 Found 99 hits of ic50 for UniProtKB: P08373
Found 99 hits of ic50 for UniProtKB: P08373 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475607
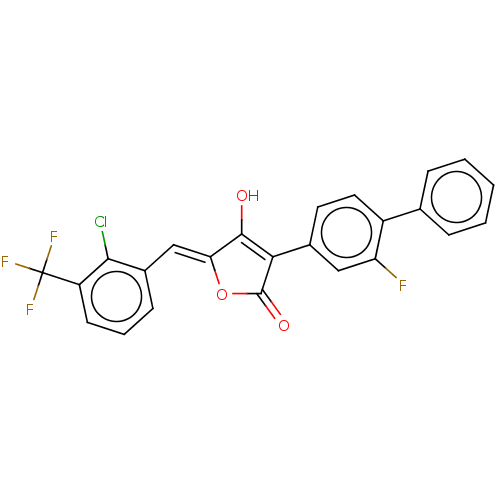
(CHEMBL199629)Show SMILES OC1=C(C(=O)O\C1=C/c1cccc(c1Cl)C(F)(F)F)c1ccc(c(F)c1)-c1ccccc1 |t:1| Show InChI InChI=1S/C24H13ClF4O3/c25-21-15(7-4-8-17(21)24(27,28)29)12-19-22(30)20(23(31)32-19)14-9-10-16(18(26)11-14)13-5-2-1-3-6-13/h1-12,30H/b19-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475601

(CHEMBL199812)Show SMILES OC1=C(C(=O)O\C1=C/c1cc(ccc1Cl)C(F)(F)F)c1ccc(c(F)c1)-c1ccccc1 |t:1| Show InChI InChI=1S/C24H13ClF4O3/c25-18-9-7-16(24(27,28)29)10-15(18)12-20-22(30)21(23(31)32-20)14-6-8-17(19(26)11-14)13-4-2-1-3-5-13/h1-12,30H/b20-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475590

(CHEMBL370421)Show SMILES OC1=C(C(=O)O\C1=C/c1cc(ccc1F)C(F)(F)F)c1ccc(c(F)c1)-c1ccccc1 |t:1| Show InChI InChI=1S/C24H13F5O3/c25-18-9-7-16(24(27,28)29)10-15(18)12-20-22(30)21(23(31)32-20)14-6-8-17(19(26)11-14)13-4-2-1-3-5-13/h1-12,30H/b20-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475599

(CHEMBL200097)Show SMILES Cc1ccccc1-c1ccc(\C=C2/OC(O)=C(C2=O)c2cc(Cl)cc(Cl)c2)cc1C |c:16| Show InChI InChI=1S/C25H18Cl2O3/c1-14-5-3-4-6-20(14)21-8-7-16(9-15(21)2)10-22-24(28)23(25(29)30-22)17-11-18(26)13-19(27)12-17/h3-13,29H,1-2H3/b22-10- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM119080
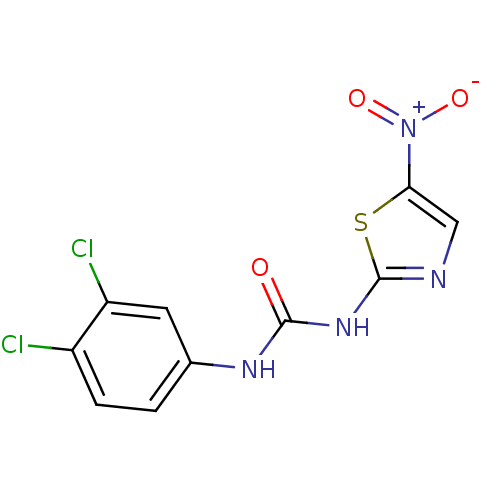
(MurB inhibitor (compound 19))Show SMILES [O-][N+](=O)c1cnc(NC(=O)Nc2ccc(Cl)c(Cl)c2)s1 Show InChI InChI=1S/C10H6Cl2N4O3S/c11-6-2-1-5(3-7(6)12)14-9(17)15-10-13-4-8(20-10)16(18)19/h1-4H,(H2,13,14,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana, A?kerceva 7, 1000 Ljubljana, Slovenia
| |
Bioorg Chem 55: 2-15 (2014)
Article DOI: 10.1016/j.bioorg.2014.03.008
BindingDB Entry DOI: 10.7270/Q2ZG6QWR |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475582
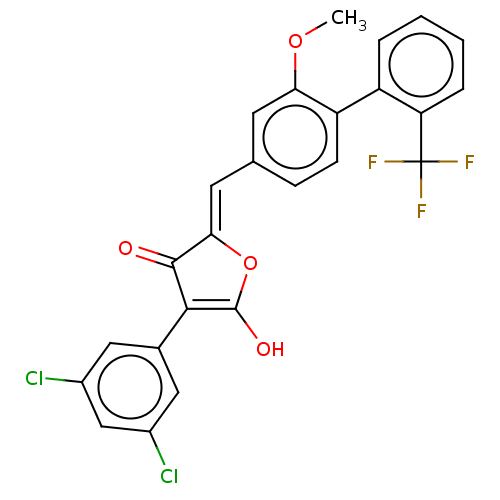
(CHEMBL199511)Show SMILES COc1cc(\C=C2/OC(O)=C(C2=O)c2cc(Cl)cc(Cl)c2)ccc1-c1ccccc1C(F)(F)F |c:9| Show InChI InChI=1S/C25H15Cl2F3O4/c1-33-20-8-13(6-7-18(20)17-4-2-3-5-19(17)25(28,29)30)9-21-23(31)22(24(32)34-21)14-10-15(26)12-16(27)11-14/h2-12,32H,1H3/b21-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM119079

