

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347330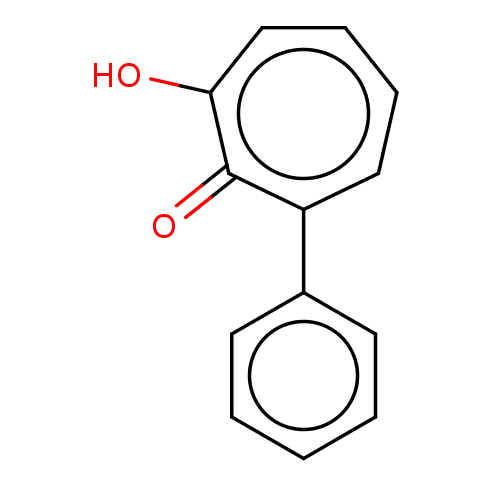 (MO-OH-PHE | US9790158, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50105327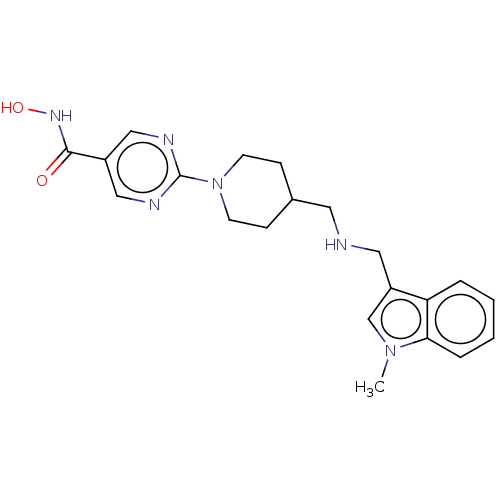 (JNJ-26481585 | Quisinostat) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human HDAC4 | Bioorg Med Chem 23: 5151-5 (2015) Article DOI: 10.1016/j.bmc.2014.12.066 BindingDB Entry DOI: 10.7270/Q2B859V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347451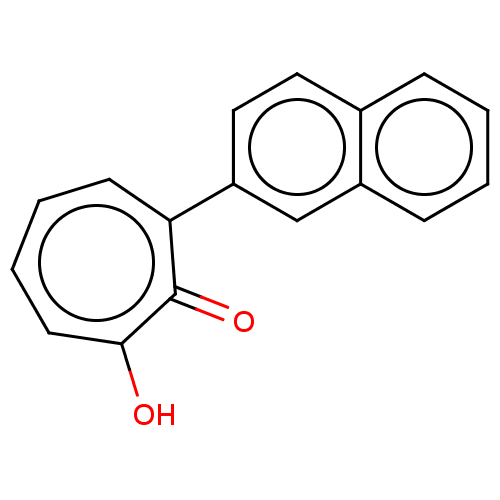 (MO-OH-NAP | US9790158, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347456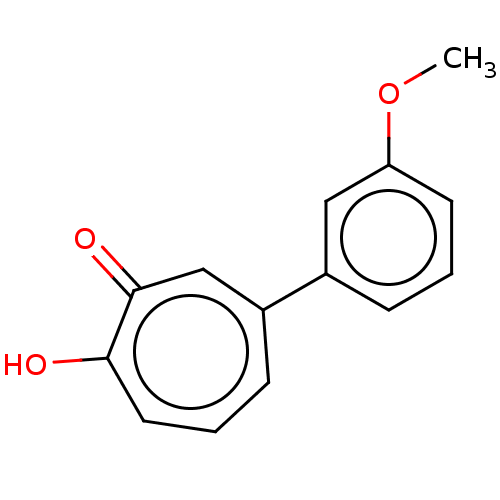 (US9790158, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50353233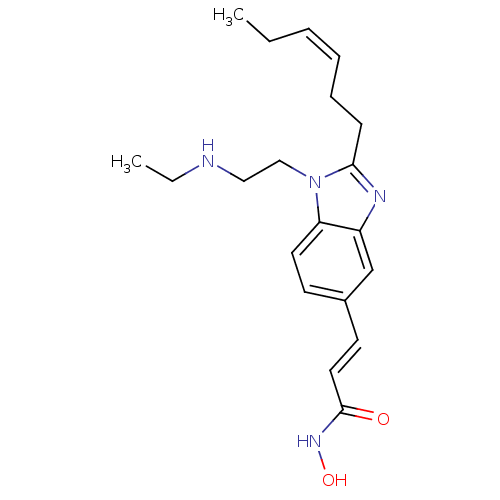 (CHEMBL1830536) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347452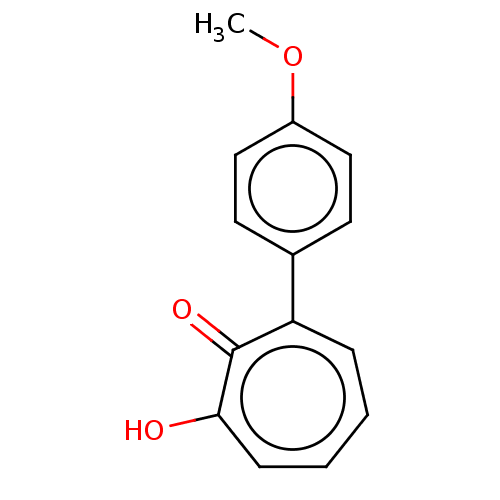 (MO-OH-SM | US9790158, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM79632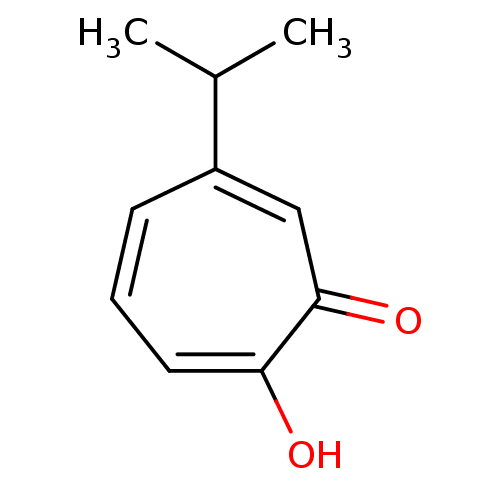 (2-hydroxy-6-isopropyl-cyclohepta-2,4,6-trien-1-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 3.