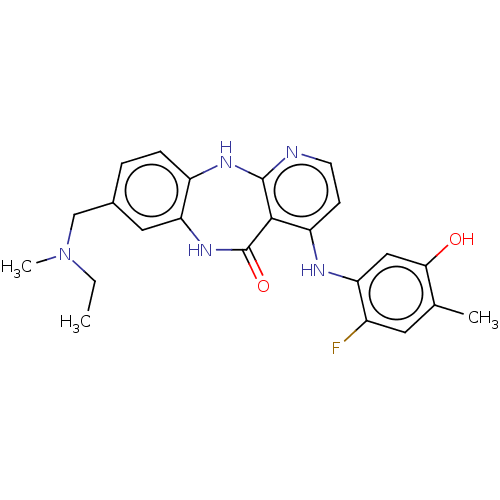
(8-{[Ethyl(methyl)amino]methyl}-4-[(2-fluoro-5-hydr...)Show SMILES CCN(C)Cc1ccc2Nc3nccc(Nc4cc(O)c(C)cc4F)c3C(=O)Nc2c1 Show InChI InChI=1S/C23H24FN5O2/c1-4-29(3)12-14-5-6-16-19(10-14)28-23(31)21-17(7-8-25-22(21)27-16)26-18-11-20(30)13(2)9-15(18)24/h5-11,30H,4,12H2,1-3H3,(H,28,31)(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.487 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4817

(N-[2-(dimethylamino)ethyl]-5-{[(3Z)-5-fluoro-2-oxo...)Show SMILES CN(C)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C20H23FN4O2/c1-11-17(23-12(2)18(11)20(27)22-7-8-25(3)4)10-15-14-9-13(21)5-6-16(14)24-19(15)26/h5-6,9-10,23H,7-8H2,1-4H3,(H,22,27)(H,24,26)/b15-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM112876