(MurB inhibitor (compound 18))Show SMILES CC1(CC(=O)Nc2ccc(Cl)cc2Cl)C(=O)N(N(C1=O)c1ccc(Cl)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C24H17Cl4N3O3/c1-24(13-21(32)29-20-11-6-16(27)12-19(20)28)22(33)30(17-7-2-14(25)3-8-17)31(23(24)34)18-9-4-15(26)5-10-18/h2-12H,13H2,1H3,(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana, A?kerceva 7, 1000 Ljubljana, Slovenia
| |
Bioorg Chem 55: 2-15 (2014)
Article DOI: 10.1016/j.bioorg.2014.03.008
BindingDB Entry DOI: 10.7270/Q2ZG6QWR |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475587

(CHEMBL426651)Show SMILES OC1=C(C(=O)O\C1=C/c1ccccc1-c1ccc(Cl)c(Cl)c1)c1ccc(Cl)c(Cl)c1 |t:1| Show InChI InChI=1S/C23H12Cl4O3/c24-16-7-5-13(9-18(16)26)15-4-2-1-3-12(15)11-20-22(28)21(23(29)30-20)14-6-8-17(25)19(27)10-14/h1-11,28H/b20-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195560

(4-(4-butoxybenzoyl)-1,2-bis(4-chlorophenyl)-5-hydr...)Show SMILES CCCCOc1ccc(cc1)C(=O)c1c(O)n(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c1=O Show InChI InChI=1S/C26H22Cl2N2O4/c1-2-3-16-34-22-14-4-17(5-15-22)24(31)23-25(32)29(20-10-6-18(27)7-11-20)30(26(23)33)21-12-8-19(28)9-13-21/h4-15,32H,2-3,16H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195550
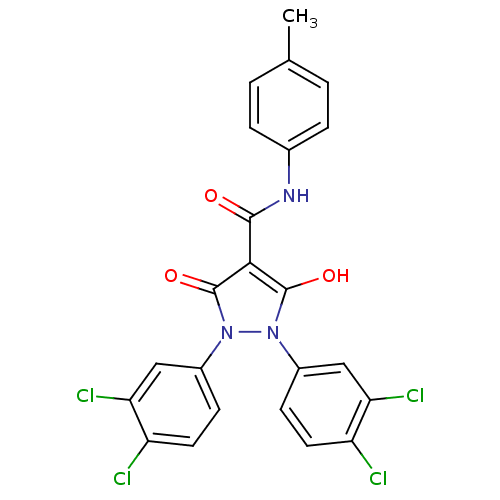
(1,2-bis(3,4-dichlorophenyl)-5-hydroxy-3-oxo-2,3-di...)Show SMILES Cc1ccc(NC(=O)c2c(O)n(-c3ccc(Cl)c(Cl)c3)n(-c3ccc(Cl)c(Cl)c3)c2=O)cc1 Show InChI InChI=1S/C23H15Cl4N3O3/c1-12-2-4-13(5-3-12)28-21(31)20-22(32)29(14-6-8-16(24)18(26)10-14)30(23(20)33)15-7-9-17(25)19(27)11-15/h2-11,32H,1H3,(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195576

(1,2-bis(3,4-dichlorophenyl)-5-hydroxy-3-oxo-2,3-di...)Show SMILES Oc1c(C(=O)Nc2ccc(Cl)c(Cl)c2)c(=O)n(-c2ccc(Cl)c(Cl)c2)n1-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H11Cl6N3O3/c23-13-4-1-10(7-16(13)26)29-20(32)19-21(33)30(11-2-5-14(24)17(27)8-11)31(22(19)34)12-3-6-15(25)18(28)9-12/h1-9,33H,(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475602
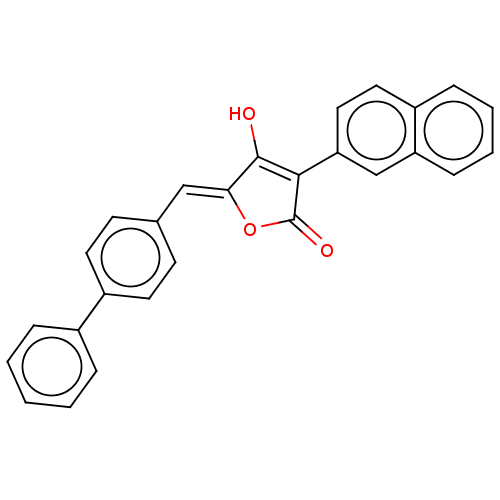
(CHEMBL199929)Show SMILES OC1=C(C(=O)O\C1=C/c1ccc(cc1)-c1ccccc1)c1ccc2ccccc2c1 |t:1| Show InChI InChI=1S/C27H18O3/c28-26-24(16-18-10-12-21(13-11-18)19-6-2-1-3-7-19)30-27(29)25(26)23-15-14-20-8-4-5-9-22(20)17-23/h1-17,28H/b24-16- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166493