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50353234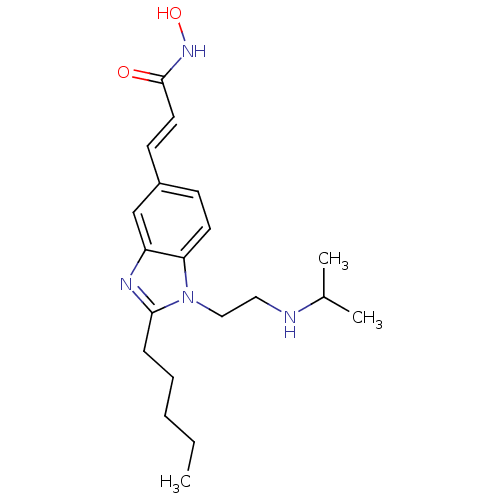 (CHEMBL1830537) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50353232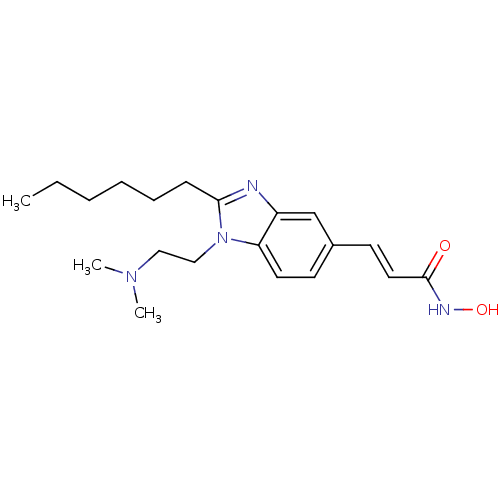 (CHEMBL1830424) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50319209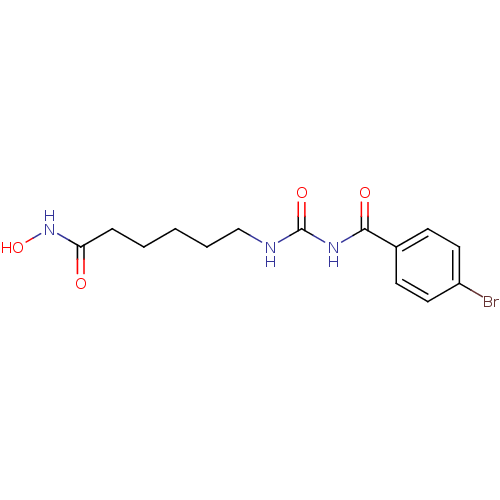 (6-[3-(4-Bromo-benzoyl)-ureido]-hexanoic acid hydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of HDAC4 | Bioorg Med Chem Lett 20: 3314-21 (2010) Article DOI: 10.1016/j.bmcl.2010.04.041 BindingDB Entry DOI: 10.7270/Q2GT5NCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50353230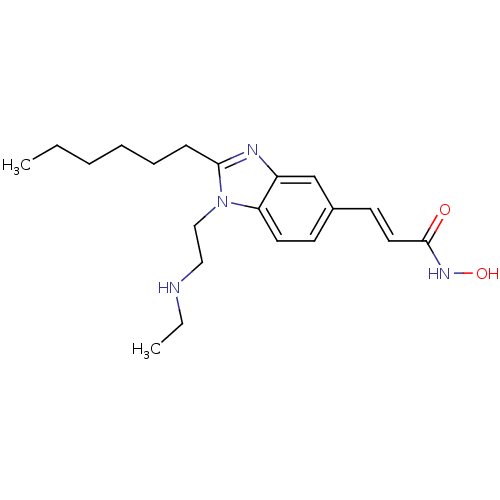 (CHEMBL1830420) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50353231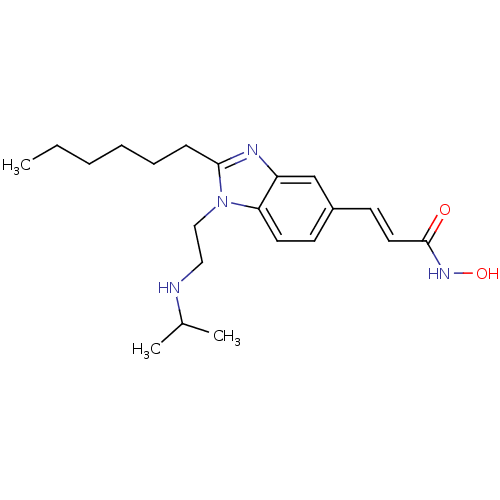 (CHEMBL1830422) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347461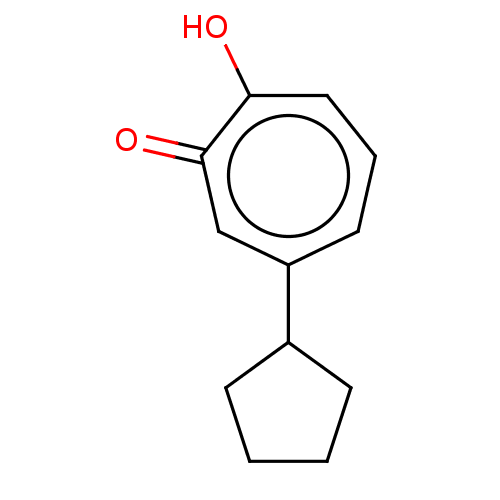 (US9790158, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50105327 (JNJ-26481585 | Quisinostat) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC4 using fluorogenic HDAC substrate class 2a after 30 mins by fluorimetrc method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50353229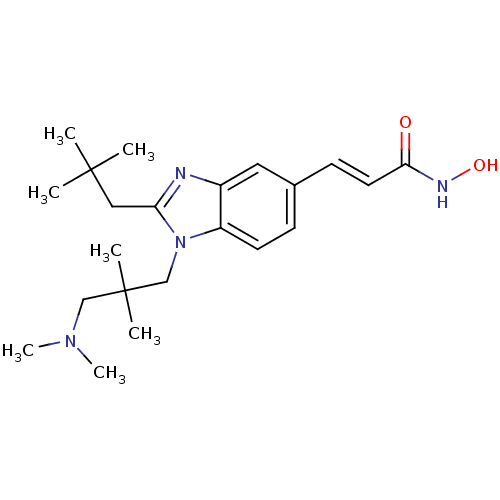 (CHEMBL1830397) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50248476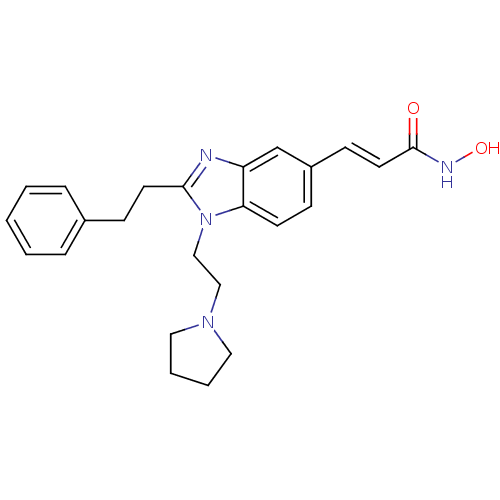 (CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50248522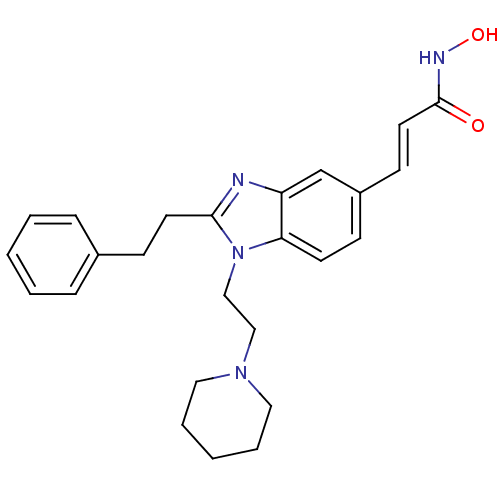 (CHEMBL489332 | N-hydroxy-3-(2-phenethyl-1-(2-(pipe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50248476 (CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC4 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50248570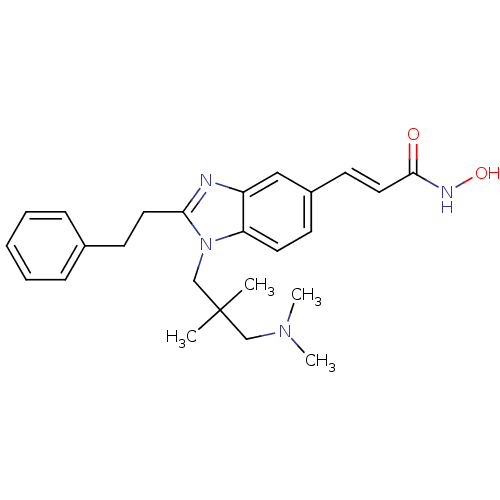 (3-(1-(3-(dimethylamino)-2,2-dimethylpropyl)-2-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50353228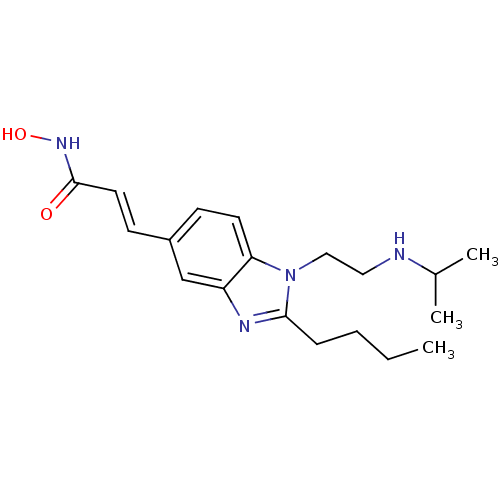 (CHEMBL1830396) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM25150 ((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of HDAC4 | Bioorg Med Chem Lett 20: 3314-21 (2010) Article DOI: 10.1016/j.bmcl.2010.04.041 BindingDB Entry DOI: 10.7270/Q2GT5NCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50105330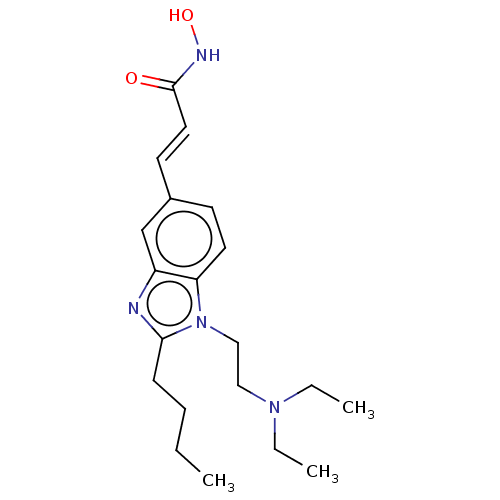 (CHEMBL1851943) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human HDAC4 | Bioorg Med Chem 23: 5151-5 (2015) Article DOI: 10.1016/j.bmc.2014.12.066 BindingDB Entry DOI: 10.7270/Q2B859V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50353227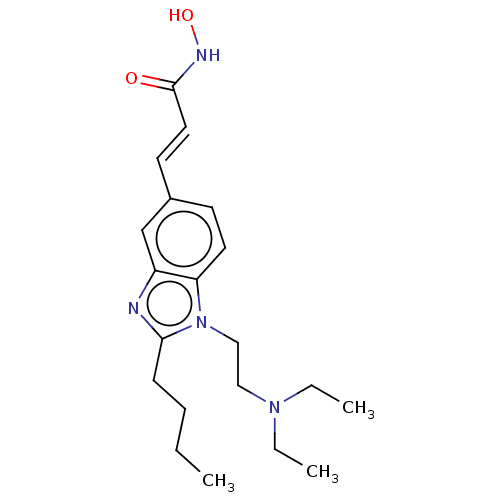 (CHEMBL3215861) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50319235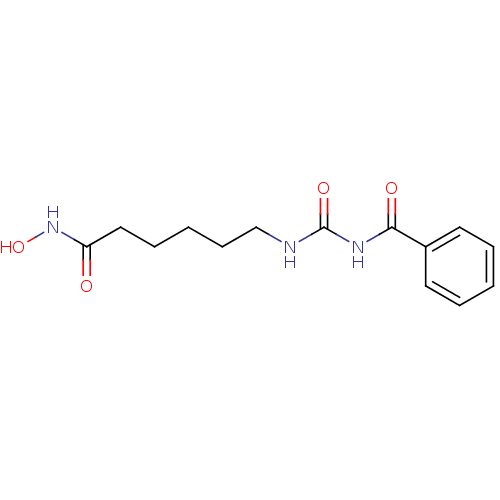 (6-(3-Benzoyl-ureido)-hexanoic acid hydroxyamide | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of HDAC4 | Bioorg Med Chem Lett 20: 3314-21 (2010) Article DOI: 10.1016/j.bmcl.2010.04.041 BindingDB Entry DOI: 10.7270/Q2GT5NCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347454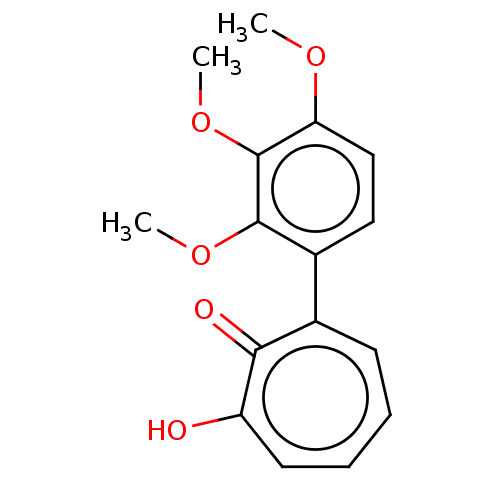 (MO-OH-TM | US9790158, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM348883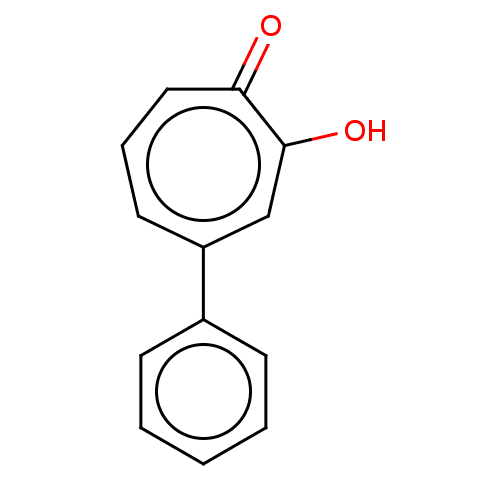 (US9790158, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 72.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50517218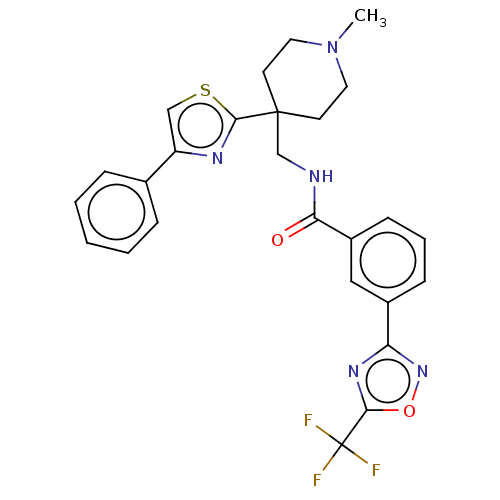 (CHEMBL4525406) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t D£sseldorf Curated by ChEMBL | Assay Description Inhibition of HDAC4 (unknown origin) | Bioorg Med Chem 27: (2019) Article DOI: 10.1016/j.bmc.2019.115087 BindingDB Entry DOI: 10.7270/Q2VM4GMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50446481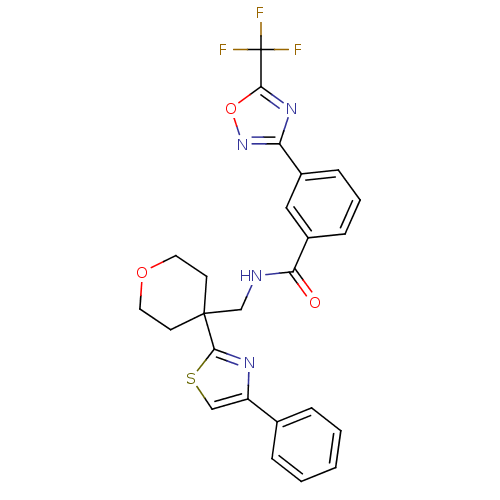 (CHEMBL3110004 | US10011611, TMP269 | US10722597, C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmacy, Faculty of Chemistry and Pharmacy , University of Regensburg , 93040 Regensburg , Germany. Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal GST-tagged HDAC4 expressed in baculovirus-infected insect cells using Boc-Lys(TFA)-AMC as substrate by flu... | J Med Chem 61: 3454-3477 (2018) Article DOI: 10.1021/acs.jmedchem.7b01593 BindingDB Entry DOI: 10.7270/Q24B33S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347460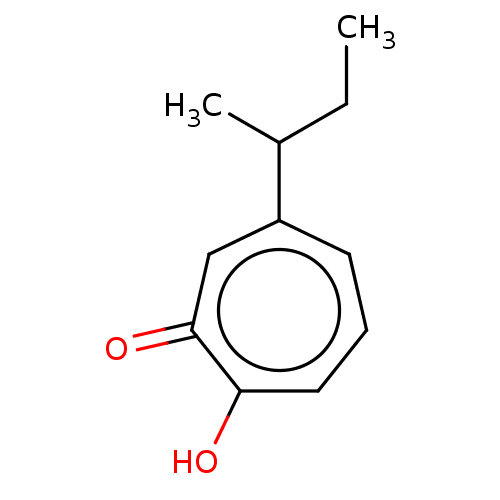 (US9790158, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 94.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50446481 (CHEMBL3110004 | US10011611, TMP269 | US10722597, C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human HDAC4 using (Boc-Lys(trifluoroacetyl)-AMC) as substrate measured after 2 hrs by Cheng-Prusoff analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00830 BindingDB Entry DOI: 10.7270/Q2F76H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC4 using fluorogenic HDAC substrate class 2a after 30 mins by fluorimetrc method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50503690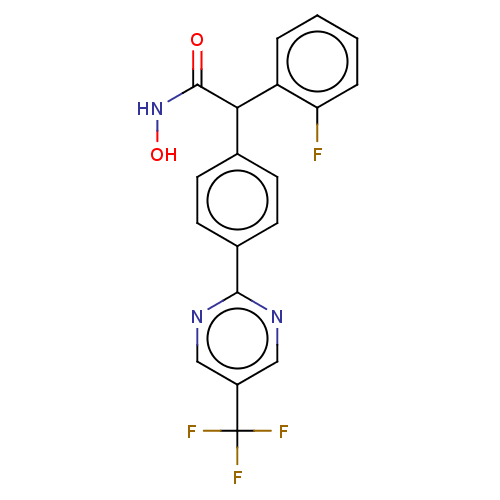 (CHEMBL4456445) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t D£sseldorf Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115108 BindingDB Entry DOI: 10.7270/Q2NV9NSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347453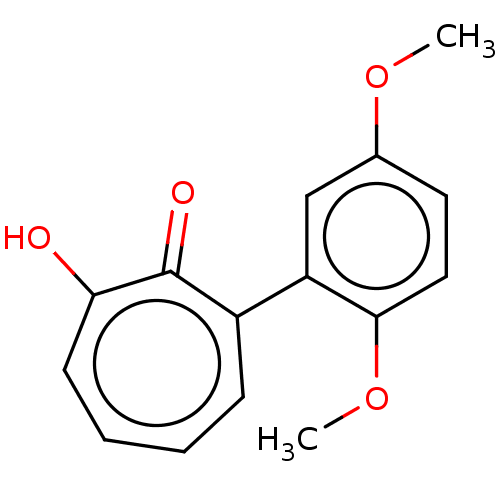 (MO-OH-DM | US9790158, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM25150 ((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human HDAC4 | Bioorg Med Chem 23: 5151-5 (2015) Article DOI: 10.1016/j.bmc.2014.12.066 BindingDB Entry DOI: 10.7270/Q2B859V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM348884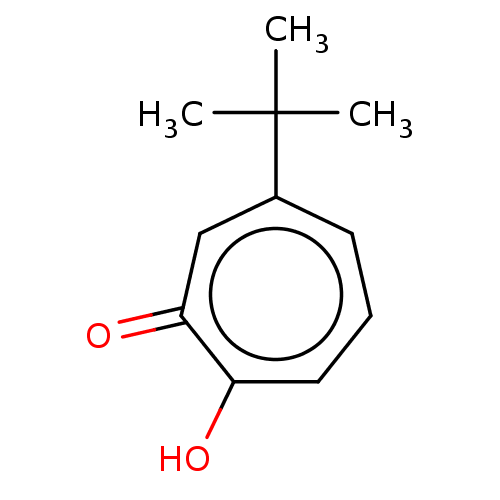 (US9790158, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 489 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347457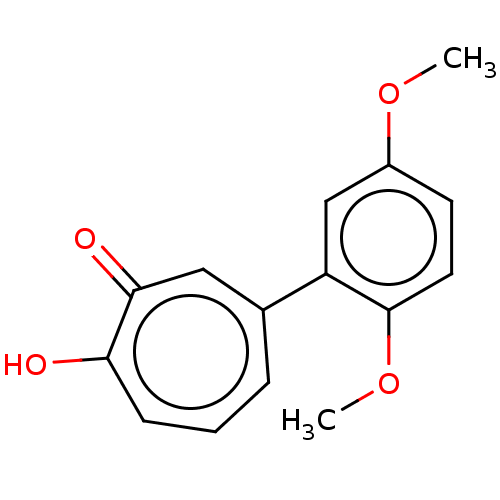 (US9790158, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 541 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human HDAC4 | Bioorg Med Chem 23: 5151-5 (2015) Article DOI: 10.1016/j.bmc.2014.12.066 BindingDB Entry DOI: 10.7270/Q2B859V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-University D£sseldorf Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged/N-terminal GST-tagged recombinant human HDAC4 (627 to 1084 residues) expressed in Baculovirus infected insect cel... | Bioorg Med Chem 27: (2019) Article DOI: 10.1016/j.bmc.2019.07.052 BindingDB Entry DOI: 10.7270/Q2VX0KZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM348884 (US9790158, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 806 