(US9695181, IIb)Show SMILES Cc1ccc(F)c(NC(=O)Nc2ccc(cc2)-c2nn(CCCCCCCC(=O)NO)c3ncnc(N)c23)c1 Show InChI InChI=1S/C27H31FN8O3/c1-17-8-13-20(28)21(15-17)33-27(38)32-19-11-9-18(10-12-19)24-23-25(29)30-16-31-26(23)36(34-24)14-6-4-2-3-5-7-22(37)35-39/h8-13,15-16,39H,2-7,14H2,1H3,(H,35,37)(H2,29,30,31)(H2,32,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
DOUBLE RIDER MEDICINE CO., LTD.
US Patent
| Assay Description
Inhibition of Compound IIa, IIb, IIc and IId on the Activity of Catalyzing Substrate Phosphorylation of KDR (VEGFR2) Protein Tyrosine Kinase at the M... |
US Patent US9695181 (2017)
BindingDB Entry DOI: 10.7270/Q2RX9971 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50381948
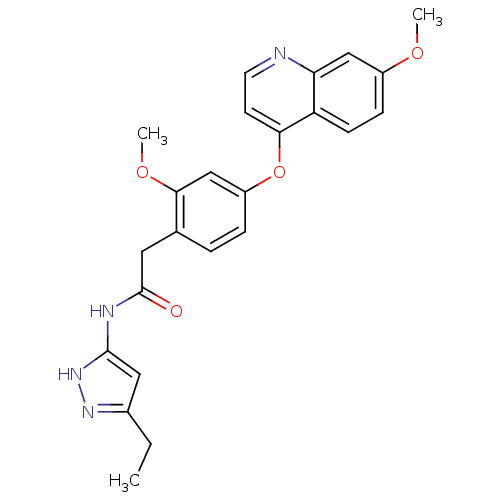
(CHEMBL2023486)Show SMILES CCc1cc(NC(=O)Cc2ccc(Oc3ccnc4cc(OC)ccc34)cc2OC)[nH]n1 Show InChI InChI=1S/C24H24N4O4/c1-4-16-12-23(28-27-16)26-24(29)11-15-5-6-18(14-22(15)31-3)32-21-9-10-25-20-13-17(30-2)7-8-19(20)21/h5-10,12-14H,4,11H2,1-3H3,(H2,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta |
Bioorg Med Chem Lett 22: 3050-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.074
BindingDB Entry DOI: 10.7270/Q298881Z |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265745
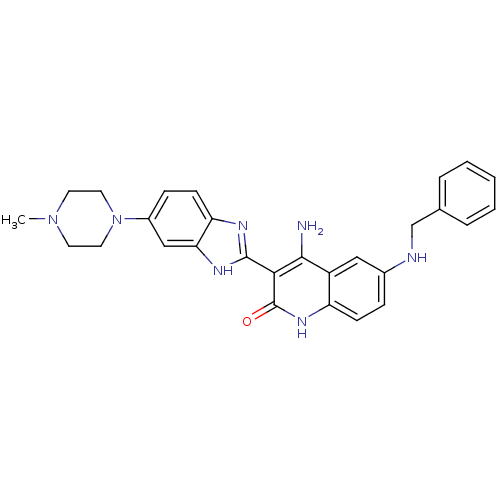
(4-Amino-6-(benzylamino)-3-(6-(4-methylpiperazin-1-...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2cc(NCc3ccccc3)ccc2[nH]c1=O Show InChI InChI=1S/C28H29N7O/c1-34-11-13-35(14-12-34)20-8-10-23-24(16-20)32-27(31-23)25-26(29)21-15-19(7-9-22(21)33-28(25)36)30-17-18-5-3-2-4-6-18/h2-10,15-16,30H,11-14,17H2,1H3,(H,31,32)(H3,29,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta (unknown origin) incubated for 1 hr by spectrophotometric analysis |
Bioorg Med Chem 24: 3483-93 (2016)
Article DOI: 10.1016/j.bmc.2016.05.057
BindingDB Entry DOI: 10.7270/Q29G5PQT |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227930
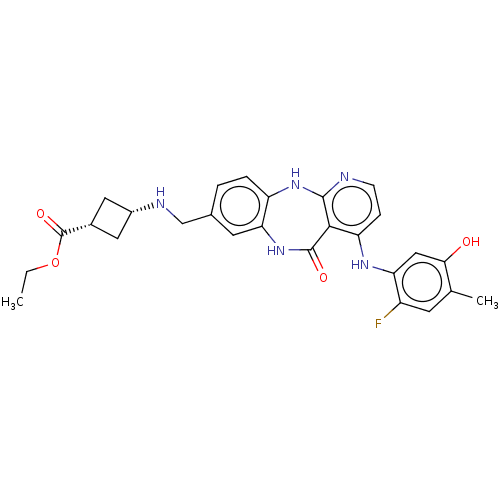
(US10047096, 191 | cis-Ethyl 3-[({4-[(2-fluoro-5-hy...)Show SMILES CCOC(=O)[C@@H]1C[C@@H](C1)NCc1ccc2Nc3nccc(Nc4cc(O)c(C)cc4F)c3C(=O)Nc2c1 |r,wU:7.9,5.4,(10.32,4.14,;10.32,2.6,;8.99,1.83,;8.99,.29,;10.32,-.48,;7.65,-.48,;6.17,-.09,;5.77,-1.57,;7.26,-1.97,;4.43,-2.34,;3.66,-3.68,;2.12,-3.68,;1.46,-5.06,;-.08,-5.18,;-.95,-3.91,;-2.45,-4.25,;-3.65,-3.29,;-4.99,-4.06,;-6.32,-3.29,;-6.32,-1.75,;-4.99,-.98,;-4.99,.56,;-6.32,1.33,;-6.32,2.87,;-7.65,3.64,;-7.65,5.18,;-8.99,2.87,;-10.32,3.64,;-8.99,1.33,;-7.65,.56,;-7.65,-.98,;-3.65,-1.75,;-2.45,-.79,;-2.85,.7,;-.95,-1.13,;-.28,-2.52,;1.26,-2.41,)| Show InChI InChI=1S/C27H28FN5O4/c1-3-37-27(36)16-10-17(11-16)30-13-15-4-5-19-22(9-15)33-26(35)24-20(6-7-29-25(24)32-19)31-21-12-23(34)14(2)8-18(21)28/h4-9,12,16-17,30,34H,3,10-11,13H2,1-2H3,(H,33,35)(H2,29,31,32)/t16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.626 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227801
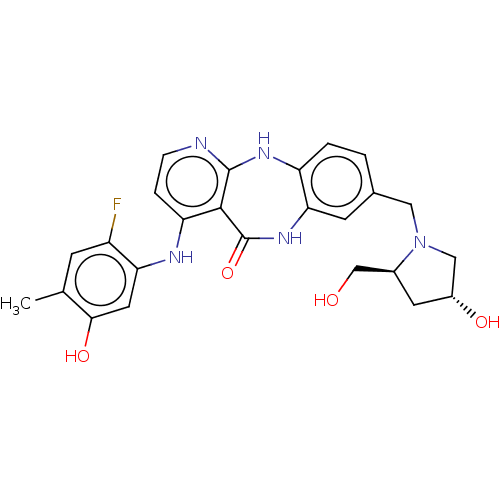
(4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-{[(...)Show SMILES Cc1cc(F)c(Nc2ccnc3Nc4ccc(CN5C[C@H](O)C[C@H]5CO)cc4NC(=O)c23)cc1O |r| Show InChI InChI=1S/C25H26FN5O4/c1-13-6-17(26)20(9-22(13)34)28-19-4-5-27-24-23(19)25(35)30-21-7-14(2-3-18(21)29-24)10-31-11-16(33)8-15(31)12-32/h2-7,9,15-16,32-34H,8,10-12H2,1H3,(H,30,35)(H2,27,28,29)/t15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265679
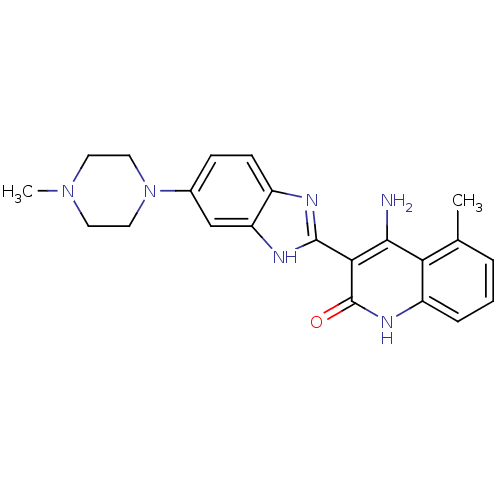
(4-Amino-5-methyl-3-[6-(4-methylpiperazinyl)benzimi...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2c(C)cccc2[nH]c1=O Show InChI InChI=1S/C22H24N6O/c1-13-4-3-5-16-18(13)20(23)19(22(29)26-16)21-24-15-7-6-14(12-17(15)25-21)28-10-8-27(2)9-11-28/h3-7,12H,8-11H2,1-2H3,(H,24,25)(H3,23,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50381949

