 Found 848 hits of ic50 for UniProtKB: Q13946
Found 848 hits of ic50 for UniProtKB: Q13946 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50042053
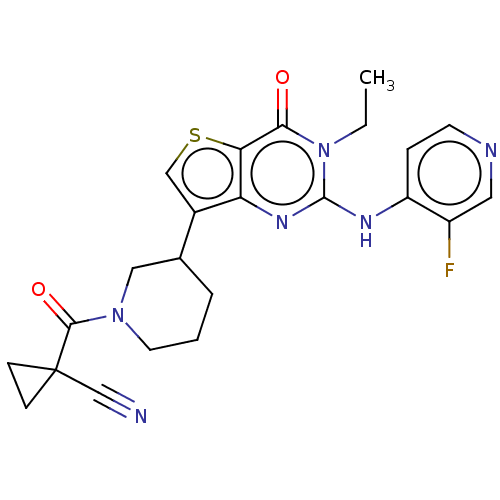
(CHEMBL3360172)Show SMILES CCn1c(Nc2ccncc2F)nc2c(csc2c1=O)C1CCCN(C1)C(=O)C1(CC1)C#N Show InChI InChI=1S/C23H23FN6O2S/c1-2-30-20(31)19-18(28-22(30)27-17-5-8-26-10-16(17)24)15(12-33-19)14-4-3-9-29(11-14)21(32)23(13-25)6-7-23/h5,8,10,12,14H,2-4,6-7,9,11H2,1H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 607-8042
Curated by ChEMBL
| Assay Description
Inhibition of PDE7A (unknown origin) |
Bioorg Med Chem Lett 25: 649-53 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.090
BindingDB Entry DOI: 10.7270/Q2542Q6P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50042036
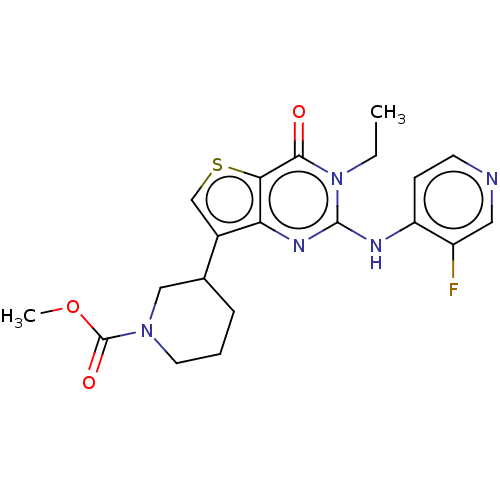
(CHEMBL3360170)Show SMILES CCn1c(Nc2ccncc2F)nc2c(csc2c1=O)C1CCCN(C1)C(=O)OC Show InChI InChI=1S/C20H22FN5O3S/c1-3-26-18(27)17-16(24-19(26)23-15-6-7-22-9-14(15)21)13(11-30-17)12-5-4-8-25(10-12)20(28)29-2/h6-7,9,11-12H,3-5,8,10H2,1-2H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 607-8042
Curated by ChEMBL
| Assay Description
Inhibition of PDE7A (unknown origin) |
Bioorg Med Chem Lett 25: 649-53 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.090
BindingDB Entry DOI: 10.7270/Q2542Q6P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50032547
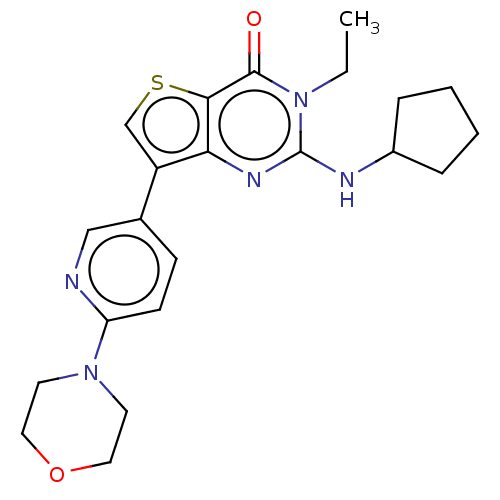
(CHEMBL3354181)Show SMILES CCn1c(NC2CCCC2)nc2c(csc2c1=O)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H27N5O2S/c1-2-27-21(28)20-19(25-22(27)24-16-5-3-4-6-16)17(14-30-20)15-7-8-18(23-13-15)26-9-11-29-12-10-26/h7-8,13-14,16H,2-6,9-12H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 607-8042
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
J Med Chem 57: 9844-54 (2014)
Article DOI: 10.1021/jm5008215
BindingDB Entry DOI: 10.7270/Q228096D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50042037
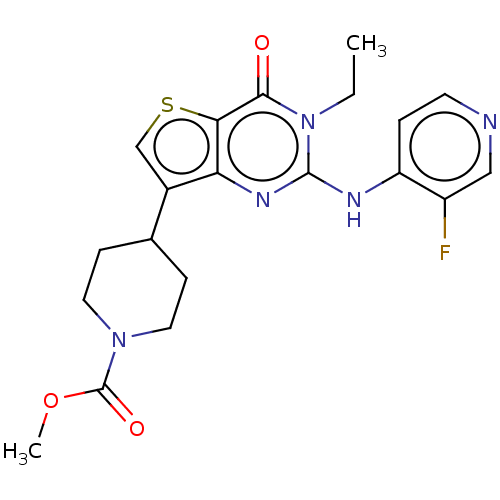
(CHEMBL3360169)Show SMILES CCn1c(Nc2ccncc2F)nc2c(csc2c1=O)C1CCN(CC1)C(=O)OC Show InChI InChI=1S/C20H22FN5O3S/c1-3-26-18(27)17-16(24-19(26)23-15-4-7-22-10-14(15)21)13(11-30-17)12-5-8-25(9-6-12)20(28)29-2/h4,7,10-12H,3,5-6,8-9H2,1-2H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 607-8042
Curated by ChEMBL
| Assay Description
Inhibition of PDE7A (unknown origin) |
Bioorg Med Chem Lett 25: 649-53 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.090
BindingDB Entry DOI: 10.7270/Q2542Q6P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50042035
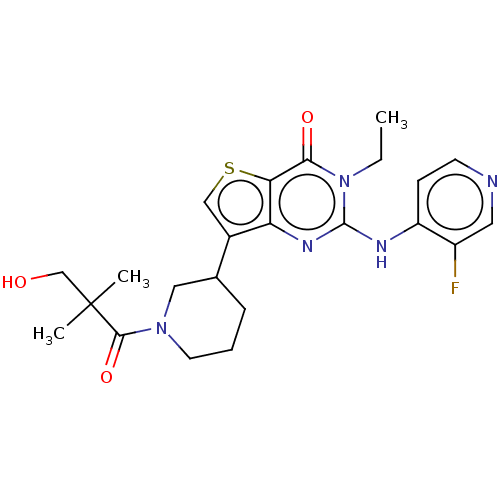
(CHEMBL3360171)Show SMILES CCn1c(Nc2ccncc2F)nc2c(csc2c1=O)C1CCCN(C1)C(=O)C(C)(C)CO Show InChI InChI=1S/C23H28FN5O3S/c1-4-29-20(31)19-18(27-22(29)26-17-7-8-25-10-16(17)24)15(12-33-19)14-6-5-9-28(11-14)21(32)23(2,3)13-30/h7-8,10,12,14,30H,4-6,9,11,13H2,1-3H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 607-8042
Curated by ChEMBL
| Assay Description
Inhibition of PDE7A (unknown origin) |
Bioorg Med Chem Lett 25: 649-53 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.090
BindingDB Entry DOI: 10.7270/Q2542Q6P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50381287
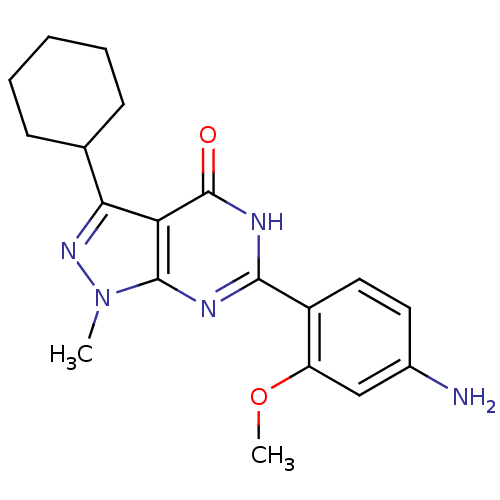
(CHEMBL2019020)Show SMILES COc1cc(N)ccc1-c1nc2n(C)nc(C3CCCCC3)c2c(=O)[nH]1 Show InChI InChI=1S/C19H23N5O2/c1-24-18-15(16(23-24)11-6-4-3-5-7-11)19(25)22-17(21-18)13-9-8-12(20)10-14(13)26-2/h8-11H,3-7,20H2,1-2H3,(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cAMP from human recombinant PDE7A |
Bioorg Med Chem Lett 22: 3223-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.025
BindingDB Entry DOI: 10.7270/Q2NP25F2 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM116309
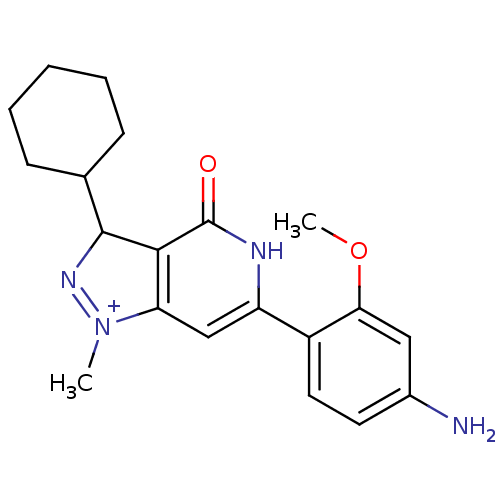
(US8637528, OM69)Show SMILES COc1cc(N)ccc1-c1cc2c(C(N=[N+]2C)C2CCCCC2)c(=O)[nH]1 |c:15| Show InChI InChI=1S/C20H24N4O2/c1-24-16-11-15(14-9-8-13(21)10-17(14)26-2)22-20(25)18(16)19(23-24)12-6-4-3-5-7-12/h8-12,19H,3-7H2,1-2H3,(H2-,21,22,25)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Omeros Corporation
US Patent
| Assay Description
The assay method used was a scintillation proximity assay (SPA) (obtained from GE Healthcare, Product Code TRKQ7100), with [3H]-cGMP as the substrate... |
US Patent US8637528 (2014)
BindingDB Entry DOI: 10.7270/Q29022FT |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50068286
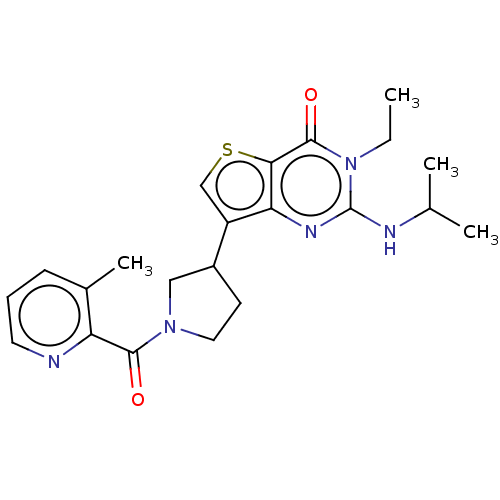
(CHEMBL3403373)Show SMILES CCn1c(NC(C)C)nc2c(csc2c1=O)C1CCN(C1)C(=O)c1ncccc1C Show InChI InChI=1S/C22H27N5O2S/c1-5-27-21(29)19-18(25-22(27)24-13(2)3)16(12-30-19)15-8-10-26(11-15)20(28)17-14(4)7-6-9-23-17/h6-7,9,12-13,15H,5,8,10-11H2,1-4H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
Bioorg Med Chem Lett 25: 1910-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.031
BindingDB Entry DOI: 10.7270/Q2NC62VM |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50068283