(4-(2-Benzylsulfanyl-ethyl)-1,2-bis-(4-chloro-pheny...)Show SMILES Oc1c(CCSCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H20Cl2N2O2S/c25-18-6-10-20(11-7-18)27-23(29)22(14-15-31-16-17-4-2-1-3-5-17)24(30)28(27)21-12-8-19(26)9-13-21/h1-13,29H,14-16H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166480
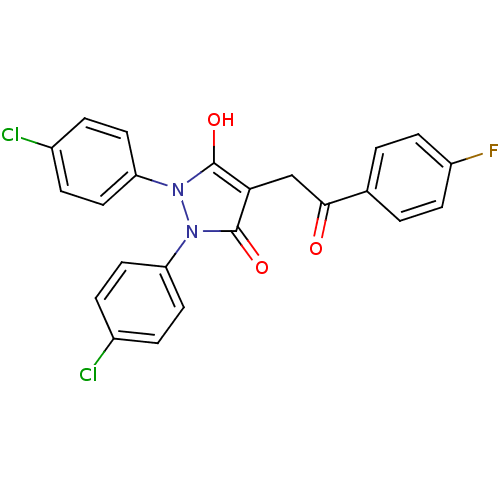
(1,2-Bis-(4-chloro-phenyl)-4-[2-(4-fluoro-phenyl)-2...)Show SMILES Oc1c(CC(=O)c2ccc(F)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H15Cl2FN2O3/c24-15-3-9-18(10-4-15)27-22(30)20(13-21(29)14-1-7-17(26)8-2-14)23(31)28(27)19-11-5-16(25)6-12-19/h1-12,30H,13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195551

(1,2-bis(4-chlorophenyl)-5-hydroxy-4-(4-trifluorome...)Show SMILES Oc1c(C(=O)c2ccc(OC(F)(F)F)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H13Cl2F3N2O4/c24-14-3-7-16(8-4-14)29-21(32)19(22(33)30(29)17-9-5-15(25)6-10-17)20(31)13-1-11-18(12-2-13)34-23(26,27)28/h1-12,32H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195567
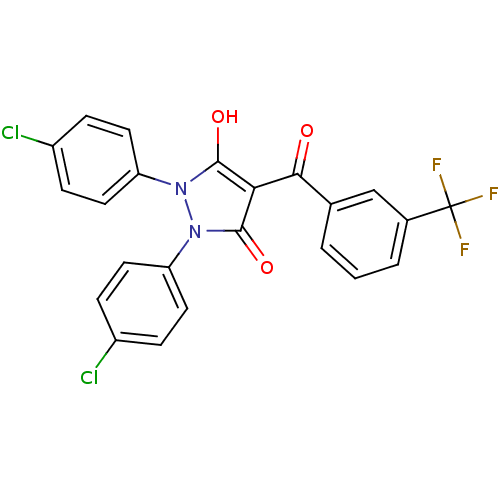
(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)c2cccc(c2)C(F)(F)F)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H13Cl2F3N2O3/c24-15-4-8-17(9-5-15)29-21(32)19(22(33)30(29)18-10-6-16(25)7-11-18)20(31)13-2-1-3-14(12-13)23(26,27)28/h1-12,32H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195557
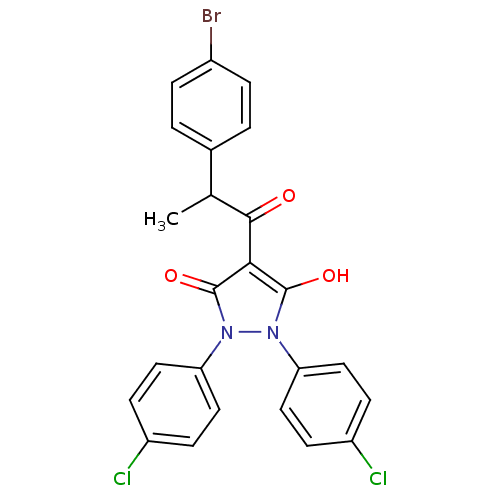
(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES CC(C(=O)c1c(O)n(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c1=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H17BrCl2N2O3/c1-14(15-2-4-16(25)5-3-15)22(30)21-23(31)28(19-10-6-17(26)7-11-19)29(24(21)32)20-12-8-18(27)9-13-20/h2-14,31H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166500
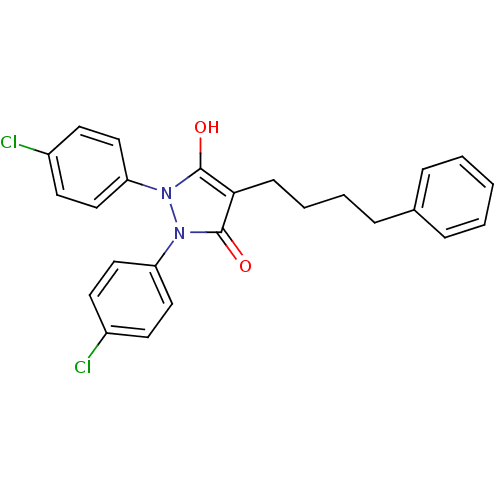
(1,2-Bis-(4-chloro-phenyl)-4-(4-phenyl-butyl)-pyraz...)Show SMILES Oc1c(CCCCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H22Cl2N2O2/c26-19-10-14-21(15-11-19)28-24(30)23(9-5-4-8-18-6-2-1-3-7-18)25(31)29(28)22-16-12-20(27)13-17-22/h1-3,6-7,10-17,30H,4-5,8-9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195555
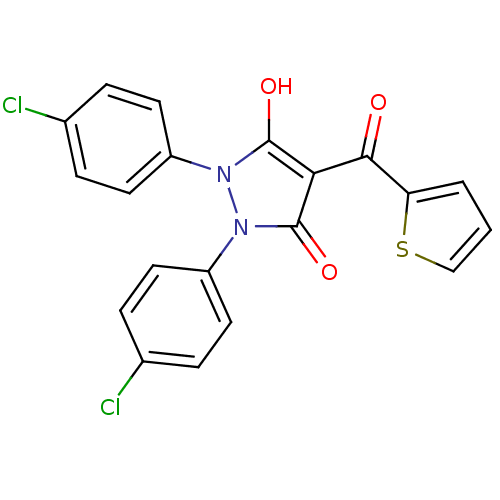
(1,2-bis(4-chlorophenyl)-5-hydroxy-4-(thiophene-2-c...)Show SMILES Oc1c(C(=O)c2cccs2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C20H12Cl2N2O3S/c21-12-3-7-14(8-4-12)23-19(26)17(18(25)16-2-1-11-28-16)20(27)24(23)15-9-5-13(22)6-10-15/h1-11,26H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166485
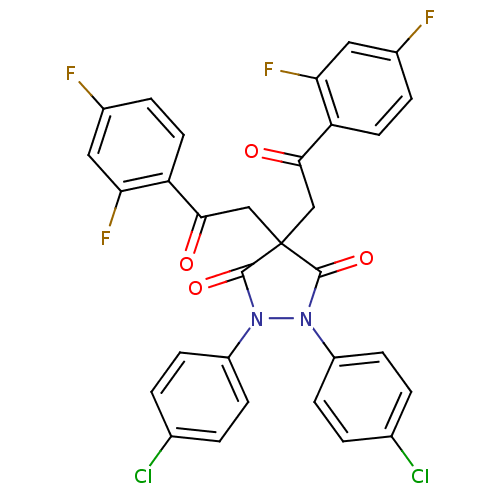
(1,2-Bis-(4-chloro-phenyl)-4,4-bis-[2-(2,4-difluoro...)Show SMILES Fc1ccc(C(=O)CC2(CC(=O)c3ccc(F)cc3F)C(=O)N(N(C2=O)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(F)c1 Show InChI InChI=1S/C31H18Cl2F4N2O4/c32-17-1-7-21(8-2-17)38-29(42)31(15-27(40)23-11-5-19(34)13-25(23)36,16-28(41)24-12-6-20(35)14-26(24)37)30(43)39(38)22-9-3-18(33)4-10-22/h1-14H,15-16H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166485

