

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50062357 (AP26113 | CHEMBL3397300) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018836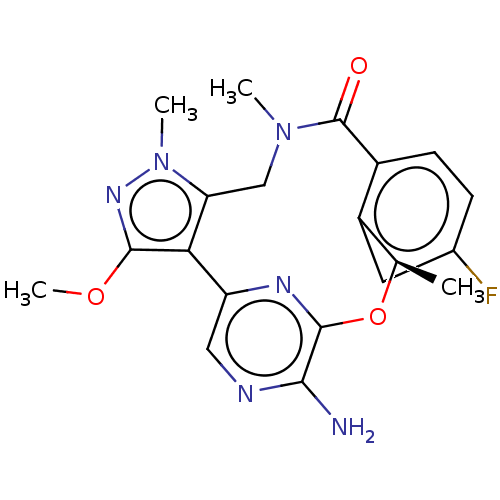 (CHEMBL3286826) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human EML4-fused ALK expressed in mouse NIH-3T3 cells assessed as phosphorylated ALK level after 1 hr by sandwich ELISA | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50185237 (CHEMBL3824308) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM482158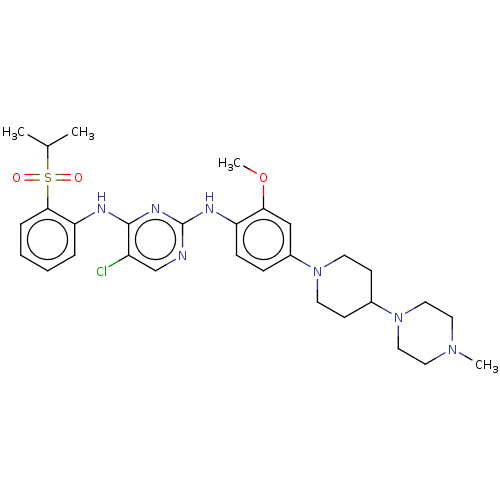 (BDBM50242742 | TAE684) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM178978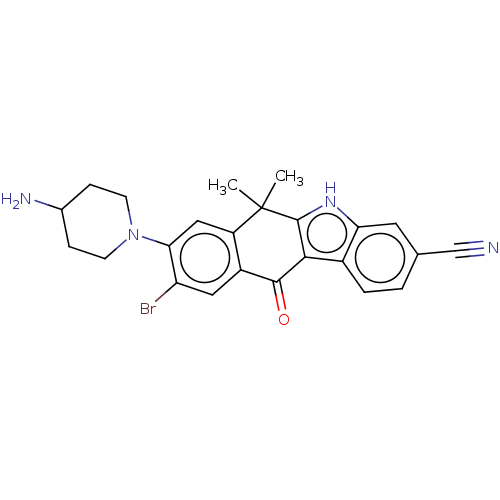 (US9126931, 277) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.168 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha US Patent | Assay Description ALK-inhibiting activity was measured by following an activity of inhibiting phosphorylation by biotinylated peptide (EGPWLEEEEEAYGWMDF). For the dete... | US Patent US9126931 (2015) BindingDB Entry DOI: 10.7270/Q22J69N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50185280 (CHEMBL3822611) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50185275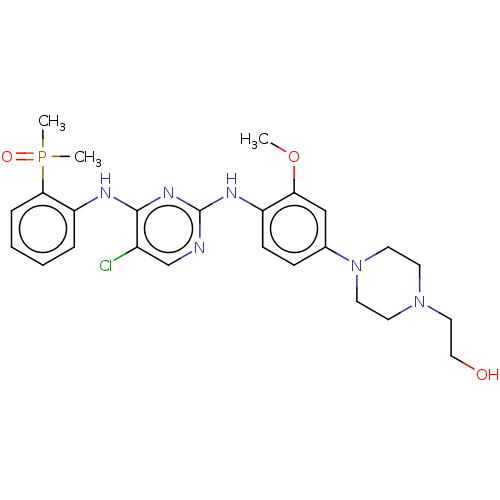 (CHEMBL3823235) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018835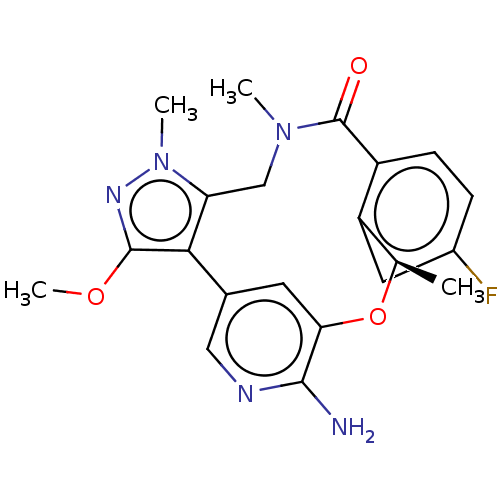 (CHEMBL3286825) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human EML4-fused ALK expressed in mouse NIH-3T3 cells assessed as phosphorylated ALK level after 1 hr by sandwich ELISA | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50448785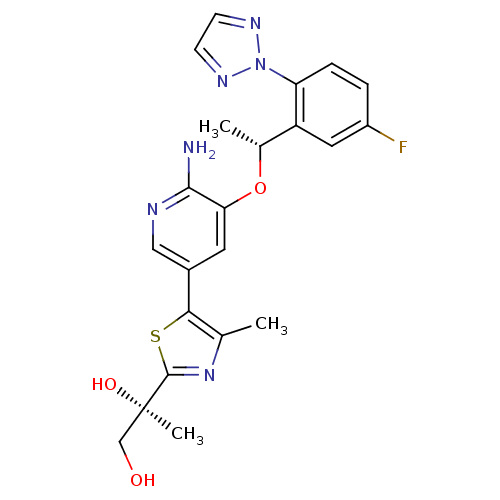 (CHEMBL3128069) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of ALK L1196M (unknown origin) | J Med Chem 62: 10927-10954 (2019) Article DOI: 10.1021/acs.jmedchem.9b00446 BindingDB Entry DOI: 10.7270/Q2S185WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436850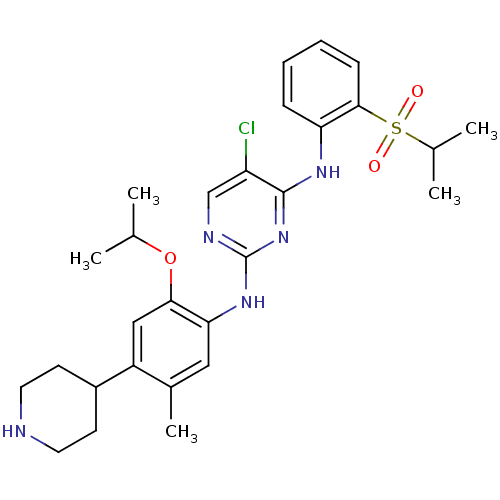 (CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of