 Found 20 hits of ec50 for UniProtKB: Q02156
Found 20 hits of ec50 for UniProtKB: Q02156 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519087
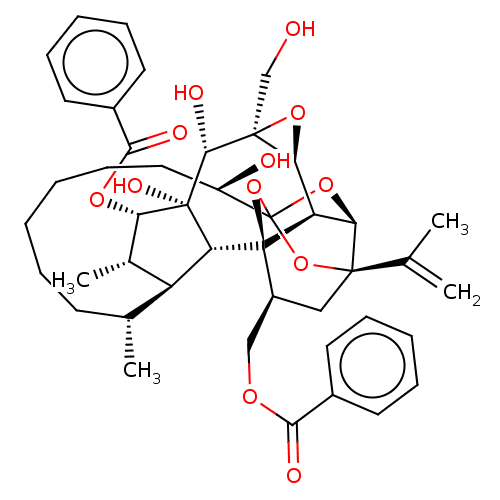
(CHEMBL3741746)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:38:35:54.55,THB:55:34:38:54.53.42,32:34:38:54.53.42,53:54:40.38.41:35,42:40:35:54.55,56:54:40.38.41:35| Show InChI InChI=1S/C44H54O12/c1-24(2)40-21-29(22-51-37(47)27-16-10-7-11-17-27)43-32-35(40)54-44(55-40,56-43)30(46)20-14-6-5-9-15-25(3)31-26(4)34(52-38(48)28-18-12-8-13-19-28)42(50,33(31)43)39(49)41(23-45)36(32)53-41/h7-8,10-13,16-19,25-26,29-36,39,45-46,49-50H,1,5-6,9,14-15,20-23H2,2-4H3/t25-,26+,29+,30-,31+,32-,33-,34+,35-,36+,39-,40-,41+,42-,43-,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human U1 cells infected with HIV-1 NL4-3 assessed as induction of HIV-1 p24 production after 72 hrs by ELISA |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519063
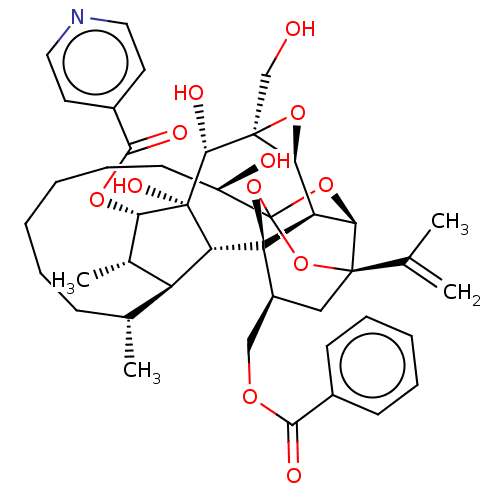
(CHEMBL4587471)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccncc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:38:54.55:35,THB:59:40:54.55:35,42:40:54.55:35,53:54:40.38.41:35,32:34:42.54.53:38| Show InChI InChI=1S/C43H53NO12/c1-23(2)39-20-28(21-51-36(47)26-13-9-7-10-14-26)42-31-34(39)54-43(55-39,56-42)29(46)15-11-6-5-8-12-24(3)30-25(4)33(52-37(48)27-16-18-44-19-17-27)41(50,32(30)42)38(49)40(22-45)35(31)53-40/h7,9-10,13-14,16-19,24-25,28-35,38,45-46,49-50H,1,5-6,8,11-12,15,20-22H2,2-4H3/t24-,25+,28+,29-,30+,31-,32-,33+,34-,35+,38-,39-,40+,41-,42-,43?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human U1 cells infected with HIV-1 NL4-3 assessed as induction of HIV-1 p24 production after 72 hrs by ELISA |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519068
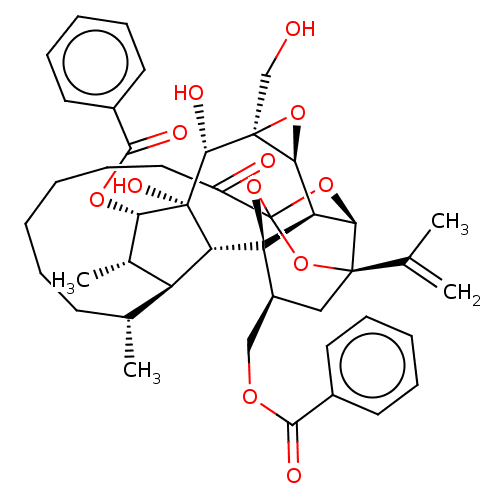
