

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50292410 ((-)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist required to inhibit Dopamine receptor D2 photoinactivation by 50% with Iodazidoclebopride using [3H]spiperone | J Med Chem 28: 405-7 (1985) BindingDB Entry DOI: 10.7270/Q2542P5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist required to inhibit Dopamine receptor D2 photoinactivation by 50% with Iodazidoclebopride using [3H]-Spiperone | J Med Chem 28: 405-7 (1985) BindingDB Entry DOI: 10.7270/Q2542P5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50029051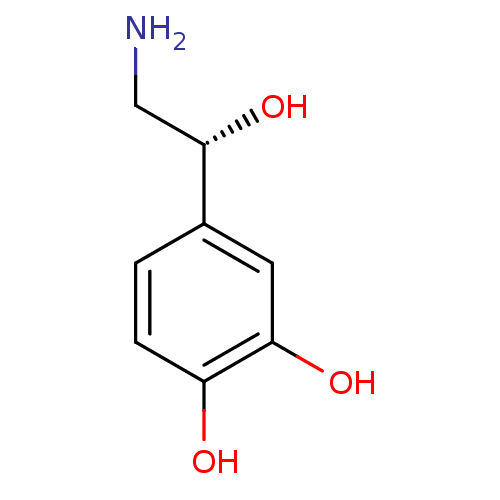 ((-)-arterenol | (-)-noradrenaline | (-)-norepineph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist required to inhibit Dopamine receptor D2 photoinactivation by 50% with Iodazidoclebopride using [3H]-Spiperone | J Med Chem 28: 405-7 (1985) BindingDB Entry DOI: 10.7270/Q2542P5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist required to inhibit Dopamine receptor D2 photoinactivation by 50% with Iodazidoclebopride using [3H]-Spiperone | J Med Chem 28: 405-7 (1985) BindingDB Entry DOI: 10.7270/Q2542P5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50366495 ((+)butaclamol | CHEMBL1255588) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist required to inhibit Dopamine receptor D2 photoinactivation by 50% with Iodazidoclebopride using [3H]spiperone | J Med Chem 28: 405-7 (1985) BindingDB Entry DOI: 10.7270/Q2542P5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50007422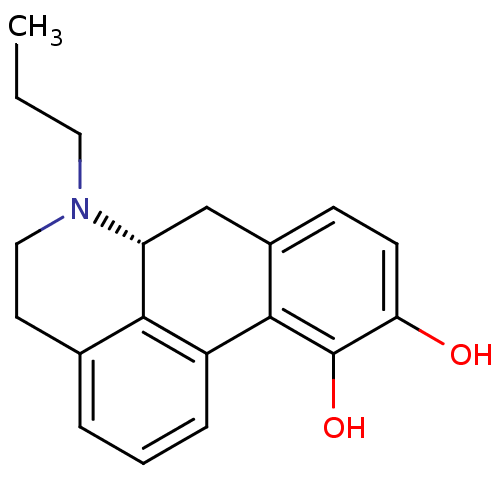 ((+)-6-Propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist required to inhibit Dopamine receptor D2 photoinactivation by 50% with Iodazidoclebopride using [3H]-Spiperone | J Med Chem 28: 405-7 (1985) BindingDB Entry DOI: 10.7270/Q2542P5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist required to inhibit Dopamine receptor D2 photoinactivation by 50% with Iodazidoclebopride using [3H]spiperone | J Med Chem 28: 405-7 (1985) BindingDB Entry DOI: 10.7270/Q2542P5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM81774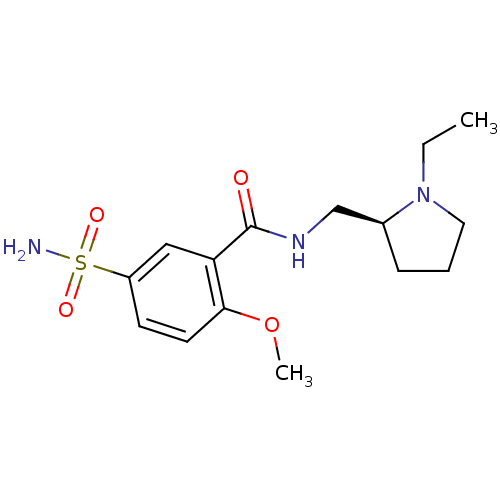 (CAS_15676-16-1 | SULPIRIDE,(+) | Sulpiride-S | Sul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist required to inhibit Dopamine receptor D2 photoinactivation by 50% with Iodazidoclebopride using [3H]spiperone | J Med Chem 28: 405-7 (1985) BindingDB Entry DOI: 10.7270/Q2542P5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM81195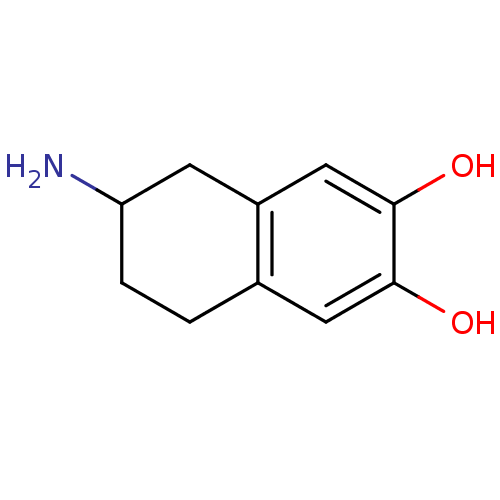 ((+/-)-2-Amino-6,7-dihydroxy-1,2,3,4-tetrahydronaph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist required to inhibit Dopamine receptor D2 photoinactivation by 50% with