(CHEMBL3403371)Show SMILES CCn1c(NC(C)C)nc2c(csc2c1=O)C1CCN(C1)C(=O)c1ccccc1 Show InChI InChI=1S/C22H26N4O2S/c1-4-26-21(28)19-18(24-22(26)23-14(2)3)17(13-29-19)16-10-11-25(12-16)20(27)15-8-6-5-7-9-15/h5-9,13-14,16H,4,10-12H2,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
Bioorg Med Chem Lett 25: 1910-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.031
BindingDB Entry DOI: 10.7270/Q2NC62VM |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50032540
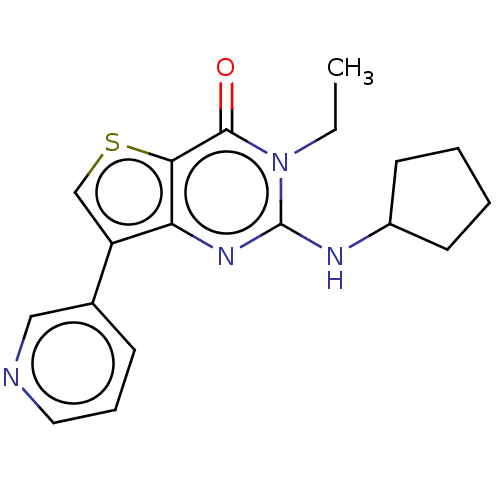
(CHEMBL3354179)Show InChI InChI=1S/C18H20N4OS/c1-2-22-17(23)16-15(21-18(22)20-13-7-3-4-8-13)14(11-24-16)12-6-5-9-19-10-12/h5-6,9-11,13H,2-4,7-8H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 607-8042
Curated by ChEMBL
| Assay Description
Inhibition of PDE7A (unknown origin) |
Bioorg Med Chem Lett 25: 649-53 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.090
BindingDB Entry DOI: 10.7270/Q2542Q6P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50032540

(CHEMBL3354179)Show InChI InChI=1S/C18H20N4OS/c1-2-22-17(23)16-15(21-18(22)20-13-7-3-4-8-13)14(11-24-16)12-6-5-9-19-10-12/h5-6,9-11,13H,2-4,7-8H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 607-8042
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
J Med Chem 57: 9844-54 (2014)
Article DOI: 10.1021/jm5008215
BindingDB Entry DOI: 10.7270/Q228096D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM140128

