

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190273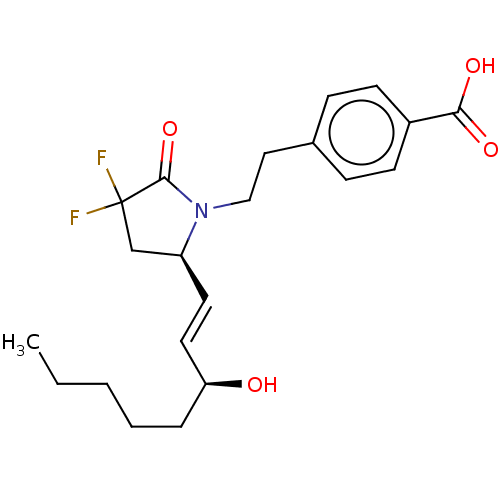 (US9180116, 21C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0820 | n/a | 0.220 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190282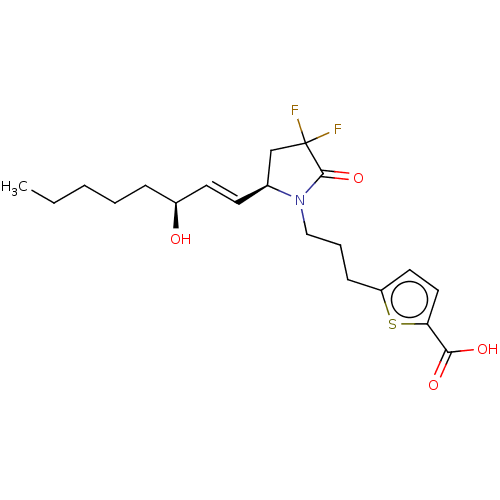 (US9180116, 33C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | 0.280 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50140261 (CHEMBL3753860) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-stimulated cAMP production after 1 hr by HTRF assa... | Bioorg Med Chem Lett 26: 931-5 (2016) Article DOI: 10.1016/j.bmcl.2015.12.057 BindingDB Entry DOI: 10.7270/Q22F7Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190272 (US9180116, 12D) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.120 | n/a | 0.320 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50101822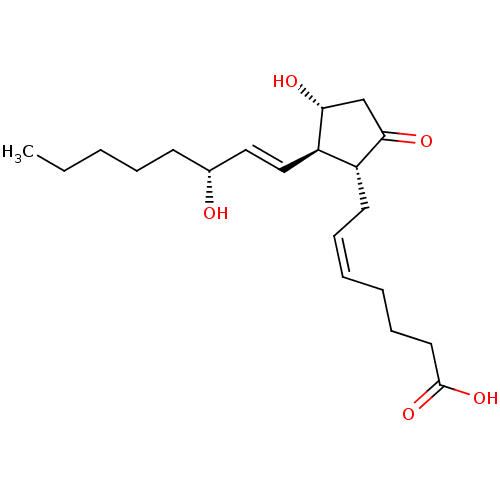 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | 0.380 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50606242 (CHEMBL5186935) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00448 BindingDB Entry DOI: 10.7270/Q2GM8CD4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50520104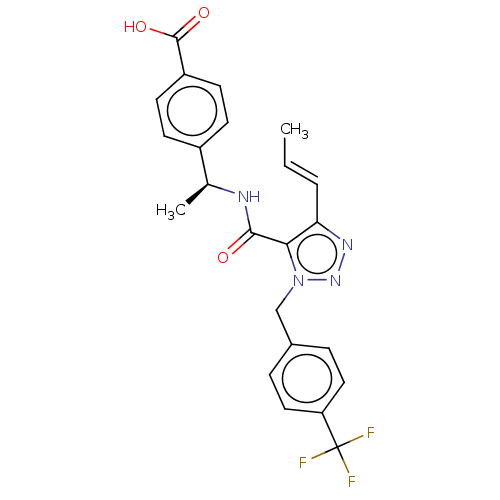 (CHEMBL4535971) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Inhibition of human EP4 transfected in human HEK293 cells co transfected with SmBit-beta-arrestin. assessed as reduction in PGE2 induced-beta-arresti... | J Med Chem 63: 569-590 (2020) Article DOI: 10.1021/acs.jmedchem.9b01269 BindingDB Entry DOI: 10.7270/Q2N58QRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cells measured after 120 mins by scintillation counting method | Bioorg Med Chem 25: 471-482 (2017) Article DOI: 10.1016/j.bmc.2016.11.014 BindingDB Entry DOI: 10.7270/Q2CF9S3S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190270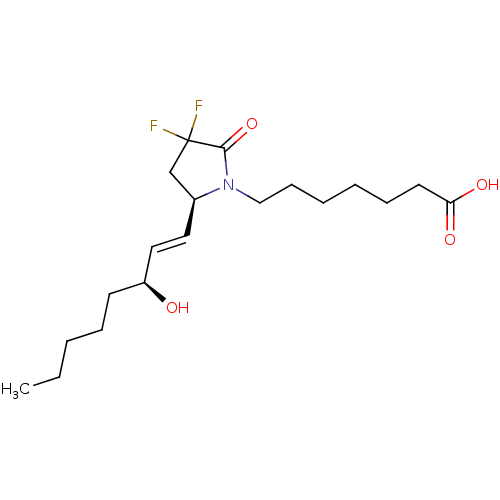 (US9180116, 9C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.210 | n/a | 0.570 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM578769 (US11479550, Example 11-15(2) | US11479550, Example...