Iodazidoclebopride using [3H]-Spiperone | J Med Chem 28: 405-7 (1985) BindingDB Entry DOI: 10.7270/Q2542P5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50007422 ((+)-6-Propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist required to inhibit Dopamine receptor D2 photoinactivation by 50% with Iodazidoclebopride using [3H]-Spiperone | J Med Chem 28: 405-7 (1985) BindingDB Entry DOI: 10.7270/Q2542P5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM319696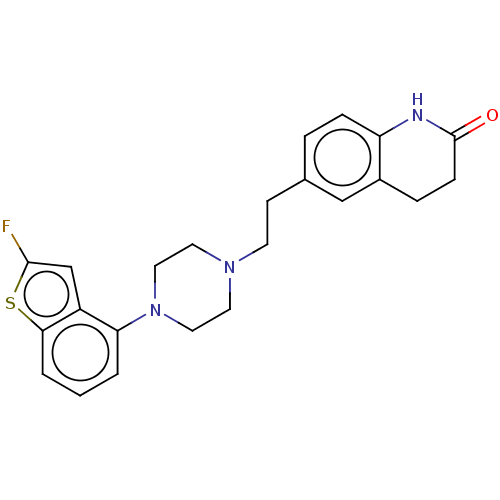 (6-(2-(4-(2-fluorobenzo[b]thiophen-4-yl)piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The D2 receptor agonism activity test (The agonism activity of test compounds on D2 receptor expressing human recombinant D2 receptor in HEK293 cells... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50511517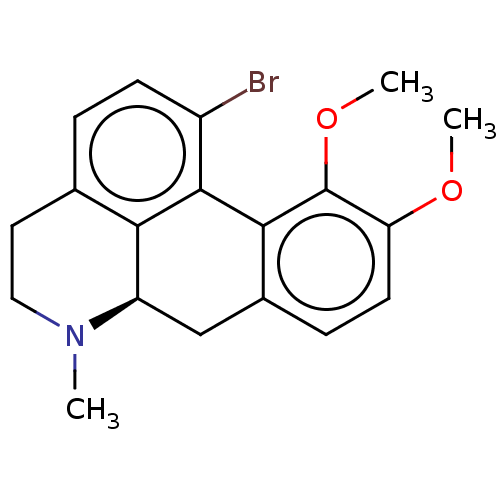 (CHEMBL4467483) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.123 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Antagonist activity at D2 long receptor (unknown origin) transfected in human HEK293T cells assessed as increase in cAMP accumulation incubated for 2... | ACS Med Chem Lett 11: 385-392 (2020) Article DOI: 10.1021/acsmedchemlett.9b00575 BindingDB Entry DOI: 10.7270/Q25X2D8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50511517 (CHEMBL4467483) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.124 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Antagonist activity at D2 long receptor (unknown origin) transfected in human HEK293T cells assessed as increase in cAMP accumulation incubated for 2... | ACS Med Chem Lett 11: 385-392 (2020) Article DOI: 10.1021/acsmedchemlett.9b00575 BindingDB Entry DOI: 10.7270/Q25X2D8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against human D2LR expressed in CHOK1 cells assessed as inhibition of pergolide-induced beta-arrestin translocation by beta-galac... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Antagonist activity at recombinant human D2 receptor expressed in CHOK1 cells assessed as inhibition of quinpirole-induced beta arrestin2 recruitment... | J Med Chem 62: 5132-5147 (2019) Article DOI: 10.1021/acs.jmedchem.9b00412 BindingDB Entry DOI: 10.7270/Q2Z03CM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM319702 (5-(2-(4-(2-fluorobenzo[b]thiophen-4-yl)piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.219 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The D2 receptor agonism activity test (The agonism activity of test compounds on D2 receptor expressing human recombinant D2 receptor in HEK293 cells... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM319695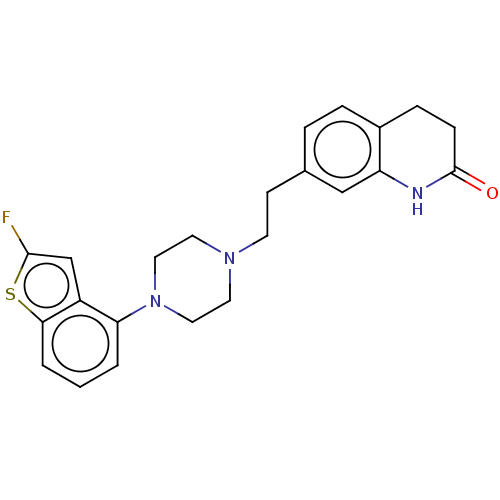 (7-(2-(4-(2-fluorobenzo[b]thiophen-4-yl)piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.242 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The D2 receptor agonism activity test (The agonism activity of test compounds on D2 receptor expressing human recombinant D2 receptor in HEK293 cells... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human recombinant dopamine D2S receptor expressed in CHO cells after 2 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50417295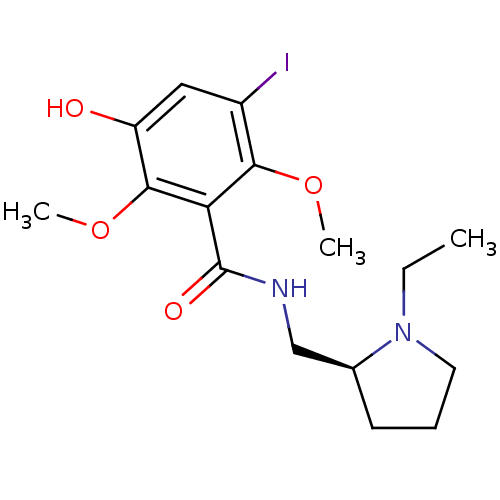 (CHEMBL1276325) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.288 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Antagonist activity at dopamine D2 receptor | Eur J Med Chem 45: 4856-62 (2010) Article DOI: 10.1016/j.ejmech.2010.07.056 BindingDB Entry DOI: 10.7270/Q2833T8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50130273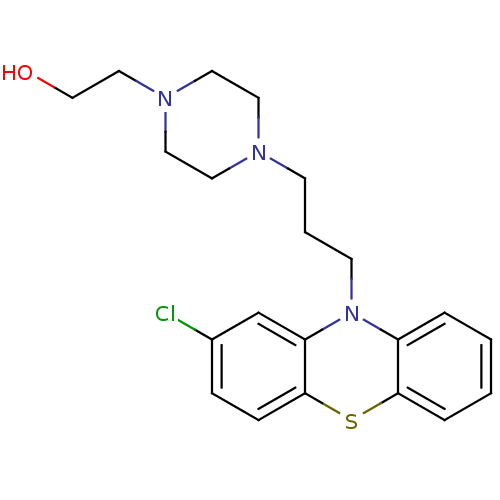 (2-(4-[3-(2-chloro-10H-phenothiazin-10-yl)propyl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | 25 |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at dopamine D2 receptor (unknown origin) expressed in CHOK1 cells coexpressing Galpha15 assessed as inhibition of agonist-induced... | J Med Chem 56: 4671-90 (2013) Article DOI: 10.1021/jm400408r BindingDB Entry DOI: 10.7270/Q2R49S50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM319701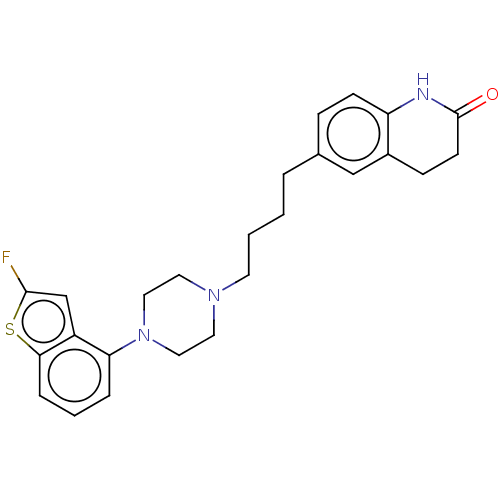 (6-(4-(4-(2-fluorobenzo[b]thiophen-4-yl)piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The D2 receptor agonism activity test (The agonism activity of test compounds on D2 receptor expressing human recombinant D2 receptor in HEK293 cells... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM319626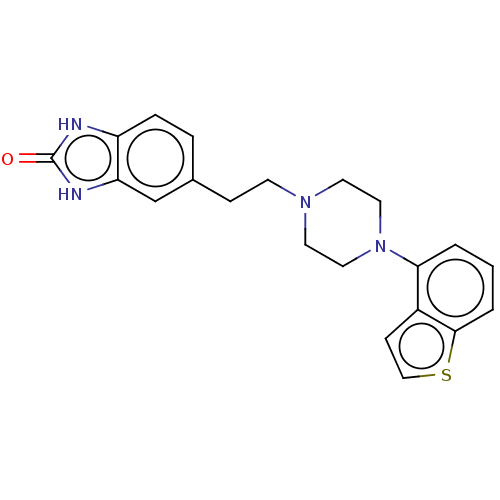 (5-(2-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.384 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The D2 receptor agonism activity test (The agonism activity of test compounds on D2 receptor expressing human recombinant D2 receptor in HEK293 cells... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM319692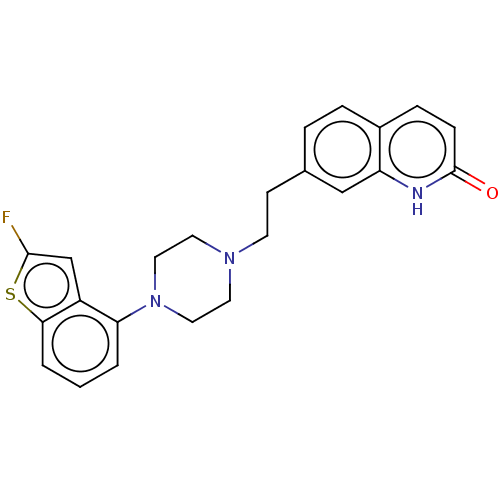 (7-(2-(4-(2-fluorobenzo[b]thiophen-4-yl)piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The D2 receptor agonism activity test (The agonism activity of test compounds on D2 receptor expressing human recombinant D2 receptor in HEK293 cells... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50417313 (CHEMBL1276585) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Antagonist activity at dopamine D2 receptor | Eur J Med Chem 45: 4856-62 (2010) Article DOI: 10.1016/j.ejmech.2010.07.056 BindingDB Entry DOI: 10.7270/Q2833T8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM319700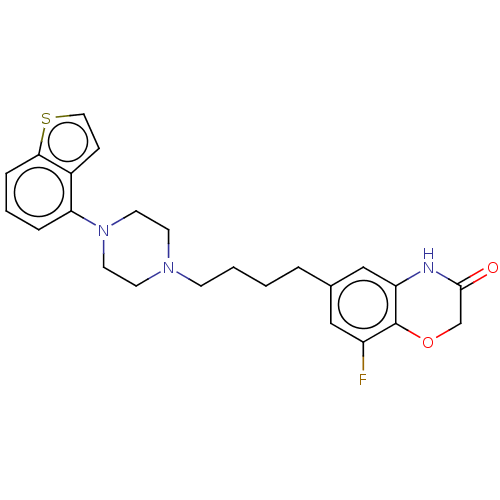 (6-(4-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.672 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The D2 receptor agonism activity test (The agonism activity of test compounds on D2 receptor expressing human recombinant D2 receptor in HEK293 cells... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50026568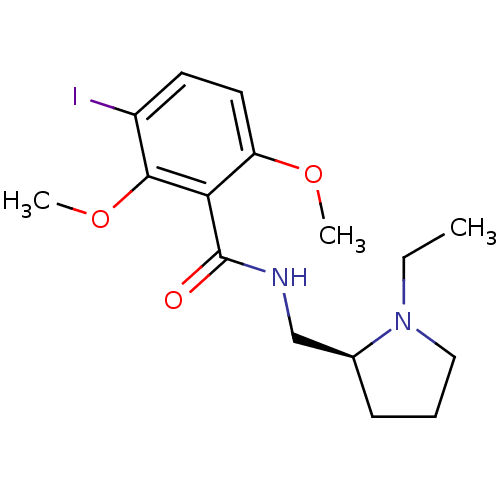 (CHEMBL23464 | N-(1-Ethyl-pyrrolidin-2-ylmethyl)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Antagonist activity at dopamine D2 receptor | Eur J Med Chem 45: 4856-62 (2010) Article DOI: 10.1016/j.ejmech.2010.07.056 BindingDB Entry DOI: 10.7270/Q2833T8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D2 short receptor transiently expressed in HEK293 cells assessed as inhibition of beta-arrestin recruitment aft... | J Med Chem 57: 4861-75 (2014) Article DOI: 10.1021/jm5004039 BindingDB Entry DOI: 10.7270/Q2SN0BH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from human recombinant D2S receptor in HEK293 cells after 60 mins by scintillation counting | J Med Chem 60: 349-361 (2017) Article DOI: 10.1021/acs.jmedchem.6b01422 BindingDB Entry DOI: 10.7270/Q27M0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50417312 (CHEMBL1276560) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Antagonist activity at dopamine D2 receptor | Eur J Med Chem 45: 4856-62 (2010) Article DOI: 10.1016/j.ejmech.2010.07.056 BindingDB Entry DOI: 10.7270/Q2833T8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM312156 (6-(4-(4-(2-Methoxyphenyl)piperazin-1-yl)butoxy)-2H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School | Assay Description Dopamine, D2S: Materials and Methods: Receptor Source: Human recombinant expressed in CHO cells Radioligand: [3H]Spiperone (20-60 Ci/mmol) C... | J Med Chem 52: 2226-32 (2009) BindingDB Entry DOI: 10.7270/Q2QZ2D9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50542261 (CHEMBL4644391) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at D2 receptor (unknown origin) by HTRF cAMP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127027 BindingDB Entry DOI: 10.7270/Q2VD731V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Antagonist activity against human recombinant dopmaine D2L receptor expressed in CHOK1 cells assessed as reduction in apomorphine-induced increase in... | Eur J Med Chem 92: 221-35 (2015) Article DOI: 10.1016/j.ejmech.2014.12.045 BindingDB Entry DOI: 10.7270/Q2Q241XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D2 receptor expressed in CHOK1 cells assessed as inhibition of apomorphine-induced calcium mobilization by radi... | Eur J Med Chem 92: 221-35 (2015) Article DOI: 10.1016/j.ejmech.2014.12.045 BindingDB Entry DOI: 10.7270/Q2Q241XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM319701 (6-(4-(4-(2-fluorobenzo[b]thiophen-4-yl)piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.06 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The D2 receptor antagonism activity test (The antagonism activity of test compounds on D2 receptor expressing human recombinant D2 receptor in HEK293... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50057761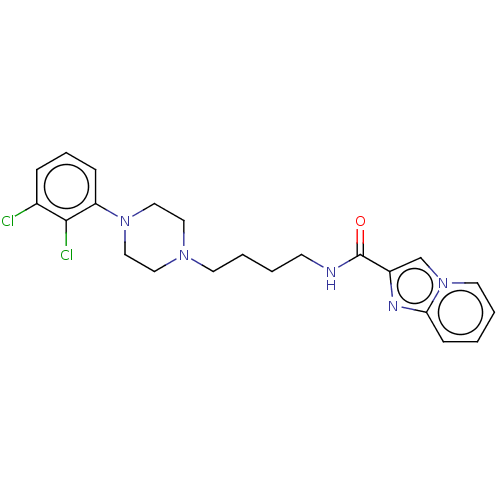 (CHEMBL3322994) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against human D2LR expressed in CHO cells assessed as inhibition of dopamine-induced [35S]GTPgammaS binding by dopamine potency s... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50208987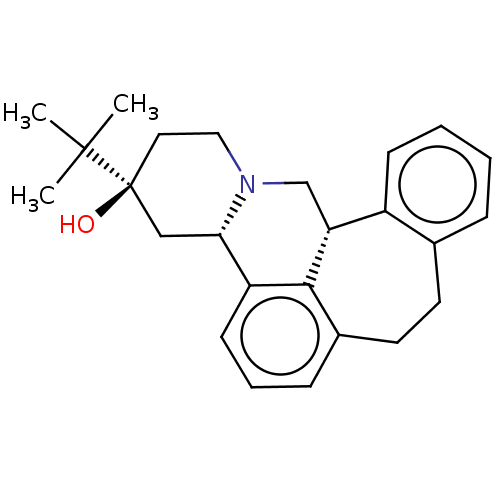 (CHEMBL3885419) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from human recombinant dopamine D2S receptor expressed in HEK293 cells measured after 60 mins by scintillation c... | Bioorg Med Chem 25: 471-482 (2017) Article DOI: 10.1016/j.bmc.2016.11.014 BindingDB Entry DOI: 10.7270/Q2CF9S3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human recombinant Dopamine D2S receptor expressed in HEK293 cells | Bioorg Med Chem 24: 1793-810 (2016) Article DOI: 10.1016/j.bmc.2016.03.006 BindingDB Entry DOI: 10.7270/Q2J67JS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50417296 (CHEMBL1276326) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Antagonist activity at dopamine D2 receptor | Eur J Med Chem 45: 4856-62 (2010) Article DOI: 10.1016/j.ejmech.2010.07.056 BindingDB Entry DOI: 10.7270/Q2833T8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50026045 ((R,S)-3-Bromo-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Antagonist activity at dopamine D2 receptor | Eur J Med Chem 45: 4856-62 (2010) Article DOI: 10.1016/j.ejmech.2010.07.056 BindingDB Entry DOI: 10.7270/Q2833T8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM319704 (5-(2-(4-(2-fluorobenzo[b]thiophen-4-yl)piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The D2 receptor agonism activity test (The agonism activity of test compounds on D2 receptor expressing human recombinant D2 receptor in HEK293 cells... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.27 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]Spiprone from human dopamine D2 receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50417297 (CHEMBL1276327) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Antagonist activity at dopamine D2 receptor | Eur J Med Chem 45: 4856-62 (2010) Article DOI: 10.1016/j.ejmech.2010.07.056 BindingDB Entry DOI: 10.7270/Q2833T8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50026565 (3-Ethyl-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,6-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Antagonist activity at dopamine D2 receptor | Eur J Med Chem 45: 4856-62 (2010) Article DOI: 10.1016/j.ejmech.2010.07.056 BindingDB Entry DOI: 10.7270/Q2833T8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM319719 (5-(4-(4-(2-fluorobenzo[b]thiophen-4-yl)piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The D2 receptor agonism activity test (The agonism activity of test compounds on D2 receptor expressing human recombinant D2 receptor in HEK293 cells... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50366495 ((+)butaclamol | CHEMBL1255588) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis Curated by ChEMBL | Assay Description Binding affinity to human dopamine D2S receptor by radioligand displacement assay | Bioorg Med Chem 21: 2764-71 (2013) Article DOI: 10.1016/j.bmc.2013.03.016 BindingDB Entry DOI: 10.7270/Q2N87C5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50417307 (CHEMBL1276494) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Antagonist activity at dopamine D2 receptor | Eur J Med Chem 45: 4856-62 (2010) Article DOI: 10.1016/j.ejmech.2010.07.056 BindingDB Entry DOI: 10.7270/Q2833T8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM81790 (Amisulpride | CAS_71675-85-9 | NSC_2159 | US101672...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Biologie Structurale | Assay Description The ability of Compound 102 to bind dopamine D2 receptors was measured in a cell-based assay. Dopamine D2 receptor cells were seeded in a half a blac... | J Med Chem 51: 1115-25 (2008) BindingDB Entry DOI: 10.7270/Q2377C1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50542263 (CHEMBL4638430) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at D2 receptor (unknown origin) by HTRF cAMP assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127027 BindingDB Entry DOI: 10.7270/Q2VD731V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM81790 (Amisulpride | CAS_71675-85-9 | NSC_2159 | US101672...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
LB PHARMACEUTICALS INC. US Patent | Assay Description The ability of Compound 102 to bind dopamine D2 receptors was measured in a cell-based assay. Dopamine D2 receptor cells were seeded in a half a blac... | US Patent US10167256 (2019) BindingDB Entry DOI: 10.7270/Q2NG4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50417311 (CHEMBL1276559) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Antagonist activity at dopamine D2 receptor | Eur J Med Chem 45: 4856-62 (2010) Article DOI: 10.1016/j.ejmech.2010.07.056 BindingDB Entry DOI: 10.7270/Q2833T8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1409 total ) | Next | Last >> |