(CHEMBL2023118)Show SMILES CCn1cc(NC(=O)Cc2ccc(Oc3ccnc4cc(OC)ccc34)cc2OC)cn1 Show InChI InChI=1S/C24H24N4O4/c1-4-28-15-17(14-26-28)27-24(29)11-16-5-6-19(13-23(16)31-3)32-22-9-10-25-21-12-18(30-2)7-8-20(21)22/h5-10,12-15H,4,11H2,1-3H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta |
Bioorg Med Chem Lett 22: 3050-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.074
BindingDB Entry DOI: 10.7270/Q298881Z |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227939
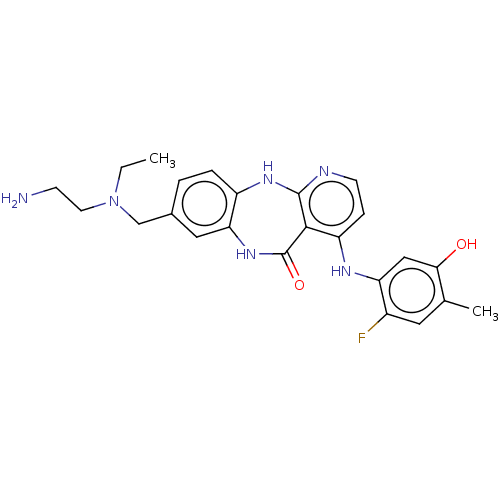
(8-{[(2-Aminoethyl)(ethyl)amino]methyl}-4-[(2-fluor...)Show SMILES CCN(CCN)Cc1ccc2Nc3nccc(Nc4cc(O)c(C)cc4F)c3C(=O)Nc2c1 Show InChI InChI=1S/C24H27FN6O2/c1-3-31(9-7-26)13-15-4-5-17-20(11-15)30-24(33)22-18(6-8-27-23(22)29-17)28-19-12-21(32)14(2)10-16(19)25/h4-6,8,10-12,32H,3,7,9,13,26H2,1-2H3,(H,30,33)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.727 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227948