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CHO cells expressing human EP4 receptor subtypes were prepared according to the methods of Nishigaki et al. (Non-Patent Literature 4), and used for e... | Citation and Details BindingDB Entry DOI: 10.7270/Q2765JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50140264 (CHEMBL3753835) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-stimulated cAMP production after 1 hr by HTRF assa... | Bioorg Med Chem Lett 26: 931-5 (2016) Article DOI: 10.1016/j.bmcl.2015.12.057 BindingDB Entry DOI: 10.7270/Q22F7Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50140253 (CHEMBL3752948) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-stimulated cAMP production after 1 hr by HTRF assa... | Bioorg Med Chem Lett 26: 931-5 (2016) Article DOI: 10.1016/j.bmcl.2015.12.057 BindingDB Entry DOI: 10.7270/Q22F7Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM578749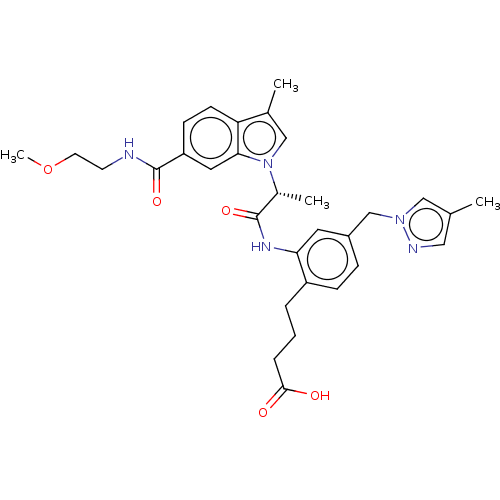 (US11479550, Example 3-9(2)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CHO cells expressing human EP4 receptor subtypes were prepared according to the methods of Nishigaki et al. (Non-Patent Literature 4), and used for e... | Citation and Details BindingDB Entry DOI: 10.7270/Q2765JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human EP4 receptor expressed in HEK293 ebna cells | Bioorg Med Chem Lett 13: 1129-32 (2003) BindingDB Entry DOI: 10.7270/Q25T3M1G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50140262 (CHEMBL3753567) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-stimulated cAMP production after 1 hr by HTRF assa... | Bioorg Med Chem Lett 26: 931-5 (2016) Article DOI: 10.1016/j.bmcl.2015.12.057 BindingDB Entry DOI: 