(US8901315, 256)Show SMILES Cc1nn(C2CCCCC2)c2sc(cc12)C(=O)Nc1ccc(nc1)N1CCNC1=O Show InChI InChI=1S/C21H24N6O2S/c1-13-16-11-17(30-20(16)27(25-13)15-5-3-2-4-6-15)19(28)24-14-7-8-18(23-12-14)26-10-9-22-21(26)29/h7-8,11-12,15H,2-6,9-10H2,1H3,(H,22,29)(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... |
US Patent US8901315 (2014)
BindingDB Entry DOI: 10.7270/Q2GM860P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50042039
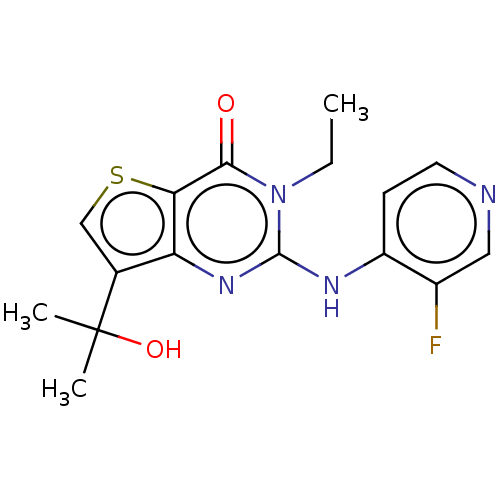
(CHEMBL3360167)Show InChI InChI=1S/C16H17FN4O2S/c1-4-21-14(22)13-12(9(8-24-13)16(2,3)23)20-15(21)19-11-5-6-18-7-10(11)17/h5-8,23H,4H2,1-3H3,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 607-8042
Curated by ChEMBL
| Assay Description
Inhibition of PDE7A (unknown origin) |
Bioorg Med Chem Lett 25: 649-53 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.090
BindingDB Entry DOI: 10.7270/Q2542Q6P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50032543
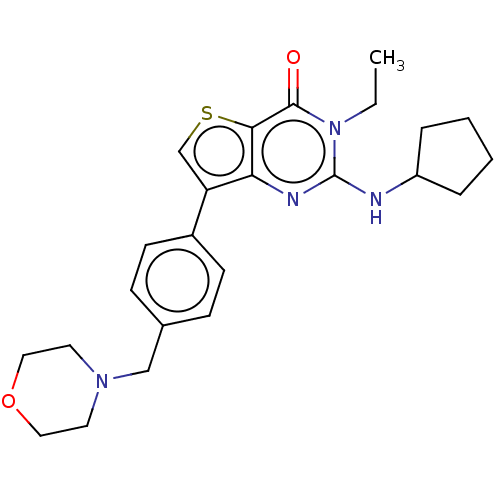
(CHEMBL3354180)Show SMILES CCn1c(NC2CCCC2)nc2c(csc2c1=O)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C24H30N4O2S/c1-2-28-23(29)22-21(26-24(28)25-19-5-3-4-6-19)20(16-31-22)18-9-7-17(8-10-18)15-27-11-13-30-14-12-27/h7-10,16,19H,2-6,11-15H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 607-8042
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
J Med Chem 57: 9844-54 (2014)
Article DOI: 10.1021/jm5008215
BindingDB Entry DOI: 10.7270/Q228096D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50068285
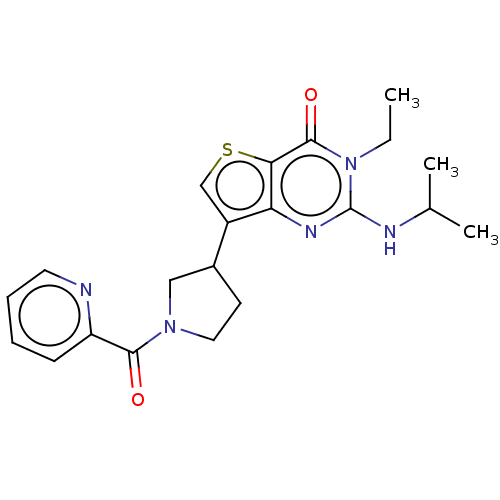
(CHEMBL3403372)Show SMILES CCn1c(NC(C)C)nc2c(csc2c1=O)C1CCN(C1)C(=O)c1ccccn1 Show InChI InChI=1S/C21H25N5O2S/c1-4-26-20(28)18-17(24-21(26)23-13(2)3)15(12-29-18)14-8-10-25(11-14)19(27)16-7-5-6-9-22-16/h5-7,9,12-14H,4,8,10-11H2,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
Bioorg Med Chem Lett 25: 1910-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.031
BindingDB Entry DOI: 10.7270/Q2NC62VM |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50397056
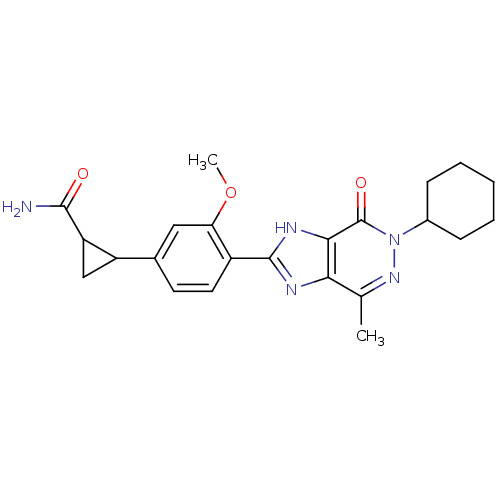
(CHEMBL2171440)Show SMILES COc1cc(ccc1-c1nc2c(C)nn(C3CCCCC3)c(=O)c2[nH]1)C1CC1C(N)=O Show InChI InChI=1S/C23H27N5O3/c1-12-19-20(23(30)28(27-12)14-6-4-3-5-7-14)26-22(25-19)15-9-8-13(10-18(15)31-2)16-11-17(16)21(24)29/h8-10,14,16-17H,3-7,11H2,1-2H3,(H2,24,29)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of cloned human recombinant PDE7A assessed as [3H]cAMP hydrolysis by radiometric assay |
Bioorg Med Chem Lett 22: 6286-91 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.077
BindingDB Entry DOI: 10.7270/Q21Z45JB |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM140095
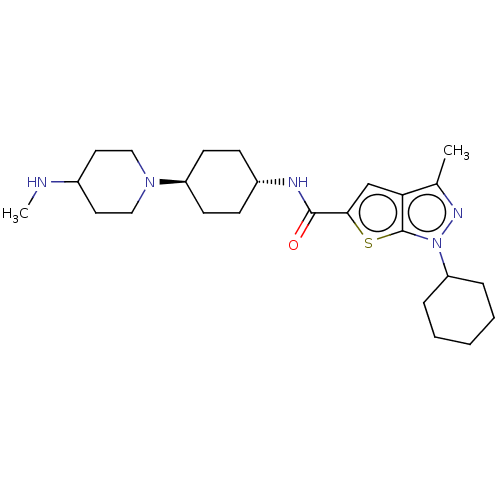
(US8901315, 179)Show SMILES CNC1CCN(CC1)[C@H]1CC[C@@H](CC1)NC(=O)c1cc2c(C)nn(C3CCCCC3)c2s1 |r,wU:11.15,wD:8.8,(9.99,4.19,;9.22,2.85,;7.68,2.85,;6.91,1.52,;5.37,1.52,;4.6,2.85,;5.37,4.19,;6.91,4.19,;3.06,2.85,;2.29,4.19,;.75,4.19,;-.02,2.85,;.75,1.52,;2.29,1.52,;-1.56,2.85,;-2.33,1.52,;-1.56,.19,;-3.87,1.52,;-4.78,2.77,;-6.24,2.29,;-7.7,2.77,;-8.47,4.1,;-8.61,1.52,;-7.7,.27,;-8.1,-1.21,;-9.59,-1.61,;-9.99,-3.1,;-8.9,-4.19,;-7.41,-3.79,;-7.01,-2.3,;-6.24,.75,;-4.78,.27,)| Show InChI InChI=1S/C25H39N5OS/c1-17-22-16-23(32-25(22)30(28-17)21-6-4-3-5-7-21)24(31)27-19-8-10-20(11-9-19)29-14-12-18(26-2)13-15-29/h16,18-21,26H,3-15H2,1-2H3,(H,27,31)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... |
US Patent US8901315 (2014)
BindingDB Entry DOI: 10.7270/Q2GM860P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50068287
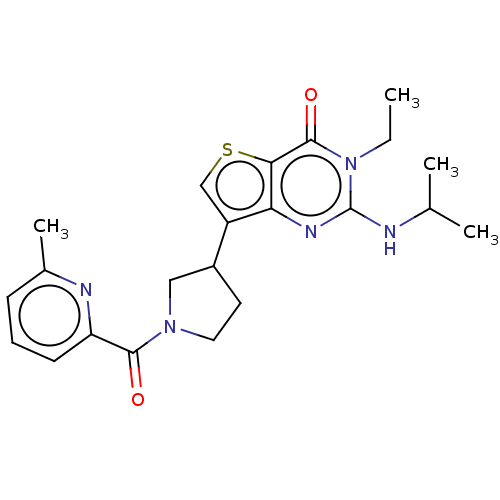
(CHEMBL3403346)Show SMILES CCn1c(NC(C)C)nc2c(csc2c1=O)C1CCN(C1)C(=O)c1cccc(C)n1 Show InChI InChI=1S/C22H27N5O2S/c1-5-27-21(29)19-18(25-22(27)23-13(2)3)16(12-30-19)15-9-10-26(11-15)20(28)17-8-6-7-14(4)24-17/h6-8,12-13,15H,5,9-11H2,1-4H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
Bioorg Med Chem Lett 25: 1910-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.031
BindingDB Entry DOI: 10.7270/Q2NC62VM |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM140100