(CHEMBL4575056)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCCC(=O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:38:54.55:35,THB:59:40:54.55:35,42:40:54.55:35,53:54:40.38.41:35,32:34:42.54.53:38| Show InChI InChI=1S/C44H52O12/c1-24(2)40-21-29(22-51-37(47)27-16-10-7-11-17-27)43-32-35(40)54-44(55-40,56-43)30(46)20-14-6-5-9-15-25(3)31-26(4)34(52-38(48)28-18-12-8-13-19-28)42(50,33(31)43)39(49)41(23-45)36(32)53-41/h7-8,10-13,16-19,25-26,29,31-36,39,45,49-50H,1,5-6,9,14-15,20-23H2,2-4H3/t25-,26+,29+,31+,32-,33-,34+,35-,36+,39-,40-,41+,42-,43-,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human U1 cells infected with HIV-1 NL4-3 assessed as induction of HIV-1 p24 production after 72 hrs by ELISA |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519085

(CHEMBL4444766)Show SMILES [H][C@@]12O[C@]1(CO)C(=O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:38:54.55:35,THB:59:40:54.55:35,42:40:54.55:35,53:54:40.38.41:35,32:34:42.54.53:38| Show InChI InChI=1S/C44H52O12/c1-24(2)40-21-29(22-51-37(47)27-16-10-7-11-17-27)43-32-35(40)54-44(55-40,56-43)30(46)20-14-6-5-9-15-25(3)31-26(4)34(52-38(48)28-18-12-8-13-19-28)42(50,33(31)43)39(49)41(23-45)36(32)53-41/h7-8,10-13,16-19,25-26,29-36,45-46,50H,1,5-6,9,14-15,20-23H2,2-4H3/t25-,26+,29+,30-,31+,32-,33-,34+,35-,36+,40-,41+,42-,43-,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.90 | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human U1 cells infected with HIV-1 NL4-3 assessed as induction of HIV-1 p24 production after 72 hrs by ELISA |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50099066
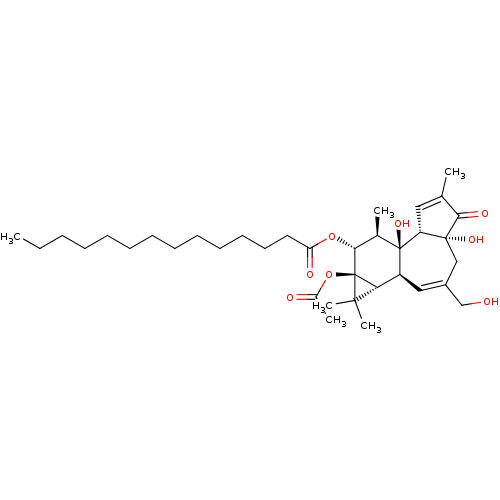
(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Activation of PKC in human platelet assessed as induction of platelet aggregation measured within 15 mins by tribidimetric method |
J Nat Prod 79: 2658-2666 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00603
BindingDB Entry DOI: 10.7270/Q24T6N3M |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50470108
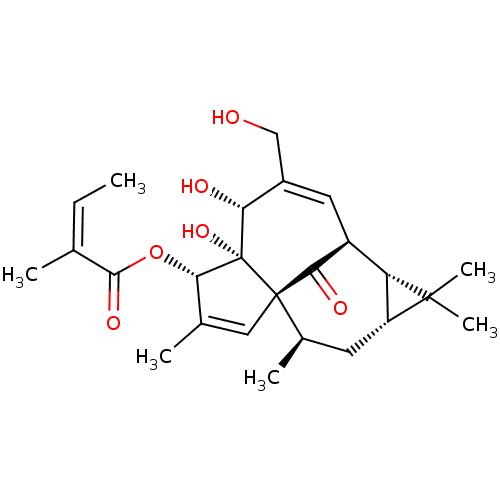