10.7270/Q22F7Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190275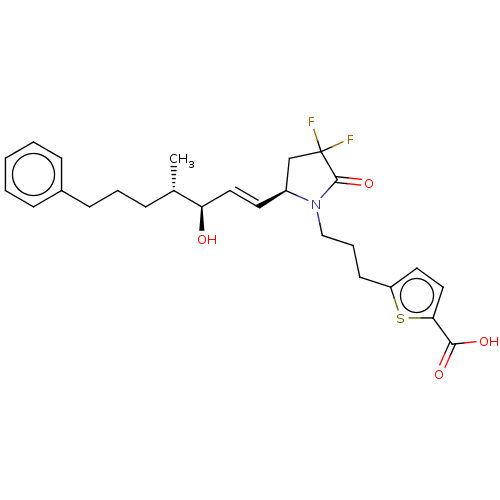 (US9180116, 28C | US9180116, 28H) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | n/a | 0.740 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM578774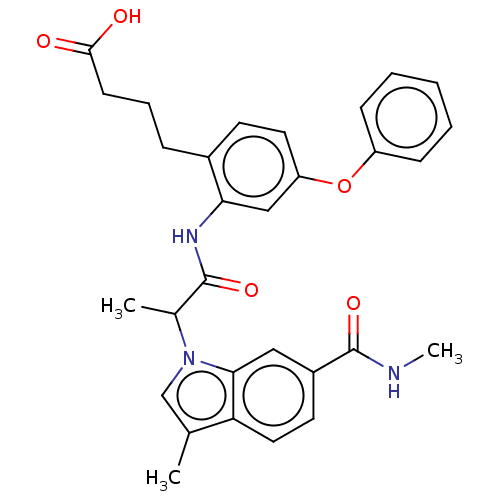 (4-[2-({2-[3-methyl-6-(methylcarbamoyl)-1H-indol-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CHO cells expressing human EP4 receptor subtypes were prepared according to the methods of Nishigaki et al. (Non-Patent Literature 4), and used for e... | Citation and Details BindingDB Entry DOI: 10.7270/Q2765JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM578746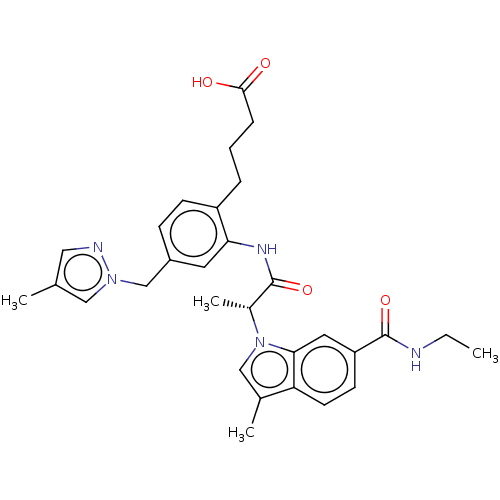 (US11479550, Example 3-3(2)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CHO cells expressing human EP4 receptor subtypes were prepared according to the methods of Nishigaki et al. (Non-Patent Literature 4), and used for e... | Citation and Details BindingDB Entry DOI: 10.7270/Q2765JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50334131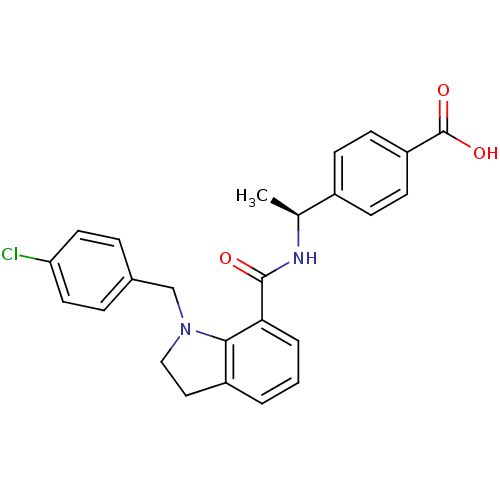 ((S)-4-(1-(1-(4-chlorobenzyl)indoline-7-carboxamido...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada Inc. Curated by ChEMBL | Assay Description Antagonist activity at human prostanoid EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-induced cAMP accumulation by scintillat... | Bioorg Med Chem Lett 21: 484-7 (2010) Article DOI: 10.1016/j.bmcl.2010.10.106 BindingDB Entry DOI: 10.7270/Q2BZ6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM640244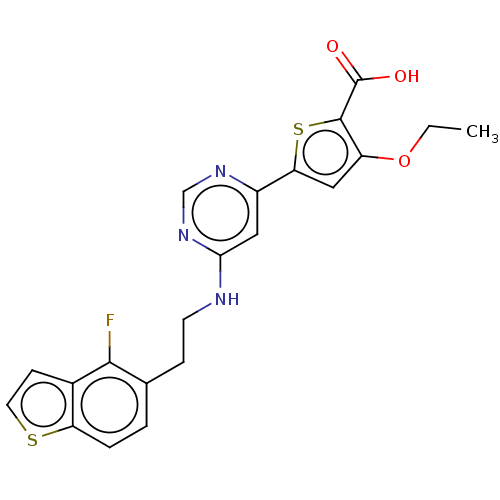 (3-Ethoxy-5-{6-[2-(4-fluoro-benzo[b]thiophen-5-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50606235 (CHEMBL5171006) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00448 BindingDB Entry DOI: 10.7270/Q2GM8CD4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM640239 (3-Ethoxy-5-{6-[2-(7-fluoro-benzo[b]thiophen-5-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM639881 (5-{6-[2-(3-Cyano-1-methyl-naphthalen-2-yl)-ethylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50606253 (CHEMBL5204626) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00448 BindingDB Entry DOI: 10.7270/Q2GM8CD4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM639880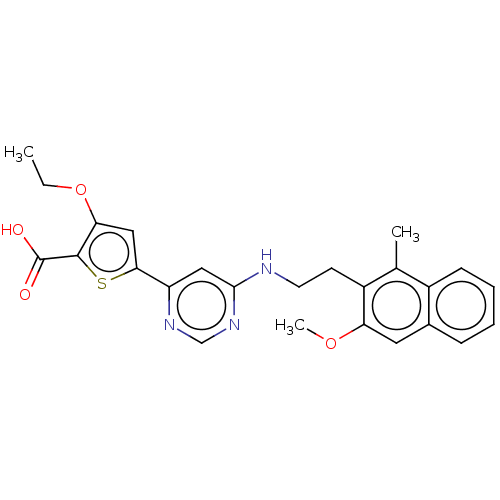 (3-Ethoxy-5-{6-[2-(3-methoxy-1-methyl-naphthalen-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50499955 (CHEMBL3741430) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor in HEK293 cells assessed as inhibition of PGE2-induced cAMP accumulation | Bioorg Med Chem Lett 26: 105-9 (2016) Article DOI: 10.1016/j.bmcl.2015.11.023 BindingDB Entry DOI: 10.7270/Q2G73HRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50319838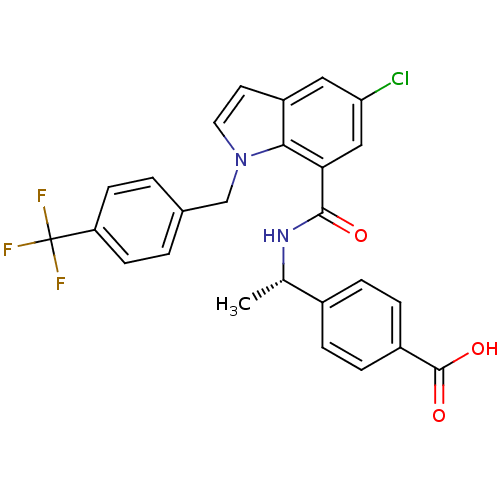 ((S)-4-(1-(5-chloro-1-(4-(trifluoromethyl)benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada Ltd Curated by ChEMBL | Assay Description Antagonist activity at human prostanoid EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-induced cAMP accumulation by scintillat... | Bioorg Med Chem Lett 20: 3760-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.065 BindingDB Entry DOI: 10.7270/Q20P106K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50334130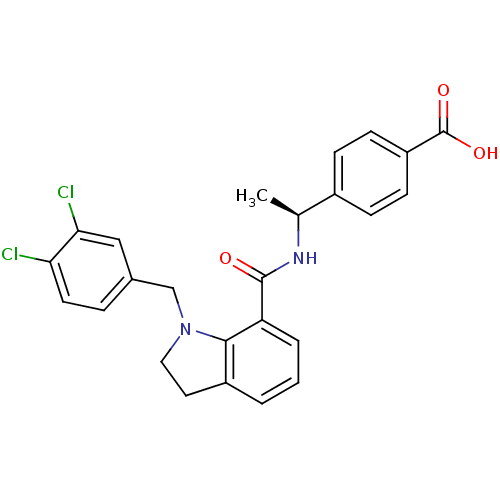 ((S)-4-(1-(1-(3,4-dichlorobenzyl)indoline-7-carboxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada Inc. Curated by ChEMBL | Assay Description Antagonist activity at human prostanoid EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-induced cAMP accumulation by scintillat... | Bioorg Med Chem Lett 21: 484-7 (2010) Article DOI: 10.1016/j.bmcl.2010.10.106 BindingDB Entry DOI: 10.7270/Q2BZ6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190267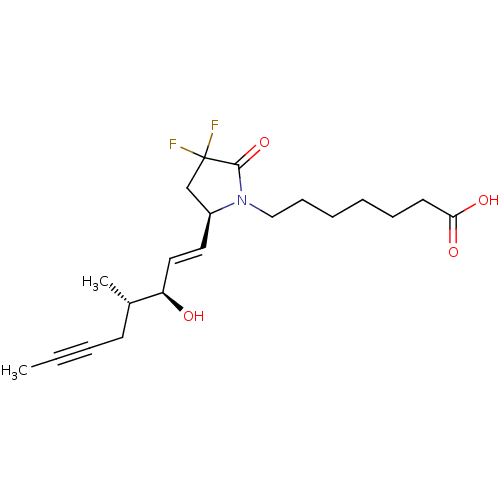 (US9180116, 1F) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.440 | n/a | 1.20 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM578743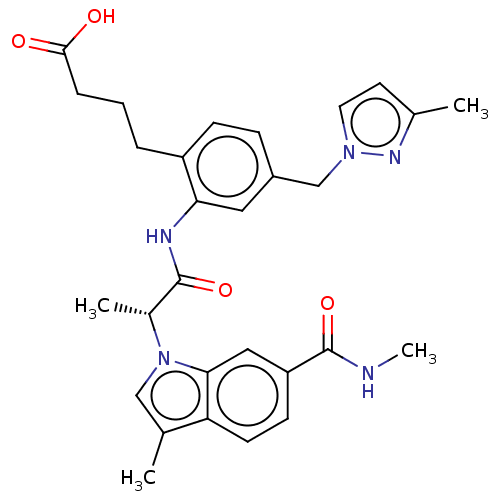 (US11479550, Example 2-6(2)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CHO cells expressing human EP4 receptor subtypes were prepared according to the methods of Nishigaki et al. (Non-Patent Literature 4), and used for e... | Citation and Details BindingDB Entry DOI: 10.7270/Q2765JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM285498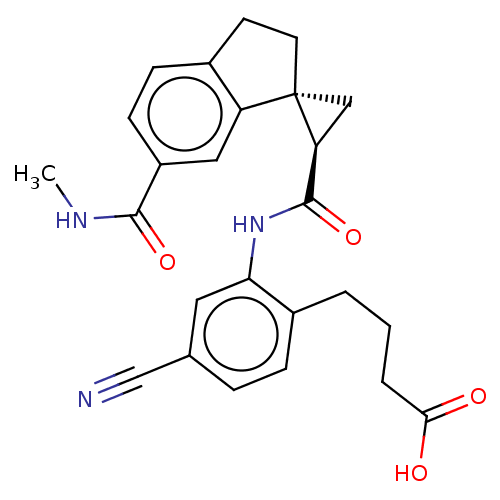 (4-[4-Cyano-2-({[(1R,2R)-6′-(methylcarbamoyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... | US Patent US10077247 (2018) BindingDB Entry DOI: 10.7270/Q22J6DWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50333748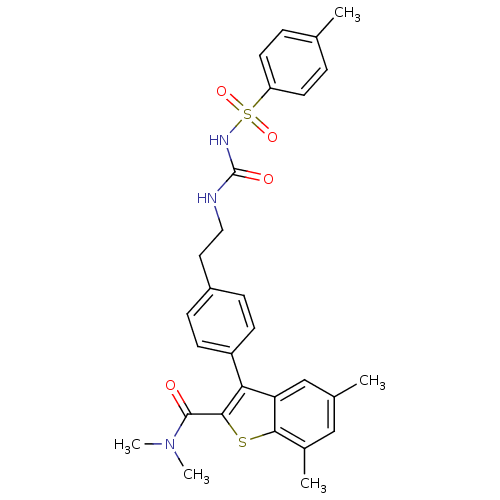 (CHEMBL1644003 | N,N,5,7-tetramethyl-3-(4-(2-(3-tos...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor in HEK293 cells by cell-based functional assay | Bioorg Med Chem Lett 21: 734-7 (2011) Article DOI: 10.1016/j.bmcl.2010.11.118 BindingDB Entry DOI: 10.7270/Q22N52JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190268 (US9180116, 2C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.490 | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM578764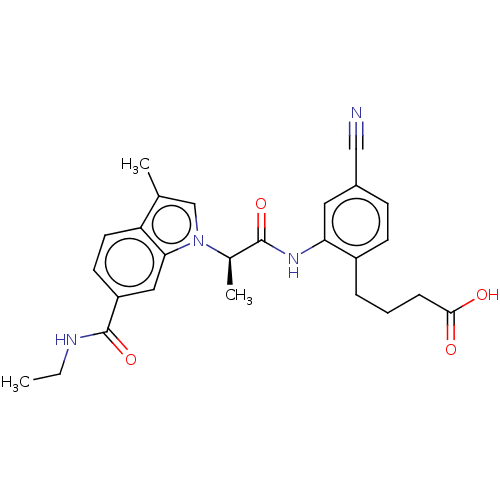 (US11479550, Example 11-1(2)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CHO cells expressing human EP4 receptor subtypes were prepared according to the methods of Nishigaki et al. (Non-Patent Literature 4), and used for e... | Citation and Details BindingDB Entry DOI: 10.7270/Q2765JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50319837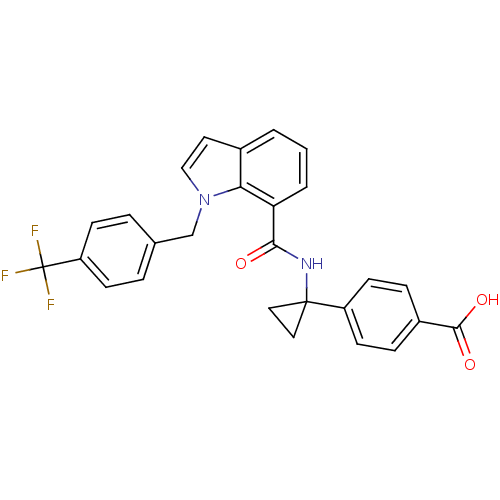 (4-(1-(1-(4-(trifluoromethyl)benzyl)-1H-indole-7-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada Ltd Curated by ChEMBL | Assay Description Antagonist activity at human prostanoid EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-induced cAMP accumulation