(8-{[(2-Aminoethyl)(methyl)amino]methyl}-4-[(2-fluo...)Show SMILES CN(CCN)Cc1ccc2Nc3nccc(Nc4cc(O)c(C)cc4F)c3C(=O)Nc2c1 Show InChI InChI=1S/C23H25FN6O2/c1-13-9-15(24)18(11-20(13)31)27-17-5-7-26-22-21(17)23(32)29-19-10-14(3-4-16(19)28-22)12-30(2)8-6-25/h3-5,7,9-11,31H,6,8,12,25H2,1-2H3,(H,29,32)(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.739 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli "Federico II"
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDGFRbeta expressed in insect cells using Ulight-Poly GAT[EAY(1:1:1)]n as substrate after 30 mins by LANCE method |
Eur J Med Chem 150: 491-505 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.080
BindingDB Entry DOI: 10.7270/Q2B27XXB |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227829
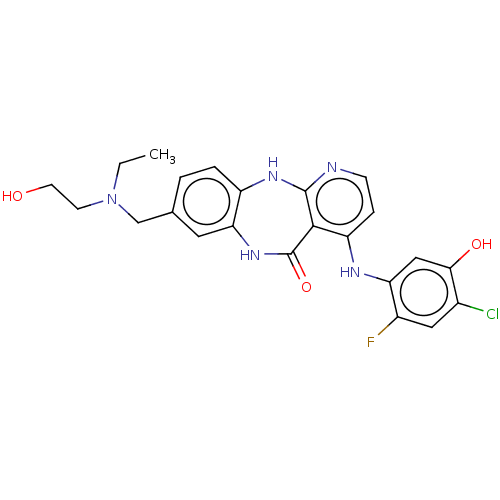
(4-[(4-Chloro-2-fluoro-5-hydroxyphenyl)amino]-8-{[e...)Show SMILES CCN(CCO)Cc1ccc2Nc3nccc(Nc4cc(O)c(Cl)cc4F)c3C(=O)Nc2c1 Show InChI InChI=1S/C23H23ClFN5O3/c1-2-30(7-8-31)12-13-3-4-16-19(9-13)29-23(33)21-17(5-6-26-22(21)28-16)27-18-11-20(32)14(24)10-15(18)25/h3-6,9-11,31-32H,2,7-8,12H2,1H3,(H,29,33)(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.793 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50362064

(CHEMBL1940108)Show SMILES COc1cccc(NC(=O)Cn2cc(Oc3ncnc4cc(OC)c(OC)cc34)cn2)c1 Show InChI InChI=1S/C22H21N5O5/c1-29-15-6-4-5-14(7-15)26-21(28)12-27-11-16(10-25-27)32-22-17-8-19(30-2)20(31-3)9-18(17)23-13-24-22/h4-11,13H,12H2,1-3H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta phosphorylation by cell based assay |
Bioorg Med Chem Lett 22: 262-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.019
BindingDB Entry DOI: 10.7270/Q24F1R6H |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227813

(US10047096, 75 | rac-4-[(2-Fluoro-5-hydroxy-4-meth...)Show SMILES CC(C)C(CO)NCc1ccc2Nc3nccc(Nc4cc(O)c(C)cc4F)c3C(=O)Nc2c1 Show InChI InChI=1S/C25H28FN5O3/c1-13(2)21(12-32)28-11-15-4-5-17-20(9-15)31-25(34)23-18(6-7-27-24(23)30-17)29-19-10-22(33)14(3)8-16(19)26/h4-10,13,21,28,32-33H,11-12H2,1-3H3,(H,31,34)(H2,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.826 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227951

(8-{[(3R)-3-Aminopiperidin-1-yl]methyl}-4-[(2-fluor...)Show SMILES Cc1cc(F)c(Nc2ccnc3Nc4ccc(CN5CCC[C@@H](N)C5)cc4NC(=O)c23)cc1O |r| Show InChI InChI=1S/C25H27FN6O2/c1-14-9-17(26)20(11-22(14)33)29-19-6-7-28-24-23(19)25(34)31-21-10-15(4-5-18(21)30-24)12-32-8-2-3-16(27)13-32/h4-7,9-11,16,33H,2-3,8,12-13,27H2,1H3,(H,31,34)(H2,28,29,30)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.829 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227936