(US8901315, 190)Show SMILES CC(=O)N1CCN(CC1)[C@H]1CC[C@@H](CC1)NC(=O)c1cc2c(C)nn(C3CCCCC3)c2s1 |r,wU:12.16,wD:9.9,(9.99,1.52,;9.22,2.85,;9.99,4.19,;7.68,2.85,;6.91,1.52,;5.37,1.52,;4.6,2.85,;5.37,4.19,;6.91,4.19,;3.06,2.85,;2.29,4.19,;.75,4.19,;-.02,2.85,;.75,1.52,;2.29,1.52,;-1.56,2.85,;-2.33,1.52,;-1.56,.19,;-3.87,1.52,;-4.78,2.77,;-6.24,2.29,;-7.7,2.77,;-8.47,4.1,;-8.61,1.52,;-7.7,.27,;-8.1,-1.21,;-9.59,-1.61,;-9.99,-3.1,;-8.9,-4.19,;-7.41,-3.79,;-7.01,-2.3,;-6.24,.75,;-4.78,.27,)| Show InChI InChI=1S/C25H37N5O2S/c1-17-22-16-23(33-25(22)30(27-17)21-6-4-3-5-7-21)24(32)26-19-8-10-20(11-9-19)29-14-12-28(13-15-29)18(2)31/h16,19-21H,3-15H2,1-2H3,(H,26,32)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... |
US Patent US8901315 (2014)
BindingDB Entry DOI: 10.7270/Q2GM860P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151638

(8''-chloro-5''-(5-hydroxy-1,2,4-oxadiazol-3-ylmeth...)Show SMILES Clc1ccc(OCc2nc(=O)o[nH]2)c2c1NC(=O)NC21CCCCC1 Show InChI InChI=1S/C16H17ClN4O4/c17-9-4-5-10(24-8-11-18-15(23)25-21-11)12-13(9)19-14(22)20-16(12)6-2-1-3-7-16/h4-5H,1-3,6-8H2,(H,18,21,23)(H2,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4627-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.010
BindingDB Entry DOI: 10.7270/Q2TT4QDX |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50042038

(CHEMBL3360168)Show InChI InChI=1S/C16H17FN4O2S/c1-2-21-15(23)14-13(10(9-24-14)4-3-7-22)20-16(21)19-12-5-6-18-8-11(12)17/h5-6,8-9,22H,2-4,7H2,1H3,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 607-8042
Curated by ChEMBL
| Assay Description
Inhibition of PDE7A (unknown origin) |
Bioorg Med Chem Lett 25: 649-53 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.090
BindingDB Entry DOI: 10.7270/Q2542Q6P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151562

(7-{5-[(Z)-Cyclohexylimino]-4-methyl-4,5-dihydro-[1...)Show SMILES Cc1nc(N)c2ccc(cc2n1)-c1nn(C)\c(=N\C2CCCCC2)s1 Show InChI InChI=1S/C18H22N6S/c1-11-20-15-10-12(8-9-14(15)16(19)21-11)17-23-24(2)18(25-17)22-13-6-4-3-5-7-13/h8-10,13H,3-7H2,1-2H3,(H2,19,20,21)/b22-18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4607-13 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.008
BindingDB Entry DOI: 10.7270/Q2377855 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50381291
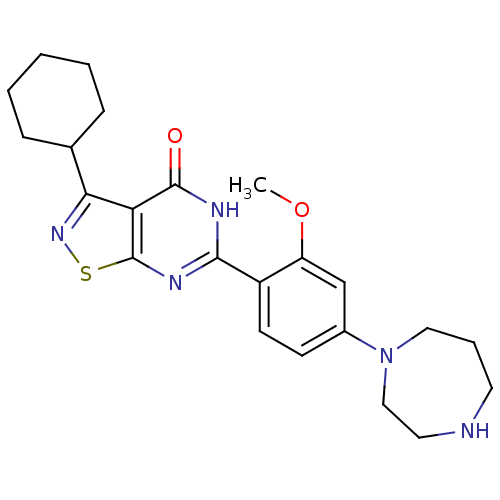
(CHEMBL2019104)Show SMILES COc1cc(ccc1-c1nc2snc(C3CCCCC3)c2c(=O)[nH]1)N1CCCNCC1 Show InChI InChI=1S/C23H29N5O2S/c1-30-18-14-16(28-12-5-10-24-11-13-28)8-9-17(18)21-25-22(29)19-20(27-31-23(19)26-21)15-6-3-2-4-7-15/h8-9,14-15,24H,2-7,10-13H2,1H3,(H,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cAMP from human recombinant PDE7A |
Bioorg Med Chem Lett 22: 3223-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.025
BindingDB Entry DOI: 10.7270/Q2NP25F2 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151635
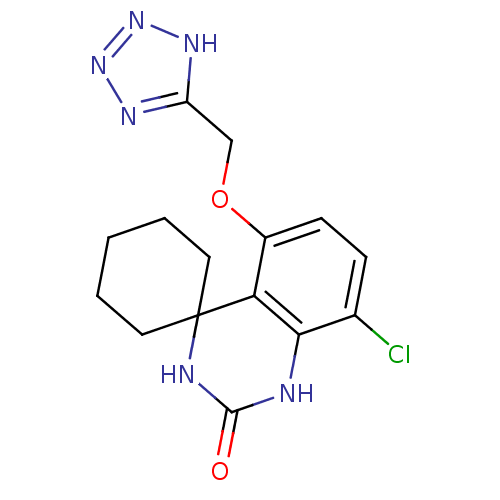
(8''-chloro-5''-(1H-1,2,3,4-tetraazol-5-ylmethoxy)s...)Show InChI InChI=1S/C15H17ClN6O2/c16-9-4-5-10(24-8-11-19-21-22-20-11)12-13(9)17-14(23)18-15(12)6-2-1-3-7-15/h4-5H,1-3,6-8H2,(H2,17,18,23)(H,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4627-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.010
BindingDB Entry DOI: 10.7270/Q2TT4QDX |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM140142
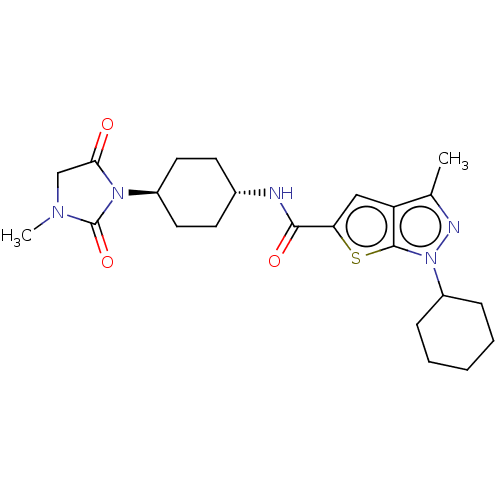
(US8901315, 275)Show SMILES CN1CC(=O)N([C@H]2CC[C@@H](CC2)NC(=O)c2cc3c(C)nn(C4CCCCC4)c3s2)C1=O |r,wU:9.12,wD:6.5,(9.15,.69,;7.81,1.46,;7.81,3,;6.35,3.48,;5.58,4.81,;5.44,2.23,;3.9,2.23,;3.13,3.56,;1.59,3.56,;.82,2.23,;1.59,.9,;3.13,.9,;-.72,2.23,;-1.49,.9,;-.72,-.44,;-3.03,.9,;-3.93,2.14,;-5.4,1.67,;-6.86,2.14,;-7.63,3.48,;-7.77,.9,;-6.86,-.35,;-7.26,-1.84,;-8.75,-2.23,;-9.15,-3.72,;-8.06,-4.81,;-6.57,-4.41,;-6.17,-2.92,;-5.4,.13,;-3.93,-.35,;6.35,.99,;5.58,-.35,)| Show InChI InChI=1S/C23H31N5O3S/c1-14-18-12-19(32-22(18)28(25-14)17-6-4-3-5-7-17)21(30)24-15-8-10-16(11-9-15)27-20(29)13-26(2)23(27)31/h12,15-17H,3-11,13H2,1-2H3,(H,24,30)/t15-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... |
US Patent US8901315 (2014)
BindingDB Entry DOI: 10.7270/Q2GM860P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50032600