ALK (unknown origin) | J Med Chem 56: 5673-4 (2014) Article DOI: 10.1021/jm401005u BindingDB Entry DOI: 10.7270/Q2B56M4Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436850 (CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of ALK (unknown origin) after 60 mins | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018830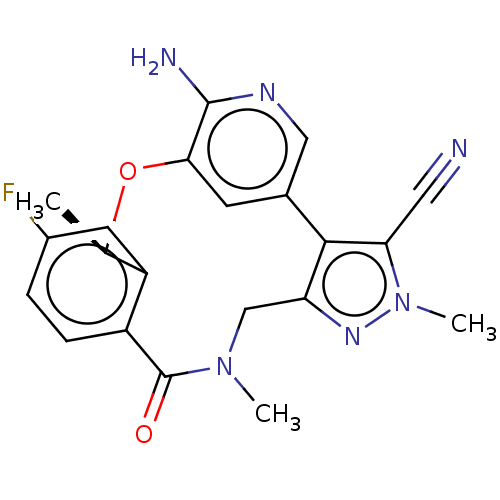 (CHEMBL3286830 | US10543199, Compound PF-06463922 |...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human EML4-fused ALK F1174L mutant expressed in mouse NIH-3T3 cells assessed as phospho-ALK level after 1 hr by sandwich ELISA | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018833 (CHEMBL3286823) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human EML4-fused ALK expressed in mouse NIH-3T3 cells assessed as phosphorylated ALK level after 1 hr by sandwich ELISA | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243386 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(7-is...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50185284 (CHEMBL3823549) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50185281 (CHEMBL3823416) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243402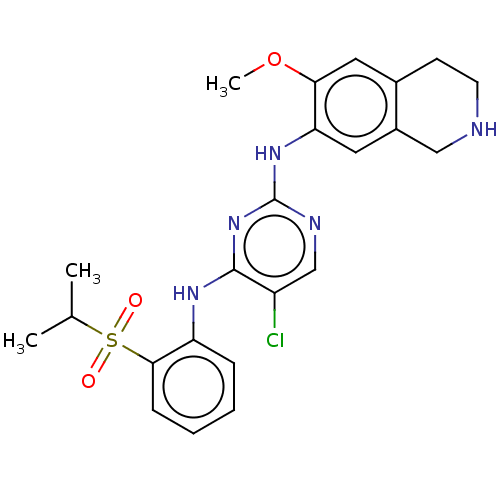 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(6-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of crizotinib-resistant ALK G1269A mutant (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescenc... | Citation and Details Article DOI: 10.1016/j.bmc.2015.12.004 BindingDB Entry DOI: 10.7270/Q269778Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243393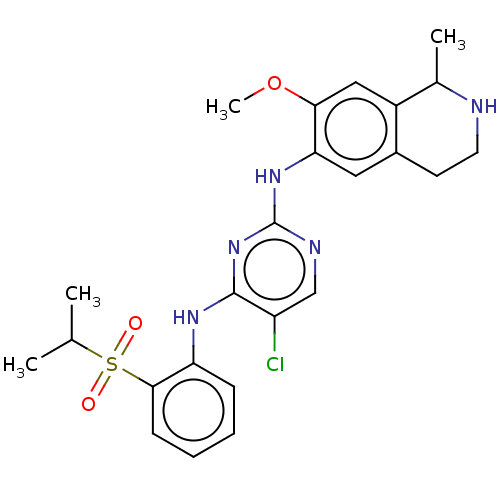 (5-chloro-N4-2-(isopropylsulfonyl)phenyl)-N2-(7-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436850 (CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ALK L1196M mutant (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113672 BindingDB Entry DOI: 10.7270/Q2DN48TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50519597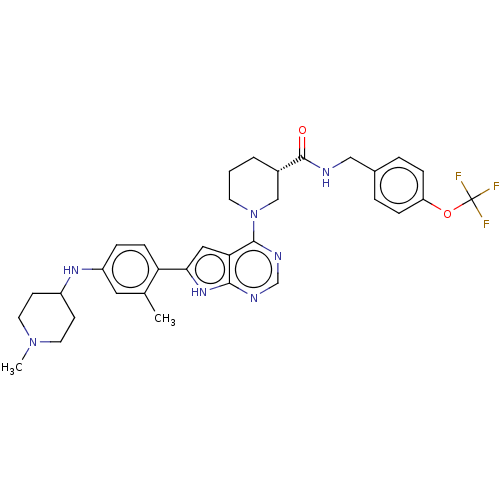 (CHEMBL4522012) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of ALK (unknown origin) by TR-FRET assay | J Med Chem 62: 10927-10954 (2019) Article DOI: 10.1021/acs.jmedchem.9b00446 BindingDB Entry DOI: 10.7270/Q2S185WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50185268 (CHEMBL3824327) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243395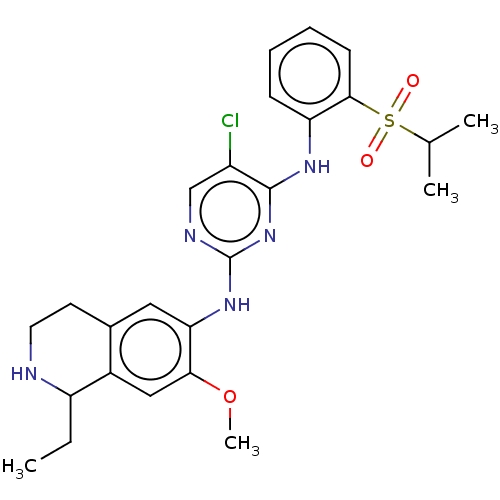 (5-chloro-N4-2-(isopropylsulfonyl)phenyl)-N2-(7-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM179071 (US9126931, 796) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha US Patent | Assay Description ALK-inhibiting activity was measured by following an activity of inhibiting phosphorylation by biotinylated peptide (EGPWLEEEEEAYGWMDF). For the dete... | US Patent US9126931 (2015) BindingDB Entry DOI: 10.7270/Q22J69N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM179072 (US9126931, 822) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha US Patent | Assay Description ALK-inhibiting activity was measured by following an activity of inhibiting phosphorylation by biotinylated peptide (EGPWLEEEEEAYGWMDF). For the dete... | US Patent US9126931 (2015) BindingDB Entry DOI: 10.7270/Q22J69N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM179073 (US9126931, 823) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha US Patent | Assay Description ALK-inhibiting activity was measured by following an activity of inhibiting phosphorylation by biotinylated peptide (EGPWLEEEEEAYGWMDF). For the dete... | US Patent US9126931 (2015) BindingDB Entry DOI: 10.7270/Q22J69N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50185290 (CHEMBL3824304) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM178992 (US9126931, 338) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha US Patent | Assay Description ALK-inhibiting activity was measured by following an activity of inhibiting phosphorylation by biotinylated peptide (EGPWLEEEEEAYGWMDF). For the dete... | US Patent US9126931 (2015) BindingDB Entry DOI: 10.7270/Q22J69N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM179026 (US9126931, 543) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha US Patent | Assay Description ALK-inhibiting activity was measured by following an activity of inhibiting phosphorylation by biotinylated peptide (EGPWLEEEEEAYGWMDF). For the dete... | US Patent US9126931 (2015) BindingDB Entry DOI: 10.7270/Q22J69N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50185286 (CHEMBL3823256) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50185283 (CHEMBL3823603) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50185272 (CHEMBL3823107) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50240271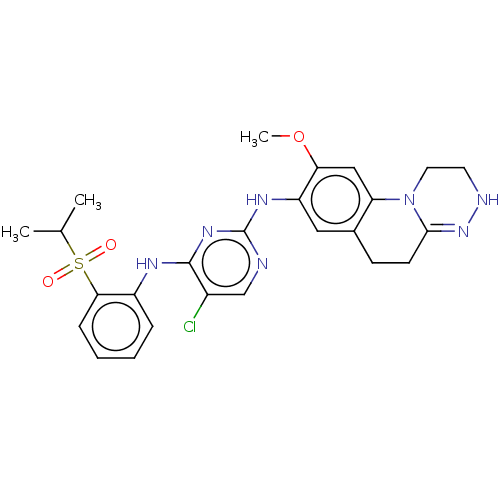 (CHEMBL4101954) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes. | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018838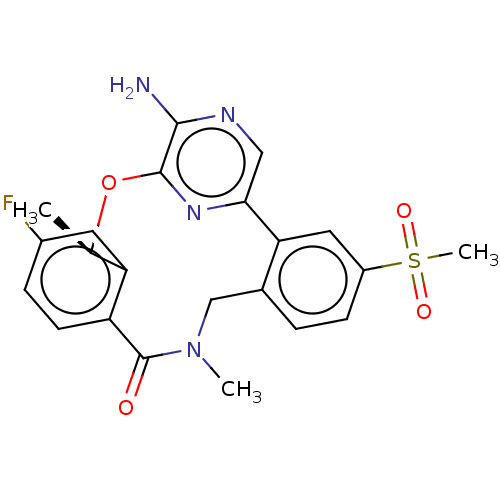 (CHEMBL3286828) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human EML4-fused ALK expressed in mouse NIH-3T3 cells assessed as phosphorylated ALK level after 1 hr by sandwich ELISA | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50185278 (CHEMBL3823017) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50185140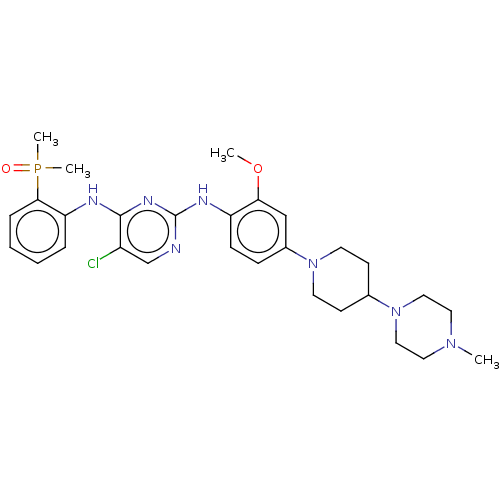 (AP-26113 | Brigatinib | US11248003, Example Brigat...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50116683 (CHEMBL3608526 | US10053458, 49) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50185245 (CHEMBL3823190) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50185239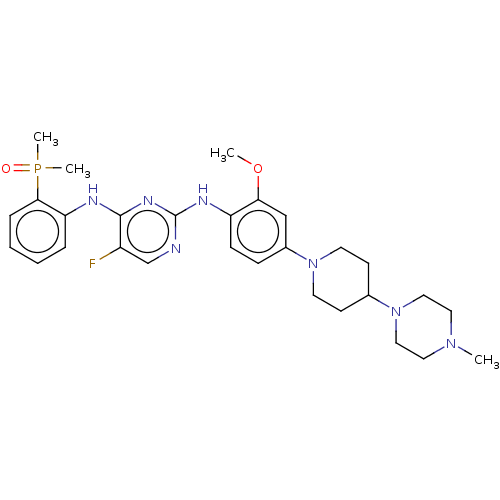 (CHEMBL3823296) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM179002 (US9126931, 403) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.391 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha US Patent | Assay Description ALK-inhibiting activity was measured by following an activity of inhibiting phosphorylation