(4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-(pi...)Show SMILES Cc1cc(F)c(Nc2ccnc3Nc4ccc(CN5CCNCC5)cc4NC(=O)c23)cc1O Show InChI InChI=1S/C24H25FN6O2/c1-14-10-16(25)19(12-21(14)32)28-18-4-5-27-23-22(18)24(33)30-20-11-15(2-3-17(20)29-23)13-31-8-6-26-7-9-31/h2-5,10-12,26,32H,6-9,13H2,1H3,(H,30,33)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.861 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50362075
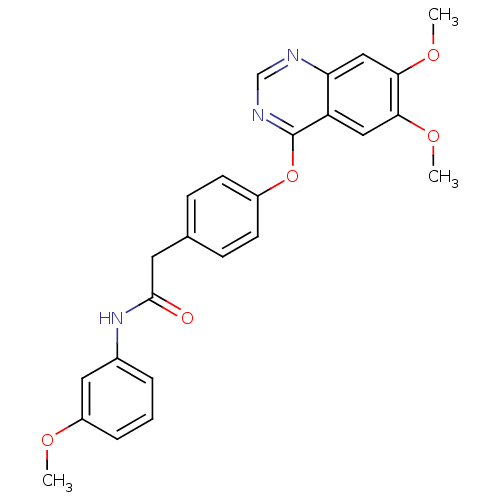
(CHEMBL1940259)Show SMILES COc1cccc(NC(=O)Cc2ccc(Oc3ncnc4cc(OC)c(OC)cc34)cc2)c1 Show InChI InChI=1S/C25H23N3O5/c1-30-19-6-4-5-17(12-19)28-24(29)11-16-7-9-18(10-8-16)33-25-20-13-22(31-2)23(32-3)14-21(20)26-15-27-25/h4-10,12-15H,11H2,1-3H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta phosphorylation by cell based assay |
Bioorg Med Chem Lett 22: 262-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.019
BindingDB Entry DOI: 10.7270/Q24F1R6H |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50604027

(CHEMBL5184634 | US20230312529, Compound 6i)Show SMILES CCN1CCN(CC1)c1ccc(Nc2ncc(C)c(Nc3ccc4cn[nH]c4c3)n2)cc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01732
BindingDB Entry DOI: 10.7270/Q2QR527K |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50381923