by scintillat... | Bioorg Med Chem Lett 20: 3760-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.065 BindingDB Entry DOI: 10.7270/Q20P106K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50334136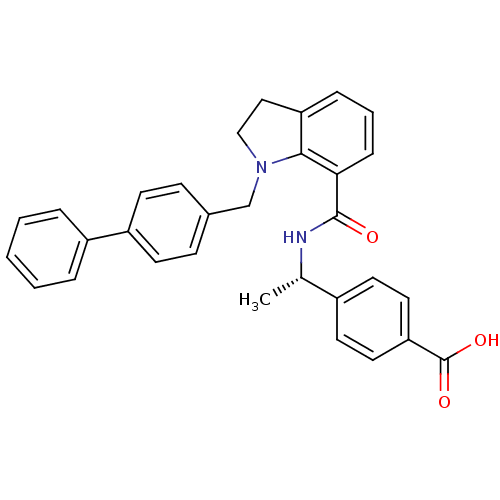 ((S)-4-(1-(1-(biphenyl-4-ylmethyl)indoline-7-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada Inc. Curated by ChEMBL | Assay Description Antagonist activity at human prostanoid EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-induced cAMP accumulation by scintillat... | Bioorg Med Chem Lett 21: 484-7 (2010) Article DOI: 10.1016/j.bmcl.2010.10.106 BindingDB Entry DOI: 10.7270/Q2BZ6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM285464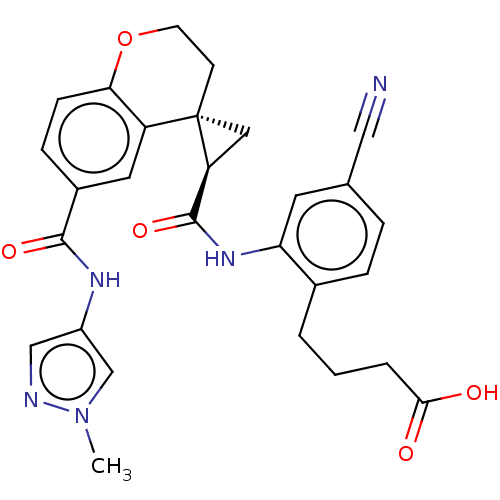 (4-{4-Cyano-2-[({2′R, 4S)-6-[(1-methyl 1H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... | US Patent US10077247 (2018) BindingDB Entry DOI: 10.7270/Q22J6DWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50520069 (CHEMBL4457944) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Antagonist activity at human EP4 expressed in CHO cells coexpressing G16-alpha assessed as intracellular calcium flux preincubated for 15 mins follow... | J Med Chem 63: 569-590 (2020) Article DOI: 10.1021/acs.jmedchem.9b01269 BindingDB Entry DOI: 10.7270/Q2N58QRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50319836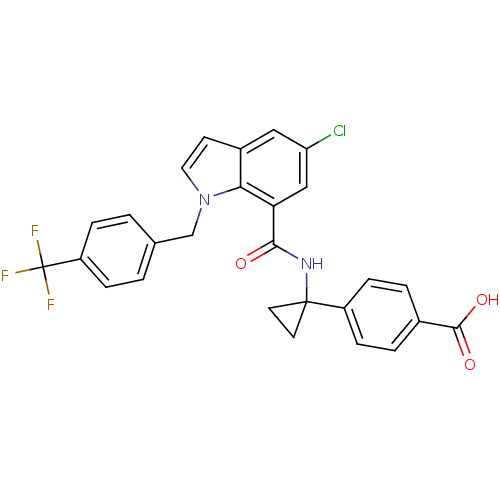 (4-(1-(5-chloro-1-(4-(trifluoromethyl)benzyl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada Ltd Curated by ChEMBL | Assay Description Antagonist activity at human prostanoid EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-induced cAMP accumulation by scintillat... | Bioorg Med Chem Lett 20: 3760-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.065 BindingDB Entry DOI: 10.7270/Q20P106K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50125405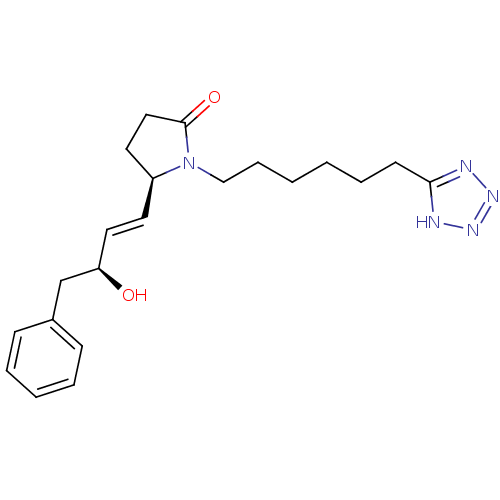 ((R)-5-((E)-(S)-3-Hydroxy-4-phenyl-but-1-enyl)-1-[6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description EP4 agonist potency utilizing a stable clone of pSV40-EP4 transfected into HEK293 cells expressing EP4 receptor | Bioorg Med Chem Lett 13: 1129-32 (2003) BindingDB Entry DOI: 10.7270/Q25T3M1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50319840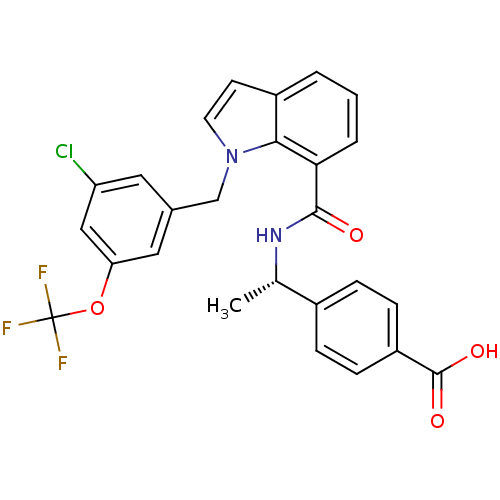 ((S)-4-(1-(1-(3-chloro-5-(trifluoromethoxy)benzyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada Ltd Curated by ChEMBL | Assay Description Antagonist activity at human prostanoid EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-induced cAMP accumulation by scintillat... | Bioorg Med Chem Lett 20: 3760-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.065 BindingDB Entry DOI: 10.7270/Q20P106K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM578751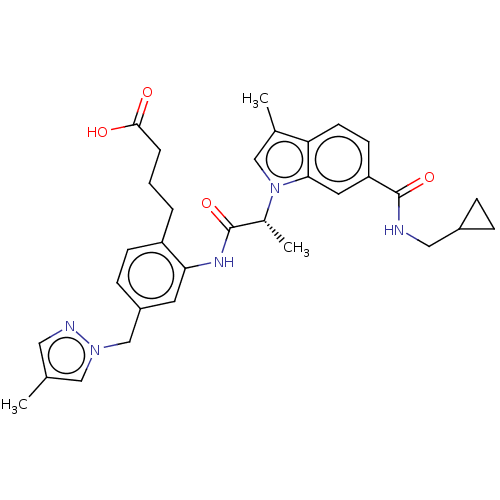 (US11479550, Example 3-20(2)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CHO cells expressing human EP4 receptor subtypes were prepared according to the methods of Nishigaki et al. (Non-Patent Literature 4), and used for e... | Citation and Details BindingDB Entry DOI: 10.7270/Q2765JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50319843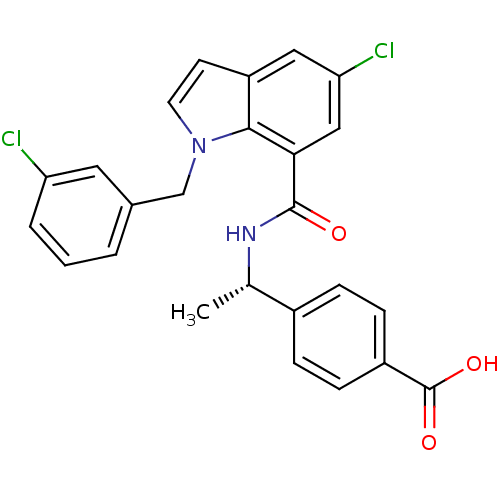 ((S)-4-(1-(5-chloro-1-(3-chlorobenzyl)-1H-indole-7-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada Ltd Curated by ChEMBL | Assay Description Antagonist activity at human prostanoid EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-induced cAMP accumulation by scintillat... | Bioorg Med Chem Lett 20: 3760-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.065 BindingDB Entry DOI: 10.7270/Q20P106K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335985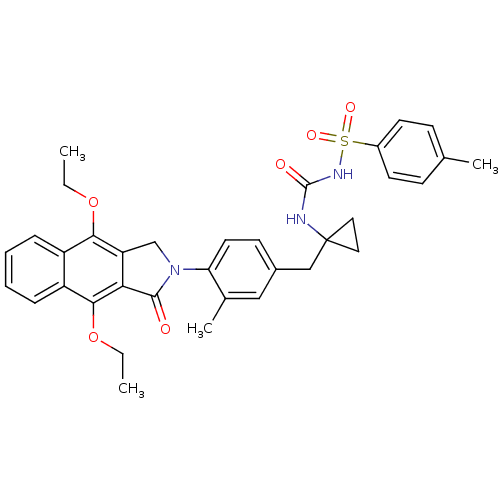 (CHEMBL1669013 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293 cells by scintillation counting | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM578756 (US11479550, Example 7-3(2)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CHO cells expressing human EP4 receptor subtypes were prepared according to the methods of Nishigaki et al. (Non-Patent Literature 4), and used for e... | Citation and Details BindingDB Entry DOI: 10.7270/Q2765JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM453713 (2-(9-Ethyl-8-fluoro-6-methyl-9H-carbazol-3-yl)-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 5. 1 Detection PrincipleBinding of prostaglandin D2 to the human PGD receptor induces activation of membrane-bound adenylate cyclases and leads to th... | US Patent US10730856 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308134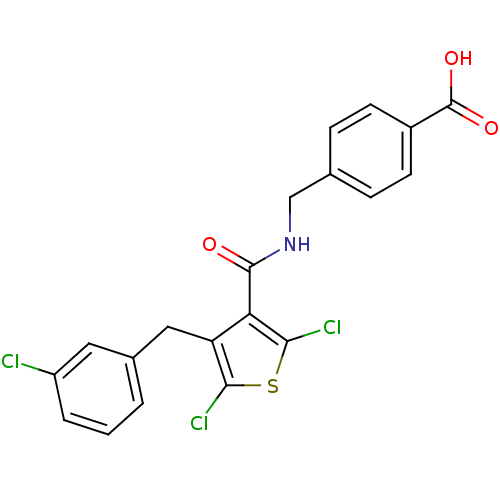 (4-[({[2,5-Dichloro-4-(3-chlorobenzyl)-3-thienyl]ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-induced cAMP accumulation by scintillation proximi... | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM578773 (US11479550, Example 12-1(2)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CHO cells expressing human EP4 receptor subtypes were prepared according to the methods of Nishigaki et al. (Non-Patent Literature 4), and used for e... | Citation and Details BindingDB Entry DOI: 10.7270/Q2765JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM578766 (US11479550, Example 11-7(2)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CHO cells expressing human EP4 receptor subtypes were prepared according to the methods of Nishigaki et al. (Non-Patent Literature 4), and used for e... | Citation and Details BindingDB Entry DOI: 10.7270/Q2765JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM285502 (US10077247, Example 20-2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... | US Patent US10077247 (2018) BindingDB Entry DOI: 10.7270/Q22J6DWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1238 total ) | Next | Last >> |