by biotinylated peptide (EGPWLEEEEEAYGWMDF). For the dete... | US Patent US9126931 (2015) BindingDB Entry DOI: 10.7270/Q22J69N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50573595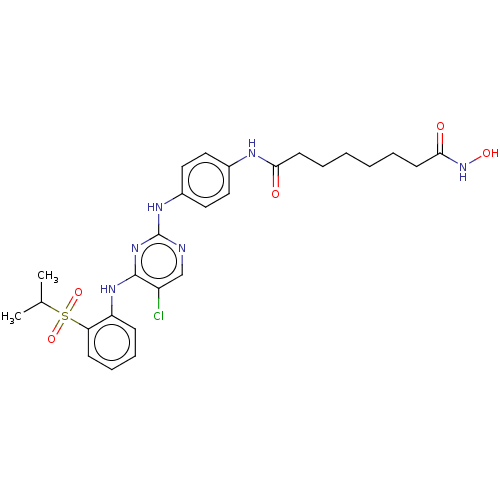 (CHEMBL4855319) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ALK G1202R mutant (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113672 BindingDB Entry DOI: 10.7270/Q2DN48TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50265869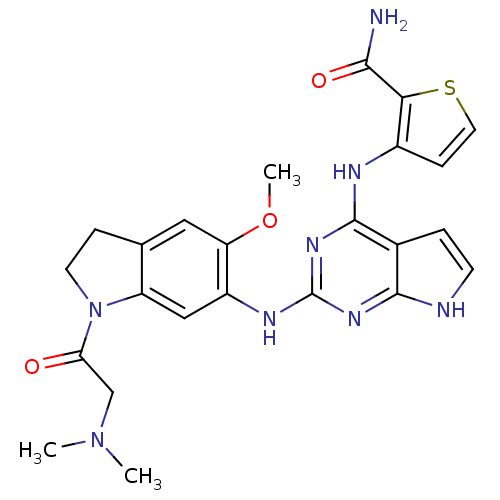 (3-(2-(1-(2-(dimethylamino)acetyl)-5-methoxyindolin...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of ALK | Bioorg Med Chem Lett 19: 373-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.065 BindingDB Entry DOI: 10.7270/Q2F190PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM179069 (US9126931, 786) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha US Patent | Assay Description ALK-inhibiting activity was measured by following an activity of inhibiting phosphorylation by biotinylated peptide (EGPWLEEEEEAYGWMDF). For the dete... | US Patent US9126931 (2015) BindingDB Entry DOI: 10.7270/Q22J69N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM178997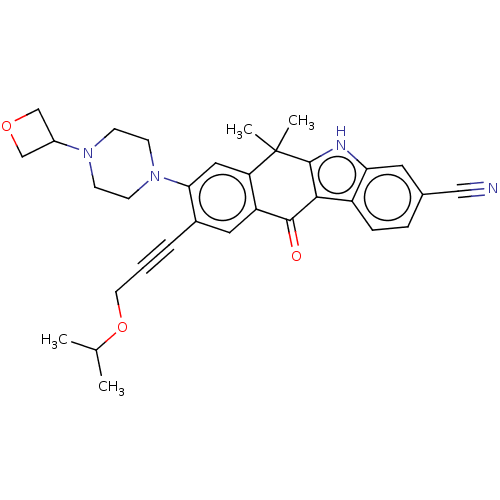 (US9126931, 359) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha US Patent | Assay Description ALK-inhibiting activity was measured by following an activity of inhibiting phosphorylation by biotinylated peptide (EGPWLEEEEEAYGWMDF). For the dete... | US Patent US9126931 (2015) BindingDB Entry DOI: 10.7270/Q22J69N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM179297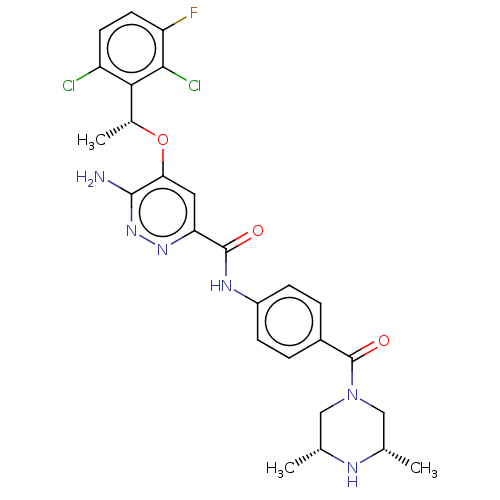 (US9126947, 18) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of ALK (unknown origin) by kinome scan-based assay | J Med Chem 62: 10927-10954 (2019) Article DOI: 10.1021/acs.jmedchem.9b00446 BindingDB Entry DOI: 10.7270/Q2S185WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50185238 (CHEMBL3823577) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM291934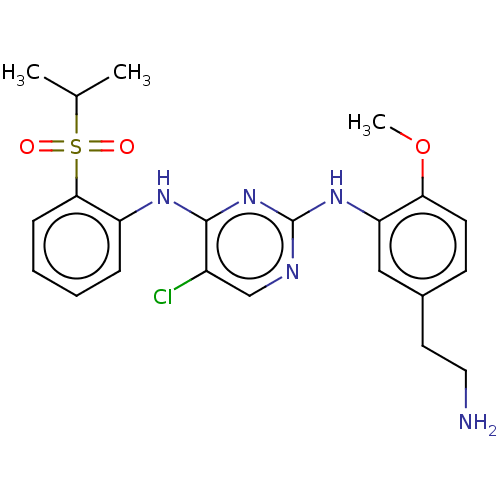 (US10100019, Example 4) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | 25 |