(CHEMBL3354171)Show InChI InChI=1S/C20H20N4OS/c1-2-24-19(25)18-17(23-20(24)22-16-7-3-4-8-16)15(13-26-18)10-9-14-6-5-11-21-12-14/h5-6,11-13,16H,2-4,7-8H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 607-8042
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
J Med Chem 57: 9844-54 (2014)
Article DOI: 10.1021/jm5008215
BindingDB Entry DOI: 10.7270/Q228096D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50068277

(CHEMBL3403358)Show InChI InChI=1S/C16H18N4OS/c1-4-20-15(21)14-13(19-16(20)18-10(2)3)12(9-22-14)11-6-5-7-17-8-11/h5-10H,4H2,1-3H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
Bioorg Med Chem Lett 25: 1910-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.031
BindingDB Entry DOI: 10.7270/Q2NC62VM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM140168
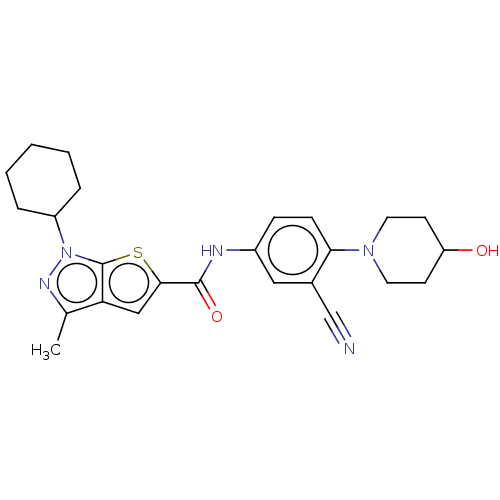
(US8901315, 373)Show SMILES Cc1nn(C2CCCCC2)c2sc(cc12)C(=O)Nc1ccc(N2CCC(O)CC2)c(c1)C#N Show InChI InChI=1S/C25H29N5O2S/c1-16-21-14-23(33-25(21)30(28-16)19-5-3-2-4-6-19)24(32)27-18-7-8-22(17(13-18)15-26)29-11-9-20(31)10-12-29/h7-8,13-14,19-20,31H,2-6,9-12H2,1H3,(H,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... |
US Patent US8901315 (2014)
BindingDB Entry DOI: 10.7270/Q2GM860P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50068261

(CHEMBL3403361)Show InChI InChI=1S/C17H20N4O2S/c1-5-21-16(22)15-14(20-17(21)19-10(2)3)12(9-24-15)11-6-7-18-13(8-11)23-4/h6-10H,5H2,1-4H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
Bioorg Med Chem Lett 25: 1910-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.031
BindingDB Entry DOI: 10.7270/Q2NC62VM |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50032529
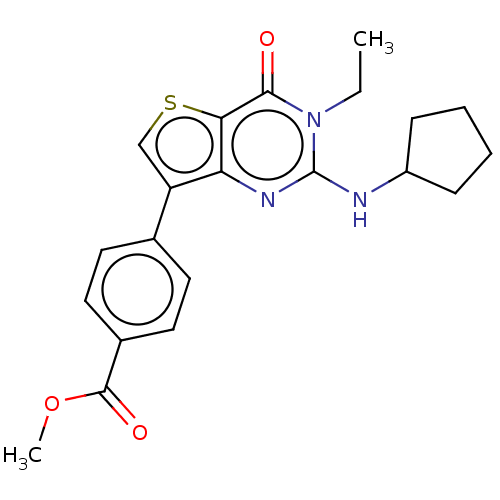
(CHEMBL3354178)Show SMILES CCn1c(NC2CCCC2)nc2c(csc2c1=O)-c1ccc(cc1)C(=O)OC Show InChI InChI=1S/C21H23N3O3S/c1-3-24-19(25)18-17(23-21(24)22-15-6-4-5-7-15)16(12-28-18)13-8-10-14(11-9-13)20(26)27-2/h8-12,15H,3-7H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 607-8042
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
J Med Chem 57: 9844-54 (2014)
Article DOI: 10.1021/jm5008215
BindingDB Entry DOI: 10.7270/Q228096D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM140155
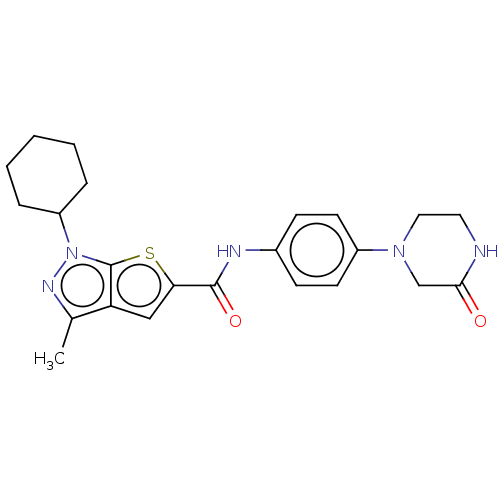
(US8901315, 341)Show SMILES Cc1nn(C2CCCCC2)c2sc(cc12)C(=O)Nc1ccc(cc1)N1CCNC(=O)C1 Show InChI InChI=1S/C23H27N5O2S/c1-15-19-13-20(31-23(19)28(26-15)18-5-3-2-4-6-18)22(30)25-16-7-9-17(10-8-16)27-12-11-24-21(29)14-27/h7-10,13,18H,2-6,11-12,14H2,1H3,(H,24,29)(H,25,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... |
US Patent US8901315 (2014)
BindingDB Entry DOI: 10.7270/Q2GM860P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50032471
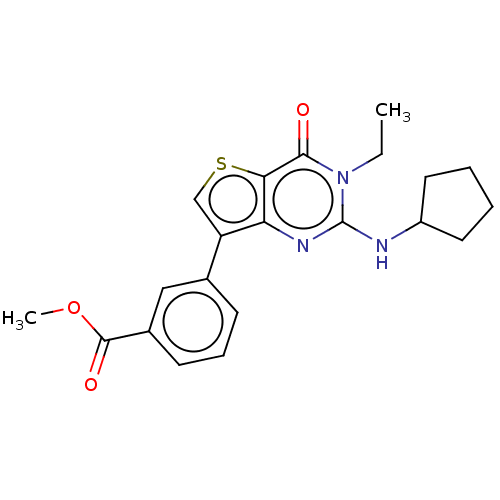
(CHEMBL3354177)Show SMILES CCn1c(NC2CCCC2)nc2c(csc2c1=O)-c1cccc(c1)C(=O)OC Show InChI InChI=1S/C21H23N3O3S/c1-3-24-19(25)18-17(23-21(24)22-15-9-4-5-10-15)16(12-28-18)13-7-6-8-14(11-13)20(26)27-2/h6-8,11-12,15H,3-5,9-10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 607-8042
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
J Med Chem 57: 9844-54 (2014)
Article DOI: 10.1021/jm5008215
BindingDB Entry DOI: 10.7270/Q228096D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50068256