(AGN 204332 | Ingenol Mebutate | PEP-005 | PEP005 |...)Show SMILES [H][C@@]12C[C@@]([H])(C)[C@]34C=C(C)[C@]([H])(OC(=O)C(\C)=C/C)[C@@]3(O)[C@]([H])(O)C(CO)=C[C@]([H])(C4=O)[C@]1([H])C2(C)C |r,c:27,t:7| Show InChI InChI=1S/C25H34O6/c1-7-12(2)22(29)31-21-13(3)10-24-14(4)8-17-18(23(17,5)6)16(20(24)28)9-15(11-26)19(27)25(21,24)30/h7,9-10,14,16-19,21,26-27,30H,8,11H2,1-6H3/b12-7-/t14-,16+,17-,18+,19-,21+,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Activation of PKC in human platelet assessed as induction of platelet aggregation measured within 15 mins by tribidimetric method |
J Nat Prod 79: 2658-2666 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00603
BindingDB Entry DOI: 10.7270/Q24T6N3M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50368315

(PROSTRATIN)Show SMILES C[C@@H]1C[C@]2(OC(C)=O)[C@H]([C@@H]3C=C(CO)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]13O)C2(C)C |r,t:10,18| Show InChI InChI=1S/C22H30O6/c1-11-6-16-20(26,18(11)25)9-14(10-23)7-15-17-19(4,5)21(17,28-13(3)24)8-12(2)22(15,16)27/h6-7,12,15-17,23,26-27H,8-10H2,1-5H3/t12-,15+,16-,17?,20-,21+,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 910 | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Activation of PKC in human platelet assessed as induction of platelet aggregation measured within 15 mins by tribidimetric method |
J Nat Prod 79: 2658-2666 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00603
BindingDB Entry DOI: 10.7270/Q24T6N3M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50470110

(CHEMBL4071723)Show SMILES [H][C@@]12C[C@@H](C)[C@]34C=C(C)[C@H](OC(=O)C(\C)=C/C)[C@@]3(O)[C@H](O)C(C)=C[C@]([H])(C4=O)[C@]1([H])C2(C)C |r,c:23,t:6| Show InChI InChI=1S/C25H34O5/c1-8-12(2)22(28)30-21-14(4)11-24-15(5)10-17-18(23(17,6)7)16(20(24)27)9-13(3)19(26)25(21,24)29/h8-9,11,15-19,21,26,29H,10H2,1-7H3/b12-8-/t15-,16+,17-,18+,19-,21+,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.04E+3 | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Activation of PKC in human platelet assessed as induction of platelet aggregation measured within 15 mins by tribidimetric method |