(1,2-Bis-(4-chloro-phenyl)-4,4-bis-[2-(2,4-difluoro...)Show SMILES Fc1ccc(C(=O)CC2(CC(=O)c3ccc(F)cc3F)C(=O)N(N(C2=O)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(F)c1 Show InChI InChI=1S/C31H18Cl2F4N2O4/c32-17-1-7-21(8-2-17)38-29(42)31(15-27(40)23-11-5-19(34)13-25(23)36,16-28(41)24-12-6-20(35)14-26(24)37)30(43)39(38)22-9-3-18(33)4-10-22/h1-14H,15-16H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana, A?kerceva 7, 1000 Ljubljana, Slovenia
| |
Bioorg Chem 55: 2-15 (2014)
Article DOI: 10.1016/j.bioorg.2014.03.008
BindingDB Entry DOI: 10.7270/Q2ZG6QWR |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195564
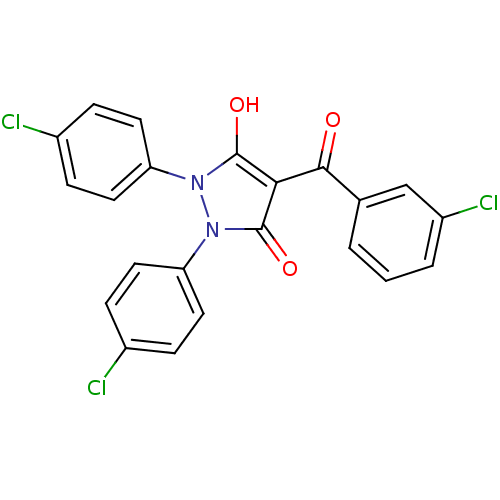
(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)c2cccc(Cl)c2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H13Cl3N2O3/c23-14-4-8-17(9-5-14)26-21(29)19(20(28)13-2-1-3-16(25)12-13)22(30)27(26)18-10-6-15(24)7-11-18/h1-12,29H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195577
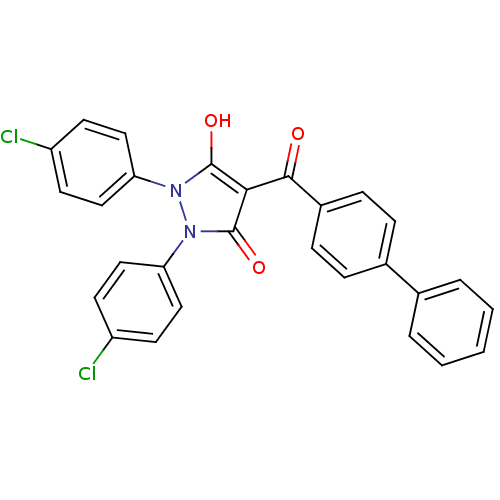
(4-(biphenyl-4-carbonyl)-1,2-bis(4-chlorophenyl)-5-...)Show SMILES Oc1c(C(=O)c2ccc(cc2)-c2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C28H18Cl2N2O3/c29-21-10-14-23(15-11-21)31-27(34)25(28(35)32(31)24-16-12-22(30)13-17-24)26(33)20-8-6-19(7-9-20)18-4-2-1-3-5-18/h1-17,34H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475596
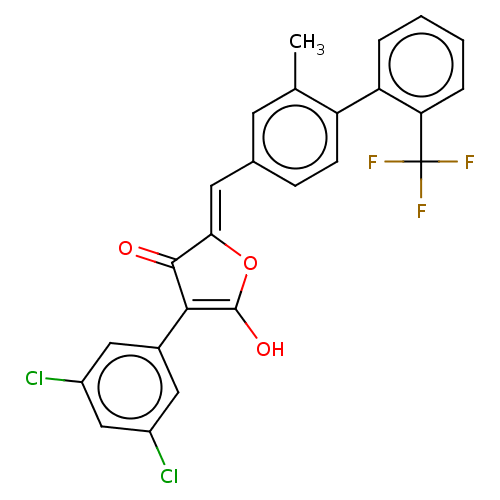
(CHEMBL200015)Show SMILES Cc1cc(\C=C2/OC(O)=C(C2=O)c2cc(Cl)cc(Cl)c2)ccc1-c1ccccc1C(F)(F)F |c:8| Show InChI InChI=1S/C25H15Cl2F3O3/c1-13-8-14(6-7-18(13)19-4-2-3-5-20(19)25(28,29)30)9-21-23(31)22(24(32)33-21)15-10-16(26)12-17(27)11-15/h2-12,32H,1H3/b21-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475591