(CHEMBL2023117)Show SMILES CCn1cc(NC(=O)Cc2ccc(Oc3ccnc4cc(OC)c(OC)cc34)cc2OC)cn1 Show InChI InChI=1S/C25H26N4O5/c1-5-29-15-17(14-27-29)28-25(30)10-16-6-7-18(11-22(16)31-2)34-21-8-9-26-20-13-24(33-4)23(32-3)12-19(20)21/h6-9,11-15H,5,10H2,1-4H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta |
Bioorg Med Chem Lett 22: 3050-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.074
BindingDB Entry DOI: 10.7270/Q298881Z |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265613
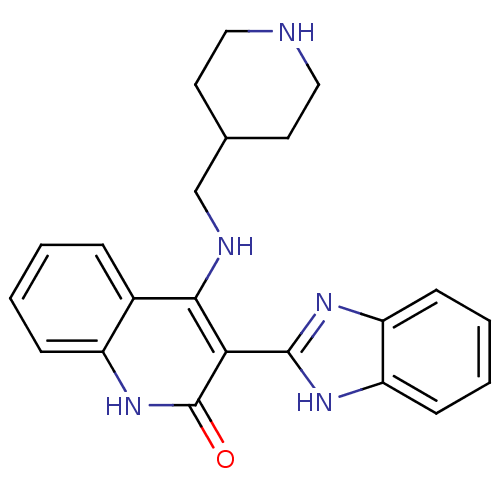
(3-(1H-benzo[d]imidazol-2-yl)-4-(piperidin-4-ylmeth...)Show SMILES O=c1[nH]c2ccccc2c(NCC2CCNCC2)c1-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H23N5O/c28-22-19(21-25-17-7-3-4-8-18(17)26-21)20(15-5-1-2-6-16(15)27-22)24-13-14-9-11-23-12-10-14/h1-8,14,23H,9-13H2,(H,25,26)(H2,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265641
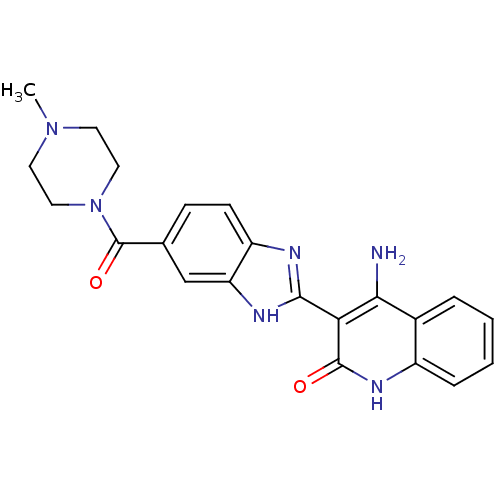
(4-Amino-3-{6-[(4-methylpiperazinyl)carbonyl]benzim...)Show SMILES CN1CCN(CC1)C(=O)c1ccc2nc([nH]c2c1)-c1c(N)c2ccccc2[nH]c1=O Show InChI InChI=1S/C22H22N6O2/c1-27-8-10-28(11-9-27)22(30)13-6-7-16-17(12-13)25-20(24-16)18-19(23)14-4-2-3-5-15(14)26-21(18)29/h2-7,12H,8-11H2,1H3,(H,24,25)(H3,23,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227792
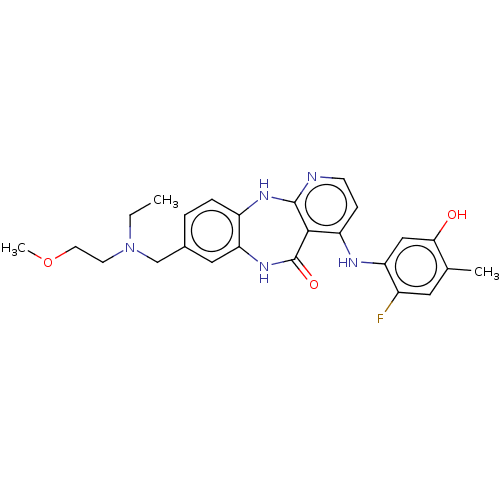
(8-{[Ethyl(2-methoxyethyl)amino]methyl}-4-[(2-fluor...)Show SMILES CCN(CCOC)Cc1ccc2Nc3nccc(Nc4cc(O)c(C)cc4F)c3C(=O)Nc2c1 Show InChI InChI=1S/C25H28FN5O3/c1-4-31(9-10-34-3)14-16-5-6-18-21(12-16)30-25(33)23-19(7-8-27-24(23)29-18)28-20-13-22(32)15(2)11-17(20)26/h5-8,11-13,32H,4,9-10,14H2,1-3H3,(H,30,33)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.905 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227782

(4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-[(4...)Show SMILES Cc1cc(F)c(Nc2ccnc3Nc4ccc(CN5CCC(O)CC5)cc4NC(=O)c23)cc1O Show InChI InChI=1S/C25H26FN5O3/c1-14-10-17(26)20(12-22(14)33)28-19-4-7-27-24-23(19)25(34)30-21-11-15(2-3-18(21)29-24)13-31-8-5-16(32)6-9-31/h2-4,7,10-12,16,32-33H,5-6,8-9,13H2,1H3,(H,30,34)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.928 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227765

(8-[(Diethylamino)methyl]-4-[(2-fluoro-5-hydroxy-4-...)Show SMILES CCN(CC)Cc1ccc2Nc3nccc(Nc4cc(O)c(C)cc4F)c3C(=O)Nc2c1 Show InChI InChI=1S/C24H26FN5O2/c1-4-30(5-2)13-15-6-7-17-20(11-15)29-24(32)22-18(8-9-26-23(22)28-17)27-19-12-21(31)14(3)10-16(19)25/h6-12,31H,4-5,13H2,1-3H3,(H,29,32)(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.953 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227790
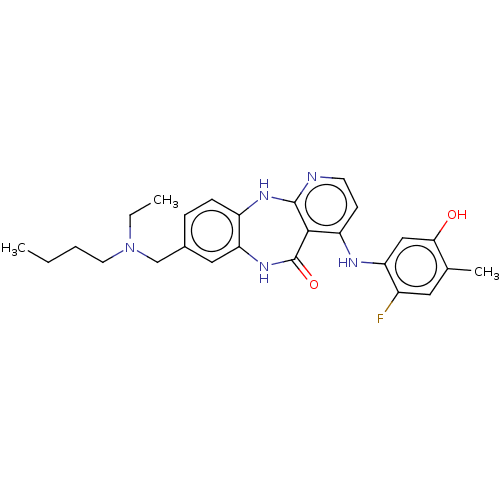
(8-{[Butyl(ethyl)amino]methyl}-4-[(2-fluoro-5-hydro...)Show SMILES CCCCN(CC)Cc1ccc2Nc3nccc(Nc4cc(O)c(C)cc4F)c3C(=O)Nc2c1 Show InChI InChI=1S/C26H30FN5O2/c1-4-6-11-32(5-2)15-17-7-8-19-22(13-17)31-26(34)24-20(9-10-28-25(24)30-19)29-21-14-23(33)16(3)12-18(21)27/h7-10,12-14,33H,4-6,11,15H2,1-3H3,(H,31,34)(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.962 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227937