J Nat Prod 79: 2658-2666 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00603
BindingDB Entry DOI: 10.7270/Q24T6N3M |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50099066

(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.56E+3 | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PKCepsilon expressed in Escherichia coli BL21(DE3) by fluorescence quenching |
Bioorg Med Chem 18: 1591-8 (2010)
Article DOI: 10.1016/j.bmc.2009.12.075
BindingDB Entry DOI: 10.7270/Q2KP834V |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50470109
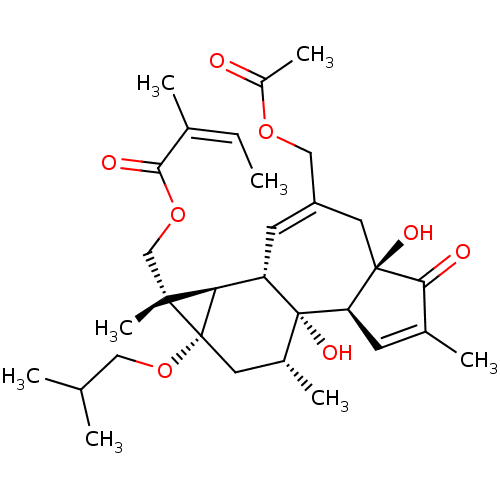
(CHEMBL4104319)Show SMILES [H][C@]12[C@]3([H])C=C(COC(C)=O)C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)C[C@@]1(OCC(C)C)[C@]2(C)COC(=O)C(\C)=C/C |r,c:17,t:4| Show InChI InChI=1S/C31H44O8/c1-9-18(4)27(34)38-16-28(8)25-23-11-22(15-37-21(7)32)13-29(35)24(10-19(5)26(29)33)31(23,36)20(6)12-30(25,28)39-14-17(2)3/h9-11,17,20,23-25,35-36H,12-16H2,1-8H3/b18-9-/t20-,23+,24-,25-,28-,29-,30+,31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.08E+3 | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Activation of PKC in human platelet assessed as induction of platelet aggregation measured within 15 mins by tribidimetric method |
J Nat Prod 79: 2658-2666 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00603
BindingDB Entry DOI: 10.7270/Q24T6N3M |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50015677
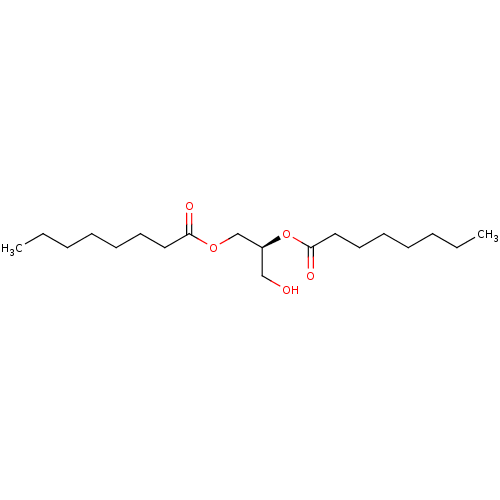
((S)-1-hydroxymethyl-2-octanoyloxy-ethyl ester | 1,...)Show InChI InChI=1S/C19H36O5/c1-3-5-7-9-11-13-18(21)23-16-17(15-20)24-19(22)14-12-10-8-6-4-2/h17,20H,3-16H2,1-2H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.19E+3 | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PKCepsilon expressed in Escherichia coli BL21(DE3) by fluorescence quenching |
Bioorg Med Chem 18: 1591-8 (2010)
Article DOI: 10.1016/j.bmc.2009.12.075
