

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human HDAC9 using pan-HDAC substrate incubated for 3 hrs by fluorescence method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00830 BindingDB Entry DOI: 10.7270/Q2F76H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM162865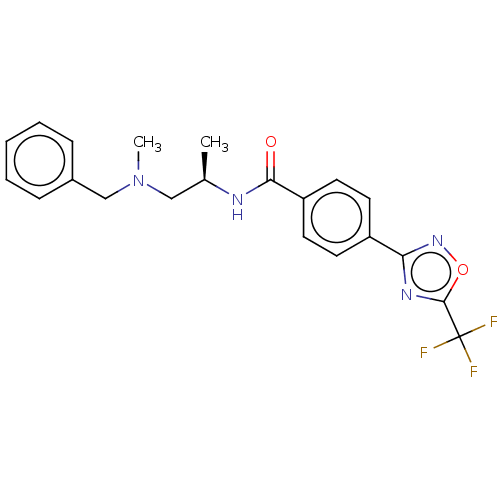 (US9056843, 156) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114011 BindingDB Entry DOI: 10.7270/Q2WS8Z98 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50268097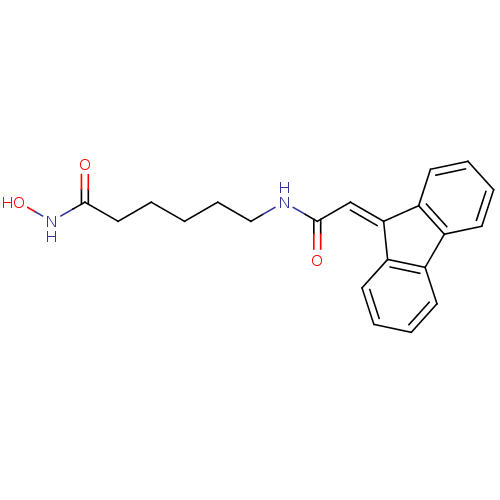 (6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC9 | Eur J Med Chem 44: 1067-85 (2009) Article DOI: 10.1016/j.ejmech.2008.06.020 BindingDB Entry DOI: 10.7270/Q2BG2NTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50563276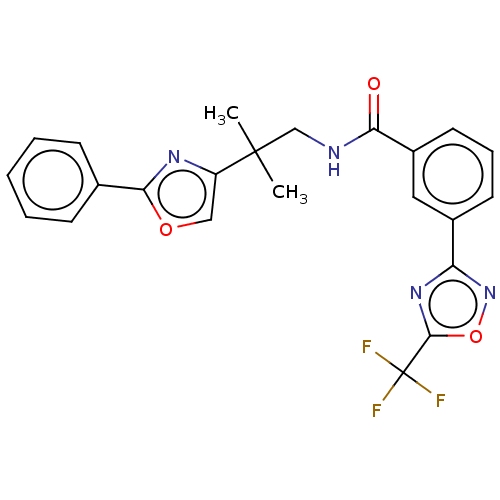 (CHEMBL4751615) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114011 BindingDB Entry DOI: 10.7270/Q2WS8Z98 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50268063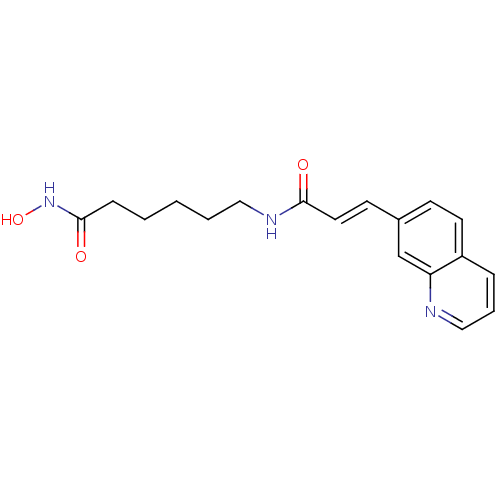 ((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC9 | Eur J Med Chem 44: 1067-85 (2009) Article DOI: 10.1016/j.ejmech.2008.06.020 BindingDB Entry DOI: 10.7270/Q2BG2NTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50268121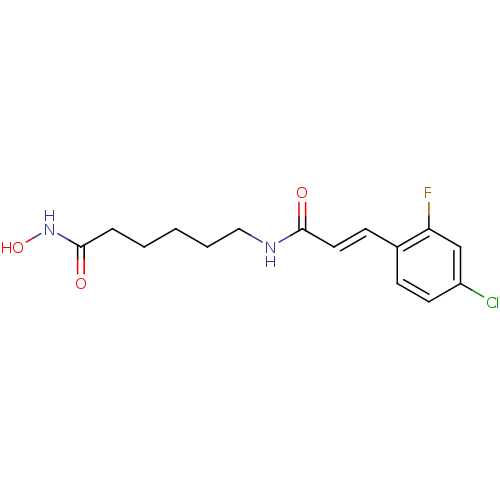 ((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC9 | Eur J Med Chem 44: 1067-85 (2009) Article DOI: 10.1016/j.ejmech.2008.06.020 BindingDB Entry DOI: 10.7270/Q2BG2NTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50268042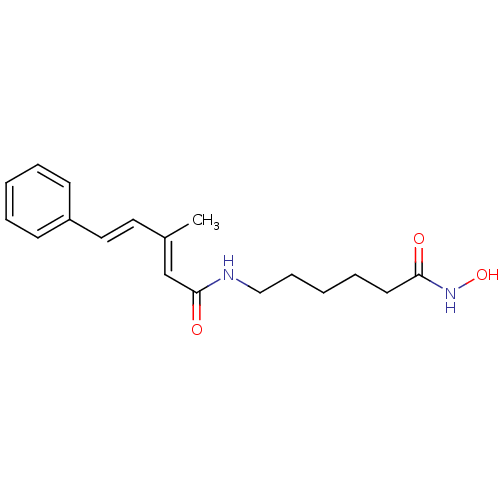 ((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC9 | Eur J Med Chem 44: 1067-85 (2009) Article DOI: 10.1016/j.ejmech.2008.06.020 BindingDB Entry DOI: 10.7270/Q2BG2NTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50044636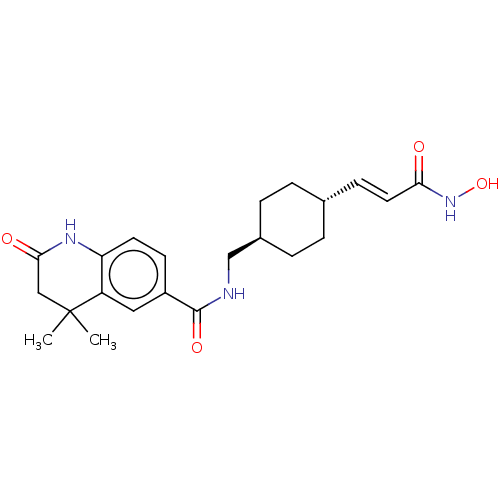 (CHEMBL3309297) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Pharmaceutical Chemicals Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human HDAC9 pre-incubated for 30 mins before substrate addition and measured after 30 mins by HDAC-Glo I/II assay | Bioorg Med Chem 22: 