Korea Research Institute of Chemical Technology US Patent | Assay Description The following experiment was performed in order to measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of ... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243402 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(6-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of crizotinib-resistant ALK C1156Y mutant (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescenc... | Citation and Details Article DOI: 10.1016/j.bmc.2015.12.004 BindingDB Entry DOI: 10.7270/Q269778Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM179033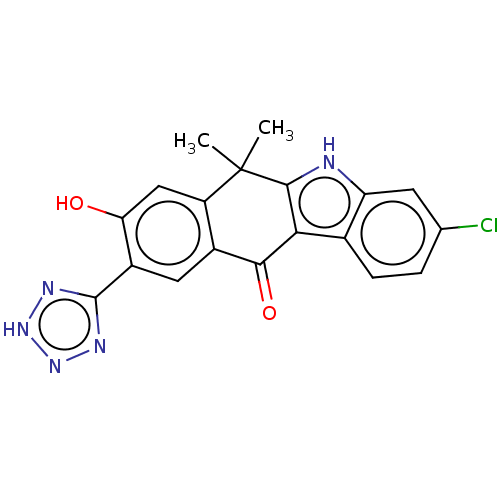 (US9126931, 601) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.456 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha US Patent | Assay Description ALK-inhibiting activity was measured by following an activity of inhibiting phosphorylation by biotinylated peptide (EGPWLEEEEEAYGWMDF). For the dete... | US Patent US9126931 (2015) BindingDB Entry DOI: 10.7270/Q22J69N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243402 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(6-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of crizotinib-resistant ALK F1174L mutant (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescenc... | Citation and Details Article DOI: 10.1016/j.bmc.2015.12.004 BindingDB Entry DOI: 10.7270/Q269778Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50554829 (CHEMBL4790570) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST tagged wild-type human ALK cytoplasmic domain (1058-1620 amino acids) expressed Sf9 cells pre-incubated for 30 mins befo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01550 BindingDB Entry DOI: 10.7270/Q2KK9GG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3485 total ) | Next | Last >> |