BindingDB Entry DOI: 10.7270/Q2KP834V |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50067040
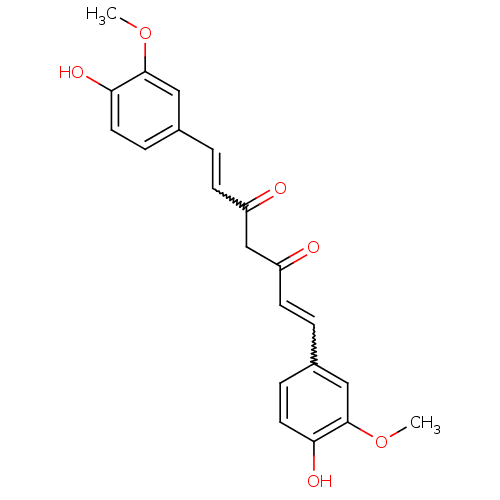
(((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...)Show SMILES COc1cc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(OC)c2)ccc1O |w:12.11,5.4| Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.81E+3 | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PKCepsilon expressed in Escherichia coli BL21(DE3) by fluorescence quenching |
Bioorg Med Chem 18: 1591-8 (2010)
Article DOI: 10.1016/j.bmc.2009.12.075
BindingDB Entry DOI: 10.7270/Q2KP834V |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50307103
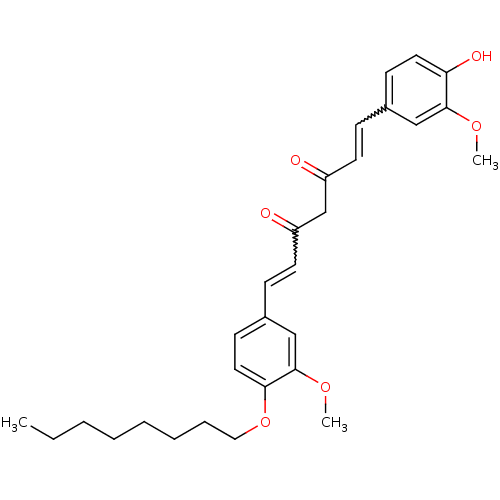
((1E,4Z,6E)-7-(4-(Hexadecyloxy)-3-methoxyphenyl)-5-...)Show SMILES CCCCCCCCOc1ccc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(OC)c2)cc1OC |w:21.21,14.14| Show InChI InChI=1S/C29H36O6/c1-4-5-6-7-8-9-18-35-27-17-13-23(20-29(27)34-3)11-15-25(31)21-24(30)14-10-22-12-16-26(32)28(19-22)33-2/h10-17,19-20,32H,4-9,18,21H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.79E+3 | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PKCepsilon expressed in Escherichia coli BL21(DE3) by fluorescence quenching |
Bioorg Med Chem 18: 1591-8 (2010)
Article DOI: 10.1016/j.bmc.2009.12.075
BindingDB Entry DOI: 10.7270/Q2KP834V |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50307104
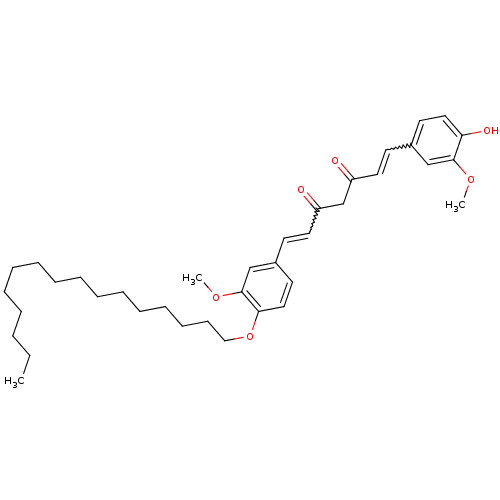
((1E,4Z,6E)-5-Hydroxy-1,7-bis(3-methoxy-4-(octyloxy...)Show SMILES CCCCCCCCCCCCCCCCOc1ccc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(OC)c2)cc1OC |w:22.22,29.29| Show InChI InChI=1S/C37H52O6/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26-43-35-25-21-31(28-37(35)42-3)19-23-33(39)29-32(38)22-18-30-20-24-34(40)36(27-30)41-2/h18-25,27-28,40H,4-17,26,29H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PKCepsilon expressed in Escherichia coli BL21(DE3) by fluorescence quenching |
Bioorg Med Chem 18: 1591-8 (2010)
Article DOI: 10.1016/j.bmc.2009.12.075