(CHEMBL3403367)Show SMILES CCn1c(NC(C)C)nc2c(csc2c1=O)C1CCCN(C1)C(C)=O Show InChI InChI=1S/C18H26N4O2S/c1-5-22-17(24)16-15(20-18(22)19-11(2)3)14(10-25-16)13-7-6-8-21(9-13)12(4)23/h10-11,13H,5-9H2,1-4H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
Bioorg Med Chem Lett 25: 1910-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.031
BindingDB Entry DOI: 10.7270/Q2NC62VM |
More data for this
Ligand-Target Pair | |
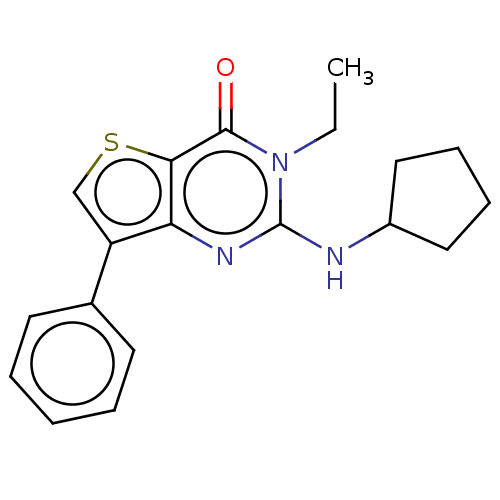
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50032607
(CHEMBL3354175)Show InChI InChI=1S/C19H21N3OS/c1-2-22-18(23)17-16(21-19(22)20-14-10-6-7-11-14)15(12-24-17)13-8-4-3-5-9-13/h3-5,8-9,12,14H,2,6-7,10-11H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 607-8042
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
J Med Chem 57: 9844-54 (2014)
Article DOI: 10.1021/jm5008215
BindingDB Entry DOI: 10.7270/Q228096D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM116310
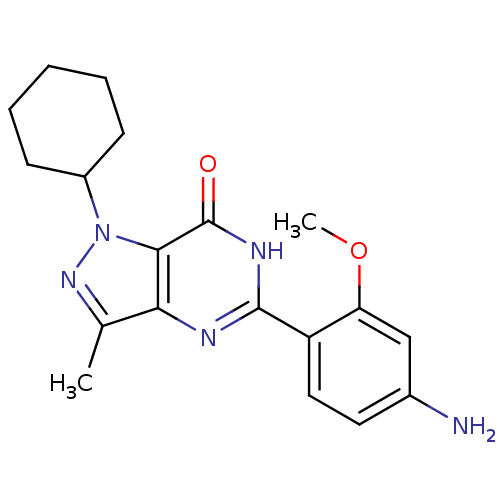
(US8637528, OM056)Show SMILES COc1cc(N)ccc1-c1nc2c(C)nn(C3CCCCC3)c2c(=O)[nH]1 Show InChI InChI=1S/C19H23N5O2/c1-11-16-17(24(23-11)13-6-4-3-5-7-13)19(25)22-18(21-16)14-9-8-12(20)10-15(14)26-2/h8-10,13H,3-7,20H2,1-2H3,(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.67 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Omeros Corporation
US Patent
| Assay Description
The assay method used was a scintillation proximity assay (SPA) (obtained from GE Healthcare, Product Code TRKQ7100), with [3H]-cGMP as the substrate... |
US Patent US8637528 (2014)
BindingDB Entry DOI: 10.7270/Q29022FT |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM140108

(US8901315, 203)Show SMILES CN1CC(=O)N(C1=O)c1ccc(NC(=O)c2cc3c(C)nn(C4CCCCC4)c3s2)cc1 Show InChI InChI=1S/C23H25N5O3S/c1-14-18-12-19(32-22(18)28(25-14)17-6-4-3-5-7-17)21(30)24-15-8-10-16(11-9-15)27-20(29)13-26(2)23(27)31/h8-12,17H,3-7,13H2,1-2H3,(H,24,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... |
US Patent US8901315 (2014)
BindingDB Entry DOI: 10.7270/Q2GM860P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50068282
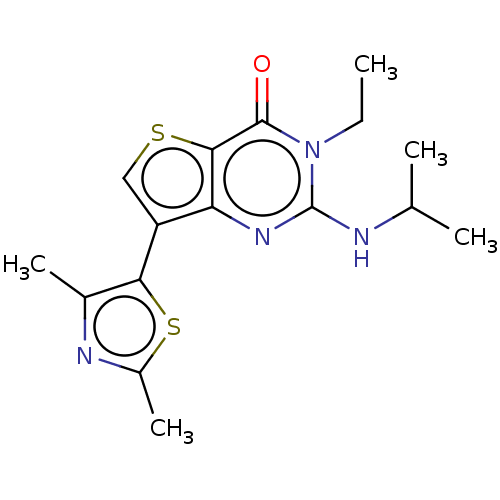
(CHEMBL3403360)Show InChI InChI=1S/C16H20N4OS2/c1-6-20-15(21)14-12(19-16(20)17-8(2)3)11(7-22-14)13-9(4)18-10(5)23-13/h7-8H,6H2,1-5H3,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
Bioorg Med Chem Lett 25: 1910-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.031
BindingDB Entry DOI: 10.7270/Q2NC62VM |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM140099
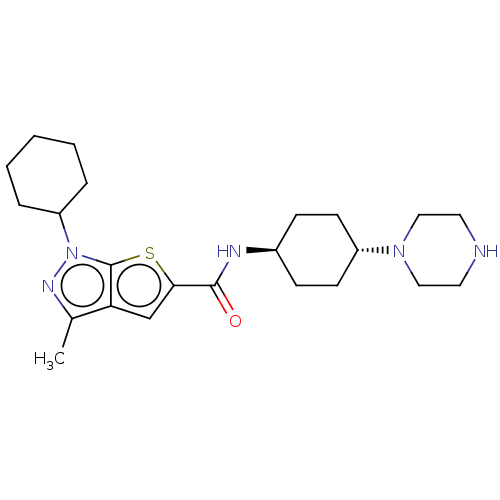
(US8901315, 188)Show SMILES Cc1nn(C2CCCCC2)c2sc(cc12)C(=O)N[C@H]1CC[C@@H](CC1)N1CCNCC1 |r,wU:18.20,wD:21.27,(-7.32,4.1,;-6.55,2.77,;-7.46,1.52,;-6.55,.27,;-6.95,-1.21,;-8.44,-1.61,;-8.83,-3.1,;-7.75,-4.19,;-6.26,-3.79,;-5.86,-2.3,;-5.09,.75,;-3.62,.27,;-2.72,1.52,;-3.62,2.77,;-5.09,2.29,;-1.18,1.52,;-.41,.19,;-.41,2.85,;1.13,2.85,;1.9,4.19,;3.44,4.19,;4.21,2.85,;3.44,1.52,;1.9,1.52,;5.75,2.85,;6.52,1.52,;8.06,1.52,;8.83,2.85,;8.06,4.19,;6.52,4.19,)| Show InChI InChI=1S/C23H35N5OS/c1-16-20-15-21(30-23(20)28(26-16)19-5-3-2-4-6-19)22(29)25-17-7-9-18(10-8-17)27-13-11-24-12-14-27/h15,17-19,24H,2-14H2,1H3,(H,25,29)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... |
US Patent US8901315 (2014)
BindingDB Entry DOI: 10.7270/Q2GM860P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM140126