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC4 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347461 (US9790158, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC4 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50105329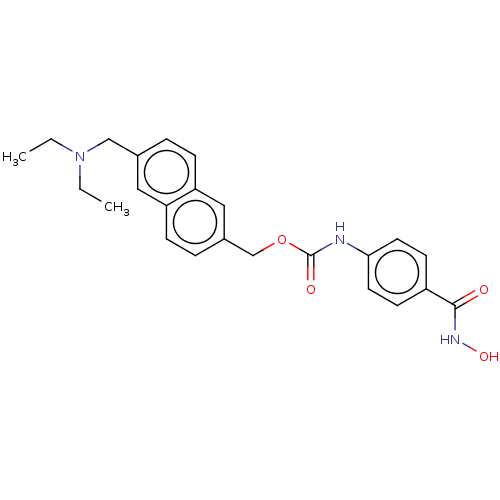 (CHEMBL1213492) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human HDAC4 | Bioorg Med Chem 23: 5151-5 (2015) Article DOI: 10.1016/j.bmc.2014.12.066 BindingDB Entry DOI: 10.7270/Q2B859V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of HDAC4 (unknown origin) | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 BindingDB Entry DOI: 10.7270/Q2HT2STK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human HDAC4 | Bioorg Med Chem 23: 5151-5 (2015) Article DOI: 10.1016/j.bmc.2014.12.066 BindingDB Entry DOI: 10.7270/Q2B859V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50446481 (CHEMBL3110004 | US10011611, TMP269 | US10722597, C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-University D£sseldorf Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged/N-terminal GST-tagged recombinant human HDAC4 (627 to 1084 residues) expressed in Baculovirus infected insect cel... | Bioorg Med Chem 27: (2019) Article DOI: 10.1016/j.bmc.2019.07.052 BindingDB