BindingDB Entry DOI: 10.7270/Q2KP834V |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50067030
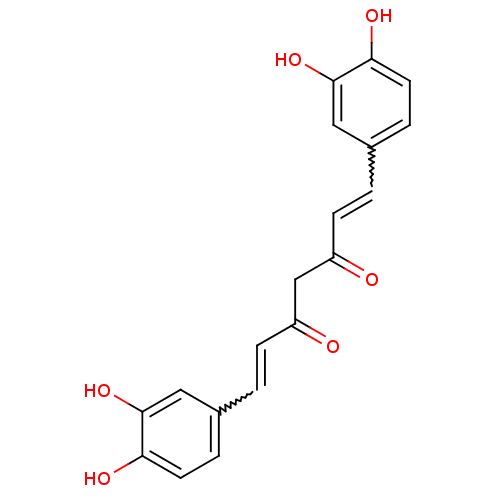
((1E,4Z,6E)-1,7-Bis-(3,4-dihydroxy-phenyl)-5-hydrox...)Show SMILES Oc1ccc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(O)c2)cc1O |w:5.4,13.13| Show InChI InChI=1S/C19H16O6/c20-14(5-1-12-3-7-16(22)18(24)9-12)11-15(21)6-2-13-4-8-17(23)19(25)10-13/h1-10,22-25H,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PKCepsilon expressed in Escherichia coli BL21(DE3) by fluorescence quenching |
Bioorg Med Chem 18: 1591-8 (2010)
Article DOI: 10.1016/j.bmc.2009.12.075
BindingDB Entry DOI: 10.7270/Q2KP834V |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50163745
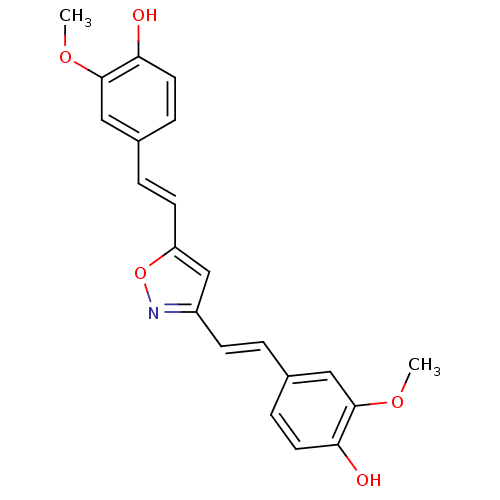
(4,40-(1E,10E)-2,20-(Isoxazole-3,5-diyl)bis(ethene-...)Show SMILES COc1cc(\C=C\c2cc(\C=C\c3ccc(O)c(OC)c3)on2)ccc1O Show InChI InChI=1S/C21H19NO5/c1-25-20-11-14(5-9-18(20)23)3-7-16-13-17(27-22-16)8-4-15-6-10-19(24)21(12-15)26-2/h3-13,23-24H,1-2H3/b7-3+,8-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Binding affinity to PKCepsilon C1B subdomain after 1 hr by fluorescence quenching analysis |
Bioorg Med Chem 19: 6196-202 (2011)
Article DOI: 10.1016/j.bmc.2011.09.011
BindingDB Entry DOI: 10.7270/Q2BK1CST |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50307105
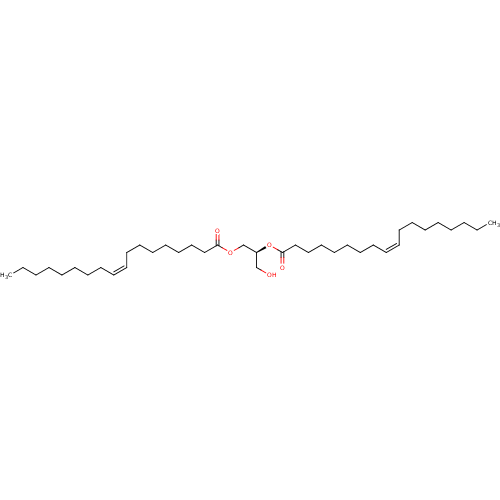
(1,2-dioleoyl-sn-glycerol | CHEMBL590047)Show SMILES CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@H](CO)OC(=O)CCCCCCC\C=C/CCCCCCCC |r| Show InChI InChI=1S/C39H72O5/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-38(41)43-36-37(35-40)44-39(42)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h17-20,37,40H,3-16,21-36H2,1-2H3/b19-17-,20-18-/t37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PKCepsilon expressed in Escherichia coli BL21(DE3) by fluorescence quenching |
Bioorg Med Chem 18: 1591-8 (2010)
Article DOI: 10.1016/j.bmc.2009.12.075