(US8901315, 247)Show SMILES Cc1nn(C2CCCCC2)c2sc(cc12)C(=O)NC1CC[C@H](CN2C(=O)CNC2=O)CC1 |r,wD:21.24,(-7.32,4.1,;-6.55,2.77,;-7.46,1.52,;-6.55,.27,;-6.95,-1.21,;-8.44,-1.61,;-8.83,-3.1,;-7.75,-4.19,;-6.26,-3.79,;-5.86,-2.3,;-5.09,.75,;-3.62,.27,;-2.72,1.52,;-3.62,2.77,;-5.09,2.29,;-1.18,1.52,;-.41,.19,;-.41,2.85,;1.13,2.85,;1.9,4.19,;3.44,4.19,;4.21,2.85,;5.75,2.85,;6.52,1.52,;6.05,.06,;4.72,-.71,;7.29,-.85,;8.54,.06,;8.06,1.52,;8.83,2.85,;3.44,1.52,;1.9,1.52,)| Show InChI InChI=1S/C23H31N5O3S/c1-14-18-11-19(32-22(18)28(26-14)17-5-3-2-4-6-17)21(30)25-16-9-7-15(8-10-16)13-27-20(29)12-24-23(27)31/h11,15-17H,2-10,12-13H2,1H3,(H,24,31)(H,25,30)/t15-,16? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... |
US Patent US8901315 (2014)
BindingDB Entry DOI: 10.7270/Q2GM860P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM135582

(US8846654, 111)Show SMILES COc1ccc(Cn2c(=O)n(C3CCN(CC3)C=O)c3ccc(OC(F)F)cc3c2=O)cc1OC Show InChI InChI=1S/C24H25F2N3O6/c1-33-20-6-3-15(11-21(20)34-2)13-28-22(31)18-12-17(35-23(25)26)4-5-19(18)29(24(28)32)16-7-9-27(14-30)10-8-16/h3-6,11-12,14,16,23H,7-10,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Sanofi
US Patent
| Assay Description
The assay is carried out in 1.5 ml Eppendorf tubes comprising 40 mM of Tris-HCl (pH 7.5), 15 mM of MgCl2, 1 mM of EGTA, 0.5 mg/ml of bovine serum alb... |
US Patent US8846654 (2014)
BindingDB Entry DOI: 10.7270/Q21V5CPZ |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50068254
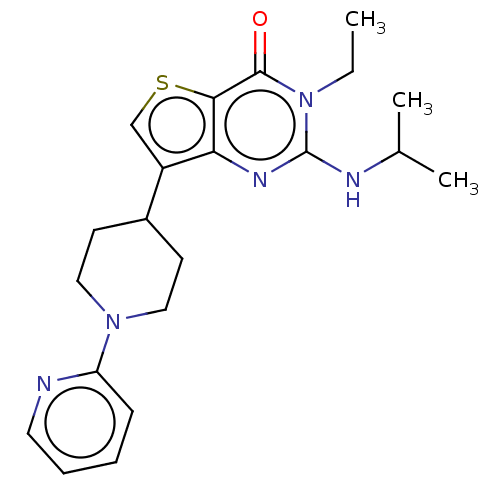
(CHEMBL3403365)Show SMILES CCn1c(NC(C)C)nc2c(csc2c1=O)C1CCN(CC1)c1ccccn1 Show InChI InChI=1S/C21H27N5OS/c1-4-26-20(27)19-18(24-21(26)23-14(2)3)16(13-28-19)15-8-11-25(12-9-15)17-7-5-6-10-22-17/h5-7,10,13-15H,4,8-9,11-12H2,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
Bioorg Med Chem Lett 25: 1910-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.031
BindingDB Entry DOI: 10.7270/Q2NC62VM |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM140098
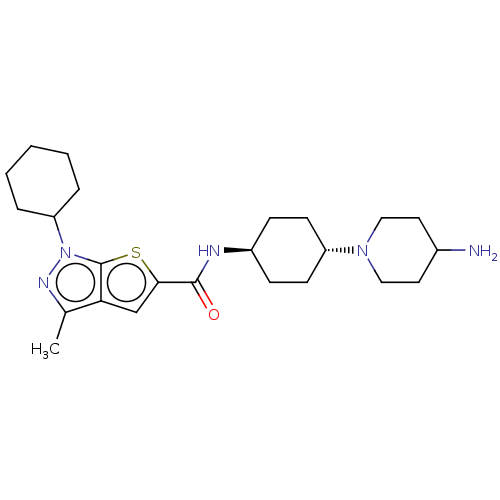
(US8901315, 184)Show SMILES Cc1nn(C2CCCCC2)c2sc(cc12)C(=O)N[C@H]1CC[C@@H](CC1)N1CCC(N)CC1 |r,wU:18.20,wD:21.27,(-8.09,4.1,;-7.32,2.77,;-8.23,1.52,;-7.32,.27,;-7.72,-1.21,;-9.21,-1.61,;-9.6,-3.1,;-8.52,-4.19,;-7.03,-3.79,;-6.63,-2.3,;-5.86,.75,;-4.39,.27,;-3.49,1.52,;-4.39,2.77,;-5.86,2.29,;-1.95,1.52,;-1.18,.19,;-1.18,2.85,;.36,2.85,;1.13,4.19,;2.67,4.19,;3.44,2.85,;2.67,1.52,;1.13,1.52,;4.98,2.85,;5.75,1.52,;7.29,1.52,;8.06,2.85,;9.6,2.85,;7.29,4.19,;5.75,4.19,)| Show InChI InChI=1S/C24H37N5OS/c1-16-21-15-22(31-24(21)29(27-16)20-5-3-2-4-6-20)23(30)26-18-7-9-19(10-8-18)28-13-11-17(25)12-14-28/h15,17-20H,2-14,25H2,1H3,(H,26,30)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... |
US Patent US8901315 (2014)
BindingDB Entry DOI: 10.7270/Q2GM860P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50381296

(CHEMBL2019109)Show SMILES COc1cc(ccc1-c1nc2snc(C3CCCCC3)c2c(=O)[nH]1)N1CCCC(O)CC1 Show InChI InChI=1S/C24H30N4O3S/c1-31-19-14-16(28-12-5-8-17(29)11-13-28)9-10-18(19)22-25-23(30)20-21(27-32-24(20)26-22)15-6-3-2-4-7-15/h9-10,14-15,17,29H,2-8,11-13H2,1H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cAMP from human recombinant PDE7A |
Bioorg Med Chem Lett 22: 3223-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.025
BindingDB Entry DOI: 10.7270/Q2NP25F2 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM140169

(US8901315, 375)Show SMILES Cc1nn(C2CCCCC2)c2sc(cc12)C(=O)Nc1ccc(N2CCC(O)CC2)c(c1)C(O)=O Show InChI InChI=1S/C25H30N4O4S/c1-15-19-14-22(34-24(19)29(27-15)17-5-3-2-4-6-17)23(31)26-16-7-8-21(20(13-16)25(32)33)28-11-9-18(30)10-12-28/h7-8,13-14,17-18,30H,2-6,9-12H2,1H3,(H,26,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... |
US Patent US8901315 (2014)
BindingDB Entry DOI: 10.7270/Q2GM860P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50032602
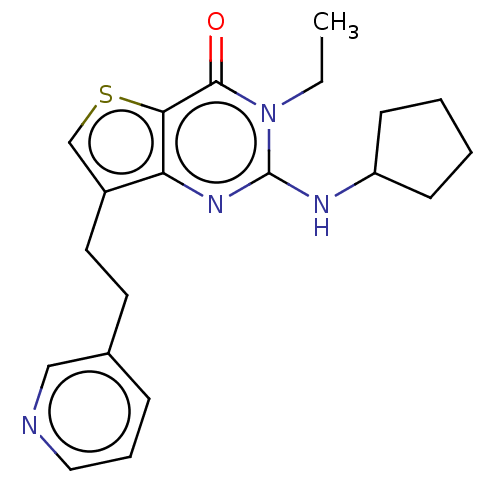
(CHEMBL3354173)Show InChI InChI=1S/C20H24N4OS/c1-2-24-19(25)18-17(23-20(24)22-16-7-3-4-8-16)15(13-26-18)10-9-14-6-5-11-21-12-14/h5-6,11-13,16H,2-4,7-10H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 607-8042
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
J Med Chem 57: 9844-54 (2014)
Article DOI: 10.1021/jm5008215
BindingDB Entry DOI: 10.7270/Q228096D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50068262