3720-31 (2014) Article DOI: 10.1016/j.bmc.2014.05.001 BindingDB Entry DOI: 10.7270/Q24T6M0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ocean University of China Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC9 (unknown origin) | J Med Chem 63: 5501-5525 (2020) Article DOI: 10.1021/acs.jmedchem.0c00442 BindingDB Entry DOI: 10.7270/Q23X89Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant HDAC9 using Boc-Lys(triflouroacetyI)-AMC substrate incubated for 2 hrs by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01472 BindingDB Entry DOI: 10.7270/Q28W3J6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro antagonistic activity against kinin-induced rabbit jugular vein contraction. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50105327 (JNJ-26481585 | Quisinostat) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length human HDAC9 expressed in SF9 baculovirus using FAM- labelled acetylated peptide as substrate measured by Electr... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112411 BindingDB Entry DOI: 10.7270/Q2JH3QVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50105327 (JNJ-26481585 | Quisinostat) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC9 (604-1066 residues) expressed in baculovirus infected Sf9 cells using FAM-RHKK-TFAc as substrate incubated for ... | ACS Med Chem Lett 11: 56-64 (2020) Article DOI: 10.1021/acsmedchemlett.9b00471 BindingDB Entry DOI: 10.7270/Q26976Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM19428 ((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human HDAC9 by fluorimetric assay | J Med Chem 53: 8387-8399 (2010) Article DOI: 10.1021/jm101092u BindingDB Entry DOI: 10.7270/Q28G8KZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM162846 (US9056843, 137) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC9 (unknown origin) using Boc Lys(TFA) as substrate by fluorogenic assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00532 BindingDB Entry DOI: 10.7270/Q2474FHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50446481 (CHEMBL3110004 | US10011611, TMP269 | US10722597, C...