(CHEMBL382328)Show SMILES OC1=C(C(=O)O\C1=C/c1cccc(c1)-c1cc(Cl)cc(Cl)c1)c1cc(Cl)cc(Cl)c1 |t:1| Show InChI InChI=1S/C23H12Cl4O3/c24-16-6-14(7-17(25)10-16)13-3-1-2-12(4-13)5-20-22(28)21(23(29)30-20)15-8-18(26)11-19(27)9-15/h1-11,28H/b20-5- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475586

(CHEMBL198030)Show SMILES OC1=C(C(=O)O\C1=C/c1ccc(cc1)-c1cc(Cl)cc(Cl)c1)c1cc(Cl)cc(Cl)c1 |t:1| Show InChI InChI=1S/C23H12Cl4O3/c24-16-6-14(7-17(25)10-16)13-3-1-12(2-4-13)5-20-22(28)21(23(29)30-20)15-8-18(26)11-19(27)9-15/h1-11,28H/b20-5- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475603
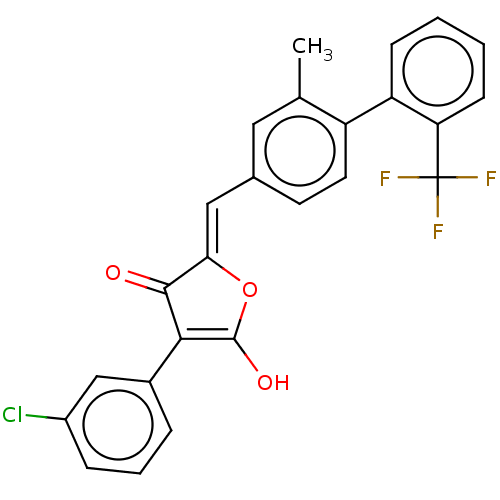
(CHEMBL199320)Show SMILES Cc1cc(\C=C2/OC(O)=C(C2=O)c2cccc(Cl)c2)ccc1-c1ccccc1C(F)(F)F |c:8| Show InChI InChI=1S/C25H16ClF3O3/c1-14-11-15(9-10-18(14)19-7-2-3-8-20(19)25(27,28)29)12-21-23(30)22(24(31)32-21)16-5-4-6-17(26)13-16/h2-13,31H,1H3/b21-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166499

(4,4-Bis-(2-benzyloxy-ethyl)-1,2-bis-(4-chloro-phen...)Show SMILES Clc1ccc(cc1)N1N(C(=O)C(CCOCc2ccccc2)(CCOCc2ccccc2)C1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C33H30Cl2N2O4/c34-27-11-15-29(16-12-27)36-31(38)33(19-21-40-23-25-7-3-1-4-8-25,20-22-41-24-26-9-5-2-6-10-26)32(39)37(36)30-17-13-28(35)14-18-30/h1-18H,19-24H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166475

(1,2-Bis-(4-chloro-phenyl)-4,4-bis-(6-phenyl-hexyl)...)Show SMILES Clc1ccc(cc1)N1N(C(=O)C(CCCCCCc2ccccc2)(CCCCCCc2ccccc2)C1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C39H42Cl2N2O2/c40-33-21-25-35(26-22-33)42-37(44)39(38(45)43(42)36-27-23-34(41)24-28-36,29-13-3-1-7-15-31-17-9-5-10-18-31)30-14-4-2-8-16-32-19-11-6-12-20-32/h5-6,9-12,17-28H,1-4,7-8,13-16,29-30H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM119076

(MurB inhibitor (compound 13))Show SMILES CCCC[C@H](N1C(SCC1=O)c1cccc(Oc2ccc(cc2)C(C)(C)C)c1)C(O)=O |r| Show InChI InChI=1S/C25H31NO4S/c1-5-6-10-21(24(28)29)26-22(27)16-31-23(26)17-8-7-9-20(15-17)30-19-13-11-18(12-14-19)25(2,3)4/h7-9,11-15,21,23H,5-6,10,16H2,1-4H3,(H,28,29)/t21-,23?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana, A?kerceva 7, 1000 Ljubljana, Slovenia
| |
Bioorg Chem 55: 2-15 (2014)
Article DOI: 10.1016/j.bioorg.2014.03.008
BindingDB Entry DOI: 10.7270/Q2ZG6QWR |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM119077