(CHEMBL3403362)Show InChI InChI=1S/C15H17N5OS/c1-4-20-14(21)13-12(19-15(20)18-9(2)3)11(7-22-13)10-5-16-8-17-6-10/h5-9H,4H2,1-3H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A expressed in insect cells assessed as inhibition of [3H]cAMP to [3H]AMP hydrolysis after 30 mins by scintillation counting |
Bioorg Med Chem Lett 25: 1910-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.031
BindingDB Entry DOI: 10.7270/Q2NC62VM |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM140122
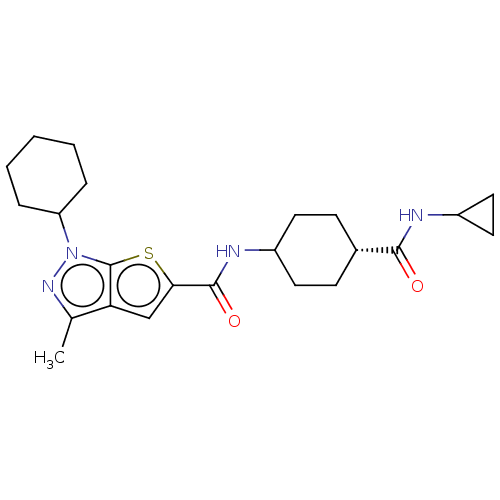
(US8901315, 238)Show SMILES Cc1nn(C2CCCCC2)c2sc(cc12)C(=O)NC1CC[C@@H](CC1)C(=O)NC1CC1 |r,wD:21.27,(-7.6,4.1,;-6.83,2.77,;-7.74,1.52,;-6.83,.27,;-7.23,-1.21,;-8.72,-1.61,;-9.12,-3.1,;-8.03,-4.19,;-6.54,-3.79,;-6.14,-2.3,;-5.37,.75,;-3.9,.27,;-3,1.52,;-3.9,2.77,;-5.37,2.29,;-1.46,1.52,;-.69,.19,;-.69,2.85,;.85,2.85,;1.62,4.19,;3.16,4.19,;3.93,2.85,;3.16,1.52,;1.62,1.52,;5.47,2.85,;6.24,4.19,;6.24,1.52,;7.78,1.52,;9.12,.75,;9.12,2.29,)| Show InChI InChI=1S/C23H32N4O2S/c1-14-19-13-20(30-23(19)27(26-14)18-5-3-2-4-6-18)22(29)25-16-9-7-15(8-10-16)21(28)24-17-11-12-17/h13,15-18H,2-12H2,1H3,(H,24,28)(H,25,29)/t15-,16? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... |
US Patent US8901315 (2014)
BindingDB Entry DOI: 10.7270/Q2GM860P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM140121
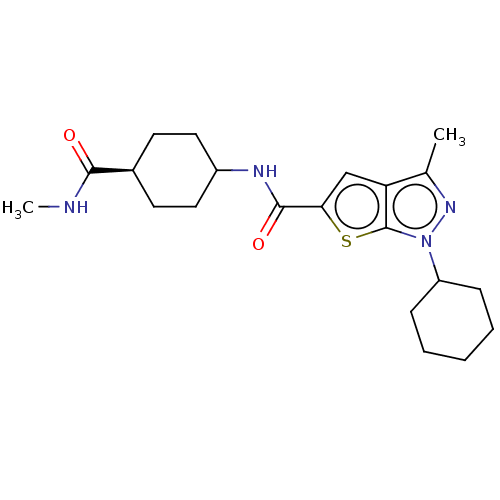
(US8901315, 237)Show SMILES CNC(=O)[C@H]1CCC(CC1)NC(=O)c1cc2c(C)nn(C3CCCCC3)c2s1 |r,wD:4.3,(8.45,1.52,;6.91,1.52,;6.14,2.85,;6.91,4.19,;4.6,2.85,;3.83,4.19,;2.29,4.19,;1.52,2.85,;2.29,1.52,;3.83,1.52,;-.02,2.85,;-.79,1.52,;-.02,.19,;-2.33,1.52,;-3.24,2.77,;-4.7,2.29,;-6.16,2.77,;-6.93,4.1,;-7.07,1.52,;-6.16,.27,;-6.56,-1.21,;-8.05,-1.61,;-8.45,-3.1,;-7.36,-4.19,;-5.87,-3.79,;-5.47,-2.3,;-4.7,.75,;-3.24,.27,)| Show InChI InChI=1S/C21H30N4O2S/c1-13-17-12-18(28-21(17)25(24-13)16-6-4-3-5-7-16)20(27)23-15-10-8-14(9-11-15)19(26)22-2/h12,14-16H,3-11H2,1-2H3,(H,22,26)(H,23,27)/t14-,15? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... |
US Patent US8901315 (2014)
BindingDB Entry DOI: 10.7270/Q2GM860P |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50381293
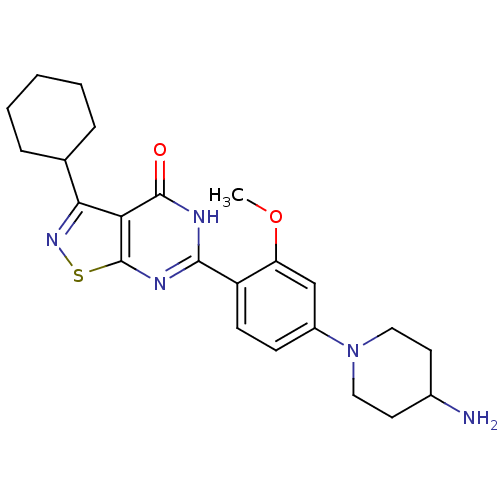
(CHEMBL2019106)Show SMILES COc1cc(ccc1-c1nc2snc(C3CCCCC3)c2c(=O)[nH]1)N1CCC(N)CC1 Show InChI InChI=1S/C23H29N5O2S/c1-30-18-13-16(28-11-9-15(24)10-12-28)7-8-17(18)21-25-22(29)19-20(27-31-23(19)26-21)14-5-3-2-4-6-14/h7-8,13-15H,2-6,9-12,24H2,1H3,(H,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cAMP from human recombinant PDE7A |
Bioorg Med Chem Lett 22: 3223-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.025
BindingDB Entry DOI: 10.7270/Q2NP25F2 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM140068
(US8901315, 112)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)c2cc3c(C)nn(C4CCCCCC4)c3s2)cc1 Show InChI InChI=1S/C25H33N5OS/c1-18-22-17-23(32-25(22)30(27-18)21-7-5-3-4-6-8-21)24(31)26-19-9-11-20(12-10-19)29-15-13-28(2)14-16-29/h9-12,17,21H,3-8,13-16H2,1-2H3,(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... |
US Patent US8901315 (2014)
BindingDB Entry DOI: 10.7270/Q2GM860P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data