(4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-{[(...)Show SMILES CN(CCO)Cc1ccc2Nc3nccc(Nc4cc(O)c(C)cc4F)c3C(=O)Nc2c1 Show InChI InChI=1S/C23H24FN5O3/c1-13-9-15(24)18(11-20(13)31)26-17-5-6-25-22-21(17)23(32)28-19-10-14(3-4-16(19)27-22)12-29(2)7-8-30/h3-6,9-11,30-31H,7-8,12H2,1-2H3,(H,28,32)(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.962 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227816

(8-{[(1,3-Dihydroxypropan-2-yl)amino]methyl}-4-[(2-...)Show SMILES Cc1cc(F)c(Nc2ccnc3Nc4ccc(CNC(CO)CO)cc4NC(=O)c23)cc1O Show InChI InChI=1S/C23H24FN5O4/c1-12-6-15(24)18(8-20(12)32)27-17-4-5-25-22-21(17)23(33)29-19-7-13(2-3-16(19)28-22)9-26-14(10-30)11-31/h2-8,14,26,30-32H,9-11H2,1H3,(H,29,33)(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.974 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227807
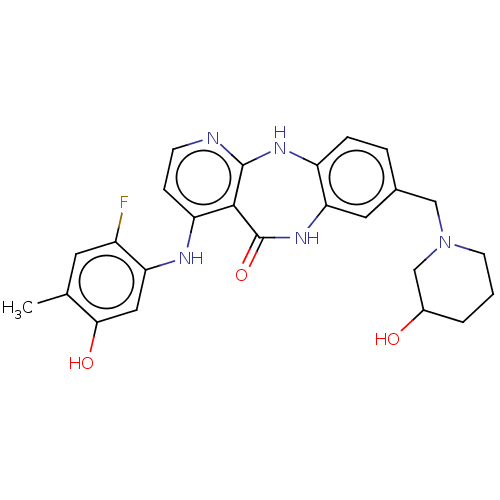
(US10047096, 69 | US10047096, 70 | US10047096, 71 |...)Show SMILES Cc1cc(F)c(Nc2ccnc3Nc4ccc(CN5CCCC(O)C5)cc4NC(=O)c23)cc1O Show InChI InChI=1S/C25H26FN5O3/c1-14-9-17(26)20(11-22(14)33)28-19-6-7-27-24-23(19)25(34)30-21-10-15(4-5-18(21)29-24)12-31-8-2-3-16(32)13-31/h4-7,9-11,16,32-33H,2-3,8,12-13H2,1H3,(H,30,34)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.985 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM227946

(4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-{[(...)Show SMILES Cc1cc(F)c(Nc2ccnc3Nc4ccc(CN5CC[C@H](O)C5)cc4NC(=O)c23)cc1O |r| Show InChI InChI=1S/C24H24FN5O3/c1-13-8-16(25)19(10-21(13)32)27-18-4-6-26-23-22(18)24(33)29-20-9-14(2-3-17(20)28-23)11-30-7-5-15(31)12-30/h2-4,6,8-10,15,31-32H,5,7,11-12H2,1H3,(H,29,33)(H2,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.995 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The kinase used was a recombinant fusion protein composed of N-terminal GST and a C-terminal fragment of human PDGFRβ (amino acids R561-L1106), expr... |
US Patent US10047096 (2018)
BindingDB Entry DOI: 10.7270/Q23R0VW4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data