(MurB inhibitor (compound 14))Show SMILES CCCC[C@@H](C(O)=O)n1c(cn(Cc2ccccc2)c1=O)-c1cccc(Oc2ccc(cc2)C(C)(C)C)c1 |r| Show InChI InChI=1S/C32H36N2O4/c1-5-6-15-28(30(35)36)34-29(22-33(31(34)37)21-23-11-8-7-9-12-23)24-13-10-14-27(20-24)38-26-18-16-25(17-19-26)32(2,3)4/h7-14,16-20,22,28H,5-6,15,21H2,1-4H3,(H,35,36)/t28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana, A?kerceva 7, 1000 Ljubljana, Slovenia
| |
Bioorg Chem 55: 2-15 (2014)
Article DOI: 10.1016/j.bioorg.2014.03.008
BindingDB Entry DOI: 10.7270/Q2ZG6QWR |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195549

(1,2-bis(3-chlorophenyl)-3,5-dioxopyrazolidine-4-ca...)Show SMILES Oc1c(C(=O)Nc2ccc(Cl)cc2)c(=O)n(-c2cccc(Cl)c2)n1-c1cccc(Cl)c1 Show InChI InChI=1S/C22H14Cl3N3O3/c23-13-7-9-16(10-8-13)26-20(29)19-21(30)27(17-5-1-3-14(24)11-17)28(22(19)31)18-6-2-4-15(25)12-18/h1-12,30H,(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195561
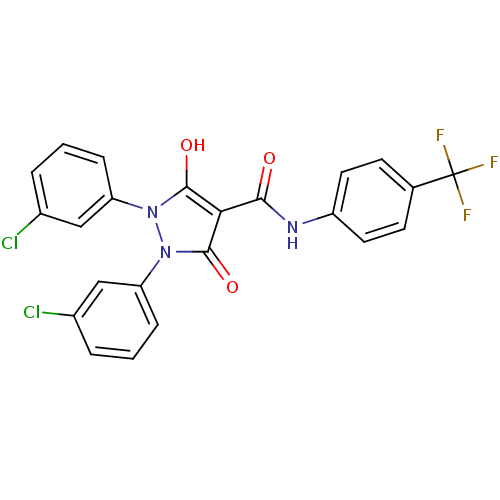
(1,2-bis(3-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)Nc2ccc(cc2)C(F)(F)F)c(=O)n(-c2cccc(Cl)c2)n1-c1cccc(Cl)c1 Show InChI InChI=1S/C23H14Cl2F3N3O3/c24-14-3-1-5-17(11-14)30-21(33)19(22(34)31(30)18-6-2-4-15(25)12-18)20(32)29-16-9-7-13(8-10-16)23(26,27)28/h1-12,33H,(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166478