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC9 (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113790 BindingDB Entry DOI: 10.7270/Q2611453 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50581678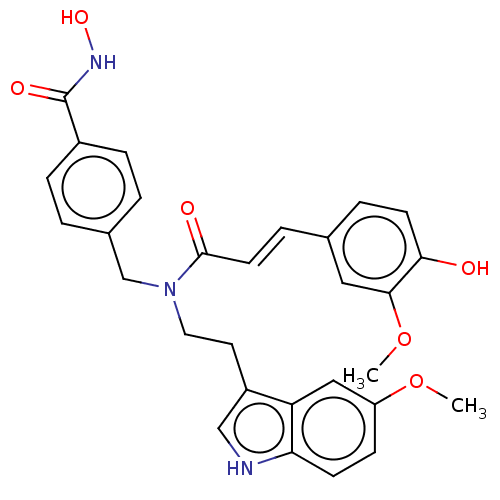 (CHEMBL5072502) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant HDAC9 (unknown origin) using Boc-Lys(trifluoroacetyl)-AMC as substrate pre-incubated for 1 hr followed by substrate additio... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01940 BindingDB Entry DOI: 10.7270/Q2WH2TVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272075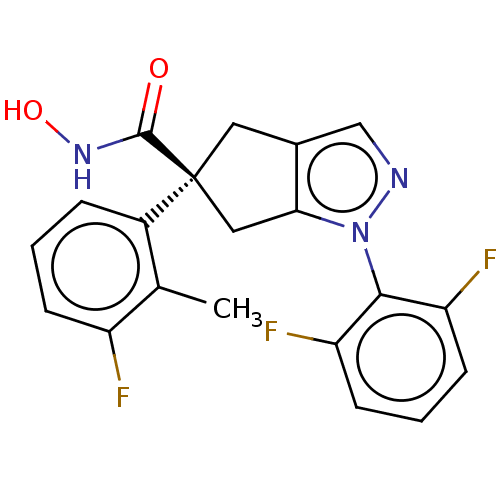 ((S)-1-(2,6-Difluorophenyl)-5-(3-fluoro-2- methylph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272075 ((S)-1-(2,6-Difluorophenyl)-5-(3-fluoro-2- methylph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM162846 (US9056843, 137) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01149 BindingDB Entry DOI: 10.7270/Q2CC14PT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272071 ((S)-1-(2-Chlorophenyl)-5-(3-fluoro-2-methylphenyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272078 ((S)-1-(2-Chloro-6-fluorophenyl)-5-(3-fluoro-2- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272071 ((S)-1-(2-Chlorophenyl)-5-(3-fluoro-2-methylphenyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272078 ((S)-1-(2-Chloro-6-fluorophenyl)-5-(3-fluoro-2- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50563276 (CHEMBL4751615) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human HDAC9 using Ac-LGK(TFA)-AMC as substrate preincubated for 15 mins followed by substrate addition and measured after 6... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01967 BindingDB Entry DOI: 10.7270/Q2DV1PMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272067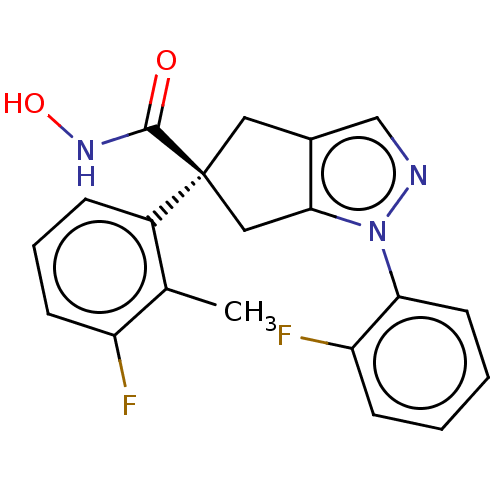 ((S)-5-(3-Fluoro-2-methylphenyl)-l-(2-fluorophenyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272074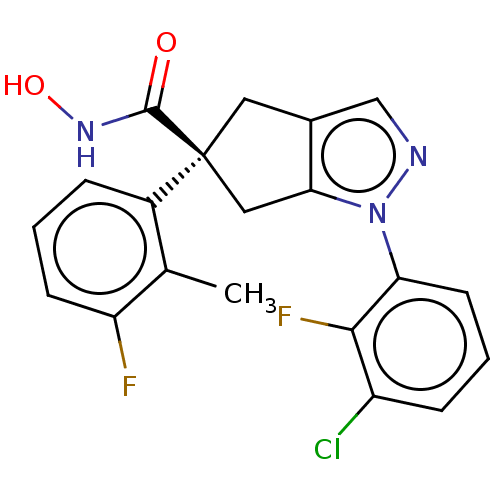 ((S)-1-(3-Chloro-2-fluorophenyl)-5-(3-fluoro-2- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272074 ((S)-1-(3-Chloro-2-fluorophenyl)-5-(3-fluoro-2- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272067 ((S)-5-(3-Fluoro-2-methylphenyl)-l-(2-fluorophenyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272079 ((S)-5-(3-Fluoro-2-methylphenyl)-1-(2-fluoro-6- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272079 ((S)-5-(3-Fluoro-2-methylphenyl)-1-(2-fluoro-6- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50446481 (CHEMBL3110004 | US10011611, TMP269 | US10722597, C...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 19.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC9 using fluorogenic substrate by fluorescence assay | Eur J Med Chem 125: 1268-1278 (2017) Article DOI: 10.1016/j.ejmech.2016.11.033 BindingDB Entry DOI: 10.7270/Q2XD145D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50563273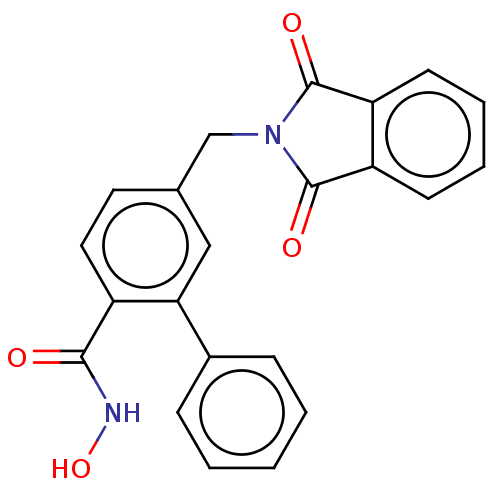 (CHEMBL4748500) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human HDAC9 using Ac-LGK(TFA)-AMC as substrate preincubated for 15 mins followed by substrate addition and measured after 6... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01967 BindingDB Entry DOI: 10.7270/Q2DV1PMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC9 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins | J Med Chem 54: 4350-64 (2011) Article DOI: 10.1021/jm2001025 BindingDB Entry DOI: 10.7270/Q2RX9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of human HDAC9 using ArgHisLysLys(Ac) fluorogenic peptide as a substrate by fluorimetric assay | J Med Chem 54: 2165-82 (2011) Article DOI: 10.1021/jm101373a BindingDB Entry DOI: 10.7270/Q2CR5TNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HDAC9 by in vitro deacetylation assay | Nat Chem Biol 6: 25-33 (2009) Article DOI: 10.1038/nchembio.275 BindingDB Entry DOI: 10.7270/Q2FF3SKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272076 ((S)-1-(2,5-Dimethylphenyl)-5-(3-fluoro-2- methylph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272076 ((S)-1-(2,5-Dimethylphenyl)-5-(3-fluoro-2- methylph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272066 ((S)-5-(3-Fluoro-2-methylphenyl)-N-hydroxy-l-(o- to...