BindingDB Entry DOI: 10.7270/Q2KP834V |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50357492
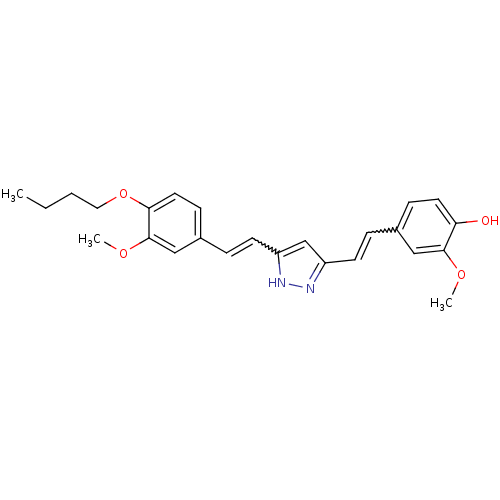
(CHEMBL1914609)Show SMILES CCCCOc1ccc(C=Cc2cc(C=Cc3ccc(O)c(OC)c3)n[nH]2)cc1OC |w:10.10,15.15| Show InChI InChI=1S/C25H28N2O4/c1-4-5-14-31-23-13-9-19(16-25(23)30-3)7-11-21-17-20(26-27-21)10-6-18-8-12-22(28)24(15-18)29-2/h6-13,15-17,28H,4-5,14H2,1-3H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Binding affinity to PKCepsilon C1B subdomain after 1 hr by fluorescence quenching analysis |
Bioorg Med Chem 19: 6196-202 (2011)
Article DOI: 10.1016/j.bmc.2011.09.011
BindingDB Entry DOI: 10.7270/Q2BK1CST |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50163748
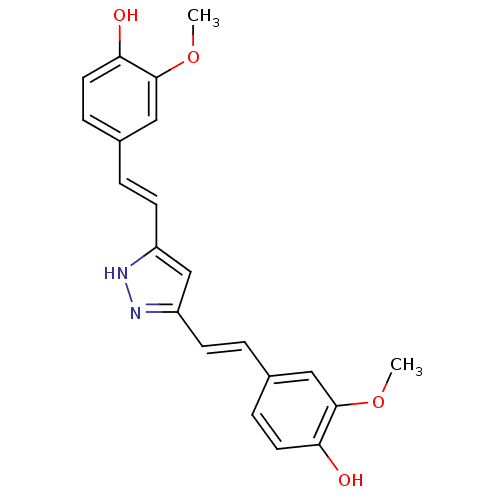
((E)-3,5-Bis[beta-(4-Hydroxy-3-methoxyphenyl)-ethen...)Show SMILES COc1cc(\C=C\c2cc(\C=C\c3ccc(O)c(OC)c3)[nH]n2)ccc1O Show InChI InChI=1S/C21H20N2O4/c1-26-20-11-14(5-9-18(20)24)3-7-16-13-17(23-22-16)8-4-15-6-10-19(25)21(12-15)27-2/h3-13,24-25H,1-2H3,(H,22,23)/b7-3+,8-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Binding affinity to PKCepsilon C1B subdomain after 1 hr by fluorescence quenching analysis |
Bioorg Med Chem 19: 6196-202 (2011)
Article DOI: 10.1016/j.bmc.2011.09.011
BindingDB Entry DOI: 10.7270/Q2BK1CST |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50357493
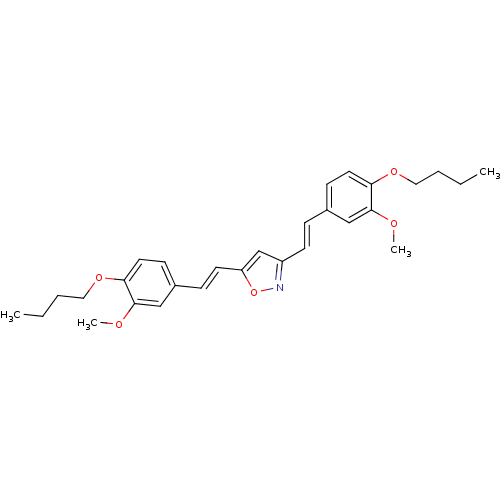
(CHEMBL1914610)Show SMILES CCCCOc1ccc(\C=C\c2cc(\C=C\c3ccc(OCCCC)c(OC)c3)on2)cc1OC Show InChI InChI=1S/C29H35NO5/c1-5-7-17-33-26-15-11-22(19-28(26)31-3)9-13-24-21-25(35-30-24)14-10-23-12-16-27(29(20-23)32-4)34-18-8-6-2/h9-16,19-21H,5-8,17-18H2,1-4H3/b13-9+,14-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.34E+4 | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Binding affinity to PKCepsilon C1B subdomain after 1 hr by fluorescence quenching analysis |
Bioorg Med Chem 19: 6196-202 (2011)
Article DOI: 10.1016/j.bmc.2011.09.011
BindingDB Entry DOI: 10.7270/Q2BK1CST |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data