(1,2-Bis-(4-chloro-phenyl)-4-(6-phenyl-hexyl)-pyraz...)Show SMILES Oc1c(CCCCCCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H26Cl2N2O2/c28-21-12-16-23(17-13-21)30-26(32)25(27(33)31(30)24-18-14-22(29)15-19-24)11-7-2-1-4-8-20-9-5-3-6-10-20/h3,5-6,9-10,12-19,32H,1-2,4,7-8,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195572
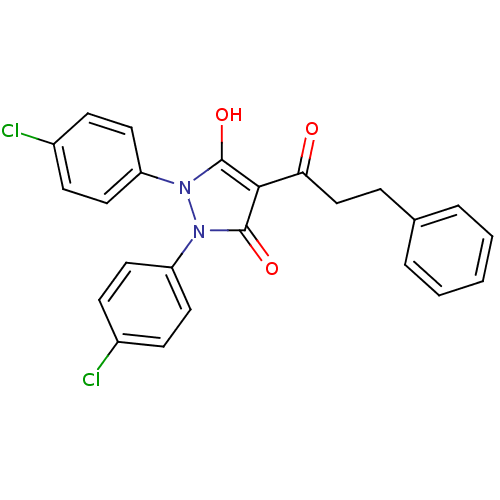
(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)CCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H18Cl2N2O3/c25-17-7-11-19(12-8-17)27-23(30)22(21(29)15-6-16-4-2-1-3-5-16)24(31)28(27)20-13-9-18(26)10-14-20/h1-5,7-14,30H,6,15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475579
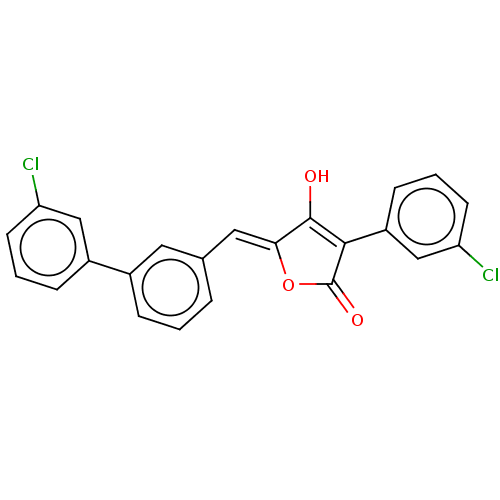
(CHEMBL382202)Show SMILES OC1=C(C(=O)O\C1=C/c1cccc(c1)-c1cccc(Cl)c1)c1cccc(Cl)c1 |t:1| Show InChI InChI=1S/C23H14Cl2O3/c24-18-8-2-6-16(12-18)15-5-1-4-14(10-15)11-20-22(26)21(23(27)28-20)17-7-3-9-19(25)13-17/h1-13,26H/b20-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195585
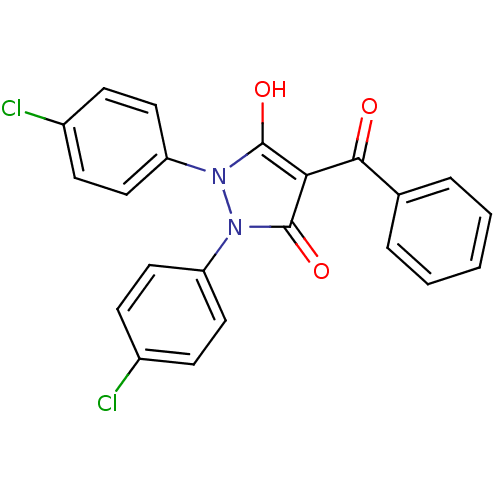
(1,2-bis(4-chlorophenyl)-3,5-dioxo-N-phenylpyrazoli...)Show SMILES Oc1c(C(=O)c2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H14Cl2N2O3/c23-15-6-10-17(11-7-15)25-21(28)19(20(27)14-4-2-1-3-5-14)22(29)26(25)18-12-8-16(24)9-13-18/h1-13,28H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50411197
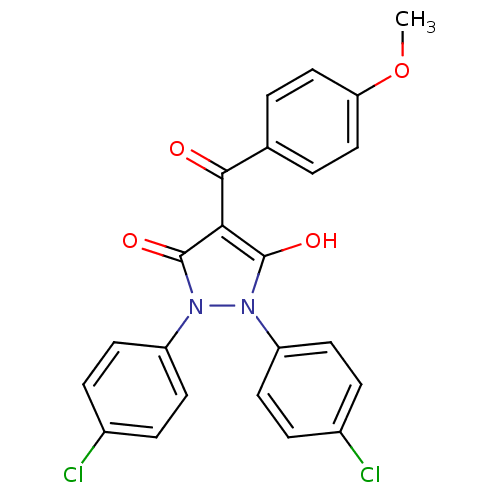
(CHEMBL2096735)Show SMILES COc1ccc(cc1)C(=O)c1c(O)n(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c1=O Show InChI InChI=1S/C23H16Cl2N2O4/c1-31-19-12-2-14(3-13-19)21(28)20-22(29)26(17-8-4-15(24)5-9-17)27(23(20)30)18-10-6-16(25)7-11-18/h2-13,29H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166498
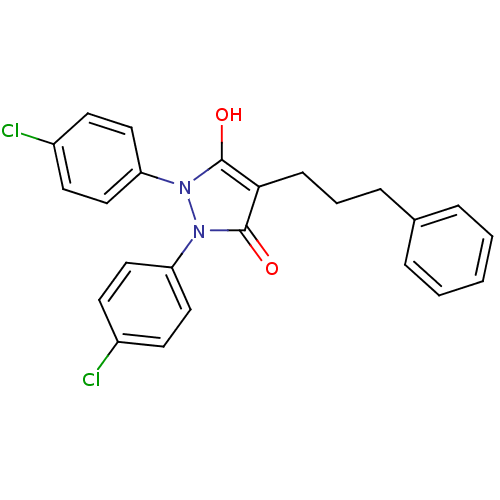
(1,2-Bis-(4-chloro-phenyl)-4-(3-phenyl-propyl)-pyra...)Show SMILES Oc1c(CCCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H20Cl2N2O2/c25-18-9-13-20(14-10-18)27-23(29)22(8-4-7-17-5-2-1-3-6-17)24(30)28(27)21-15-11-19(26)12-16-21/h1-3,5-6,9-16,29H,4,7-8H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50411197

(CHEMBL2096735)Show SMILES COc1ccc(cc1)C(=O)c1c(O)n(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c1=O Show InChI InChI=1S/C23H16Cl2N2O4/c1-31-19-12-2-14(3-13-19)21(28)20-22(29)26(17-8-4-15(24)5-9-17)27(23(20)30)18-10-6-16(25)7-11-18/h2-13,29H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166497
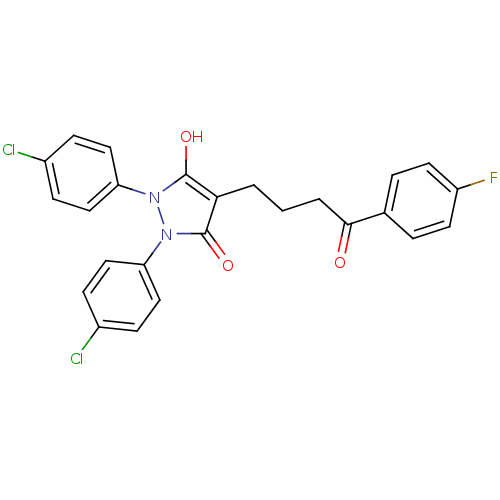
(1,2-Bis-(4-chloro-phenyl)-4-[4-(4-fluoro-phenyl)-4...)Show SMILES Oc1c(CCCC(=O)c2ccc(F)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H19Cl2FN2O3/c26-17-6-12-20(13-7-17)29-24(32)22(25(33)30(29)21-14-8-18(27)9-15-21)2-1-3-23(31)16-4-10-19(28)11-5-16/h4-15,32H,1-3H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166495