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272066 ((S)-5-(3-Fluoro-2-methylphenyl)-N-hydroxy-l-(o- to...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50446481 (CHEMBL3110004 | US10011611, TMP269 | US10722597, C...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 23.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC9 using trifluoroacetyl lysine as substrate | Eur J Med Chem 116: 126-135 (2016) Article DOI: 10.1016/j.ejmech.2016.03.046 BindingDB Entry DOI: 10.7270/Q2057HTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50044641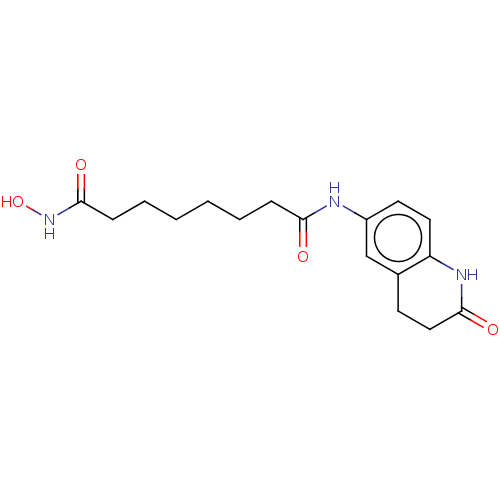 (CHEMBL3311465) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Pharmaceutical Chemicals Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human HDAC9 pre-incubated for 30 mins before substrate addition and measured after 30 mins by HDAC-Glo I/II assay | Bioorg Med Chem 22: 3720-31 (2014) Article DOI: 10.1016/j.bmc.2014.05.001 BindingDB Entry DOI: 10.7270/Q24T6M0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272077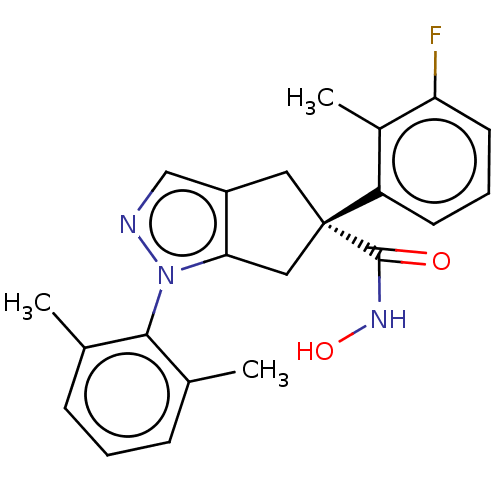 ((S)-1-(2,6-Dimethylphenyl)-5-(3-fluoro-2- methylph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272158 ((R)-1-Benzyl-4-(3-fluoro-2-methylphenyl)-N-hydroxy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272077 ((S)-1-(2,6-Dimethylphenyl)-5-(3-fluoro-2- methylph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272158 ((R)-1-Benzyl-4-(3-fluoro-2-methylphenyl)-N-hydroxy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272065 ((S)-5-(3-Fluoro-2-methylphenyl)-N-hydroxy-1-phenyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272088 ((S)-5-(3-Fluoro-2-methylphenyl)-N-hydroxy-1-(m- to...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50304782 (CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development Curated by ChEMBL | Assay Description Inhibition of human HDAC9 | Bioorg Med Chem Lett 20: 294-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.118 BindingDB Entry DOI: 10.7270/Q2DB81ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272065 ((S)-5-(3-Fluoro-2-methylphenyl)-N-hydroxy-1-phenyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 742 total ) | Next | Last >> |