Entry DOI: 10.7270/Q2VX0KZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50252395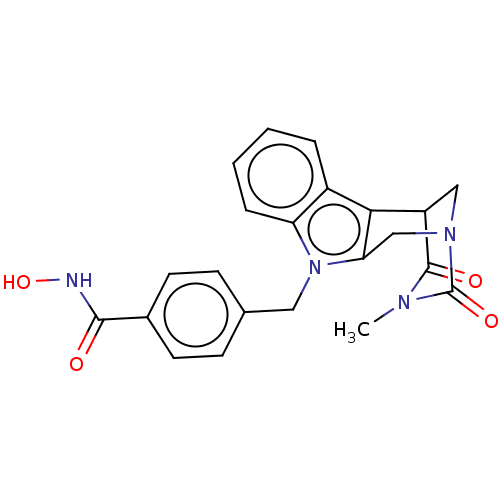 (CHEMBL4087616) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmacy, Faculty of Chemistry and Pharmacy , University of Regensburg , 93040 Regensburg , Germany. Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal GST-tagged HDAC4 expressed in baculovirus-infected insect cells using Boc-Lys(TFA)-AMC as substrate by flu... | J Med Chem 61: 3454-3477 (2018) Article DOI: 10.1021/acs.jmedchem.7b01593 BindingDB Entry DOI: 10.7270/Q24B33S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50123957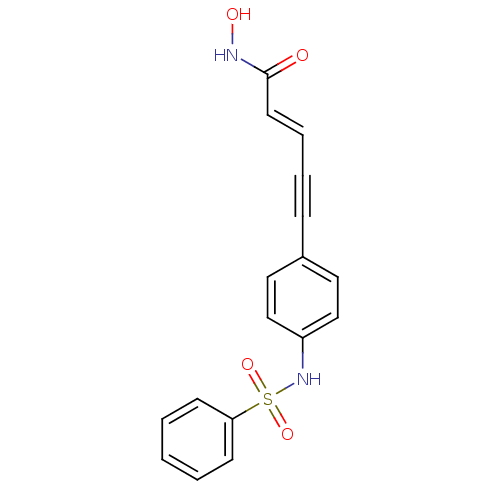 ((E)-5-(3-Benzenesulfonylamino-phenyl)-pent-2-en-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human HDAC4 | Bioorg Med Chem 23: 5151-5 (2015) Article DOI: 10.1016/j.bmc.2014.12.066 BindingDB Entry DOI: 10.7270/Q2B859V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19428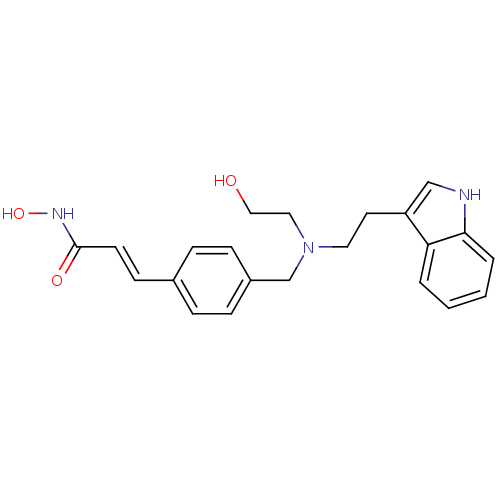 ((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human HDAC4 | Bioorg Med Chem 23: 5151-5 (2015) Article DOI: 10.1016/j.bmc.2014.12.066 BindingDB Entry DOI: 10.7270/Q2B859V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 112 total ) | Next | Last >> |