(1,2-Bis-(4-chloro-phenyl)-4-(5-phenyl-pentyl)-pyra...)Show SMILES Oc1c(CCCCCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H24Cl2N2O2/c27-20-11-15-22(16-12-20)29-25(31)24(10-6-2-5-9-19-7-3-1-4-8-19)26(32)30(29)23-17-13-21(28)14-18-23/h1,3-4,7-8,11-18,31H,2,5-6,9-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166483
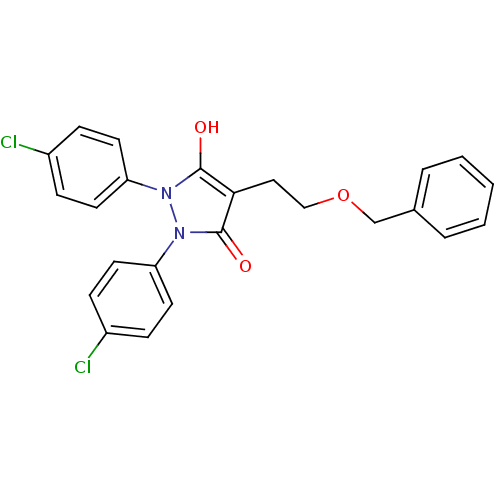
(4-(2-Benzyloxy-ethyl)-1,2-bis-(4-chloro-phenyl)-py...)Show SMILES Oc1c(CCOCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H20Cl2N2O3/c25-18-6-10-20(11-7-18)27-23(29)22(14-15-31-16-17-4-2-1-3-5-17)24(30)28(27)21-12-8-19(26)9-13-21/h1-13,29H,14-16H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166486
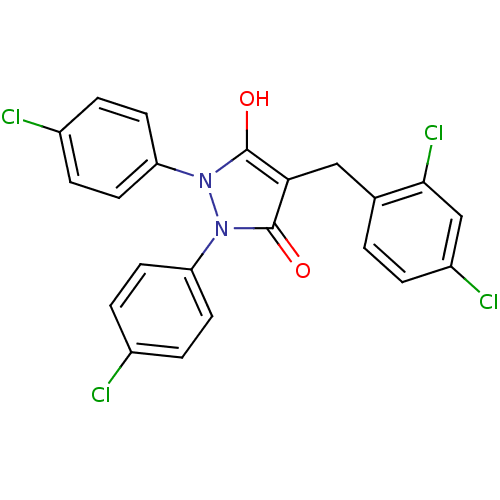
(1,2-Bis-(4-chloro-phenyl)-4-(2,4-dichloro-benzyl)-...)Show SMILES Oc1c(Cc2ccc(Cl)cc2Cl)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H14Cl4N2O2/c23-14-3-7-17(8-4-14)27-21(29)19(11-13-1-2-16(25)12-20(13)26)22(30)28(27)18-9-5-15(24)6-10-18/h1-10,12,29H,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50411196

(CHEMBL2096736)Show SMILES Oc1c(C(=O)c2ccc(Cl)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H13Cl3N2O3/c23-14-3-1-13(2-4-14)20(28)19-21(29)26(17-9-5-15(24)6-10-17)27(22(19)30)18-11-7-16(25)8-12-18/h1-12,29H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50411196

(CHEMBL2096736)Show SMILES Oc1c(C(=O)c2ccc(Cl)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H13Cl3N2O3/c23-14-3-1-13(2-4-14)20(28)19-21(29)26(17-9-5-15(24)6-10-17)27(22(19)30)18-11-7-16(25)8-12-18/h1-12,29H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166496

(1-{4-[1,2-Bis-(4-chloro-phenyl)-3,5-dioxo-pyrazoli...)Show SMILES Oc1c(Cc2ccc(cc2)C2(CC=CC=C2)C#N)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 |c:13,15| Show InChI InChI=1S/C29H21Cl2N3O2/c30-22-8-12-24(13-9-22)33-27(35)26(28(36)34(33)25-14-10-23(31)11-15-25)18-20-4-6-21(7-5-20)29(19-32)16-2-1-3-17-29/h1-16,35H,17-18H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166501
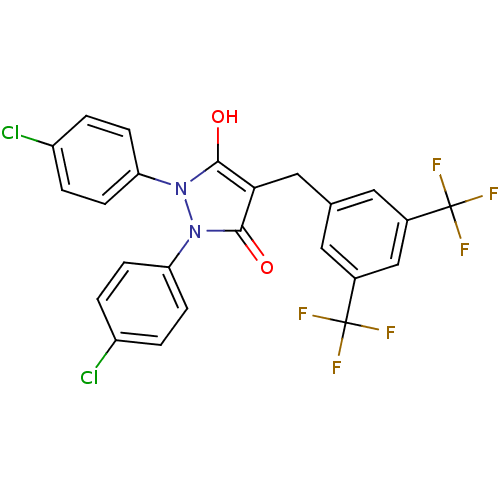
(4-(3,5-Bis-trifluoromethyl-benzyl)-1,2-bis-(4-chlo...)Show SMILES Oc1c(Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H14Cl2F6N2O2/c25-16-1-5-18(6-2-16)33-21(35)20(22(36)34(33)19-7-3-17(26)4-8-19)11-13-9-14(23(27,28)29)12-15(10-13)24(30,31)32/h1-10,12,35H,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195583

(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)c2ccccc2Cl)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H13Cl3N2O3/c23-13-5-9-15(10-6-13)26-21(29)19(20(28)17-3-1-2-4-18(17)25)22(30)27(26)16-11-7-14(24)8-12-16/h1-12,29H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475595

(CHEMBL199757)Show SMILES OC1=C(C(=O)O\C1=C/c1cccc(c1)C(F)(F)F)c1ccc(cc1)-c1ccccc1 |t:1| Show InChI InChI=1S/C24H15F3O3/c25-24(26,27)19-8-4-5-15(13-19)14-20-22(28)21(23(29)30-20)18-11-9-17(10-12-18)16-6-2-1-3-7-16/h1-14,28H/b20-14- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data