

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50259948 (CHEMBL4096462) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Saclay Curated by ChEMBL | Assay Description Inhibition of human MMP10 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate by spectrophotometric analysis | J Med Chem 60: 403-414 (2017) Article DOI: 10.1021/acs.jmedchem.6b01420 BindingDB Entry DOI: 10.7270/Q24X5B7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120222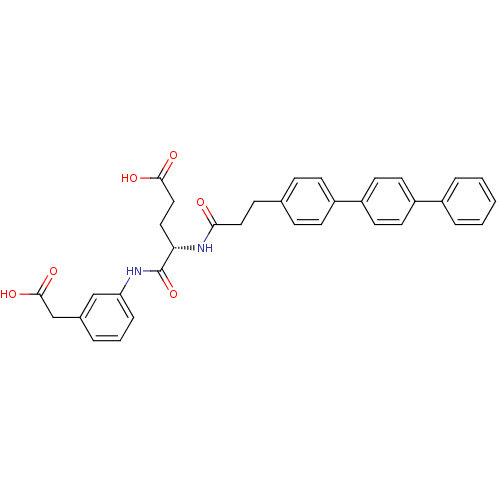 (US8691753, 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM92441 (RXP470, 1 | RXP470, Compound 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -11.0 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
Commissariat á l'Energie Atomique | Assay Description Enzyme assay using human matrix metalloproteases or ADAMTS. | J Biol Chem 287: 26647-56 (2012) Article DOI: 10.1074/jbc.M112.380782 BindingDB Entry DOI: 10.7270/Q2H993SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM92441 (RXP470, 1 | RXP470, Compound 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.30 | -11.0 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
Commissariat à l'Energie Atomique | Assay Description Enzyme assay using matrix metalloproteinases. | J Biol Chem 285: 35900-9 (2010) Article DOI: 10.1074/jbc.M110.139634 BindingDB Entry DOI: 10.7270/Q25X27HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120215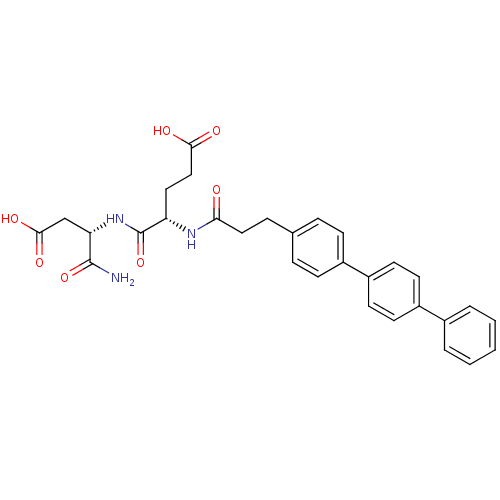 (US8691753, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120160 (US8691753, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120244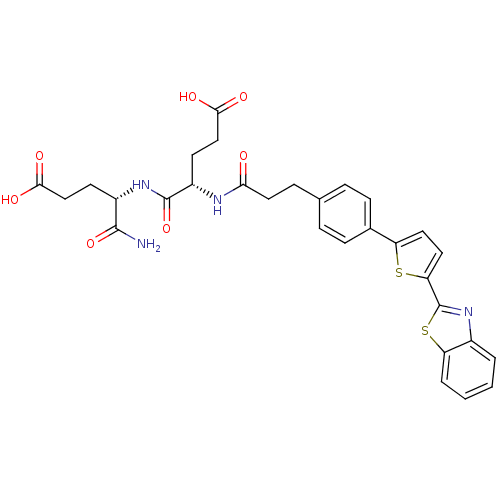 (US8691753, 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 38.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120157 (US8691753, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120155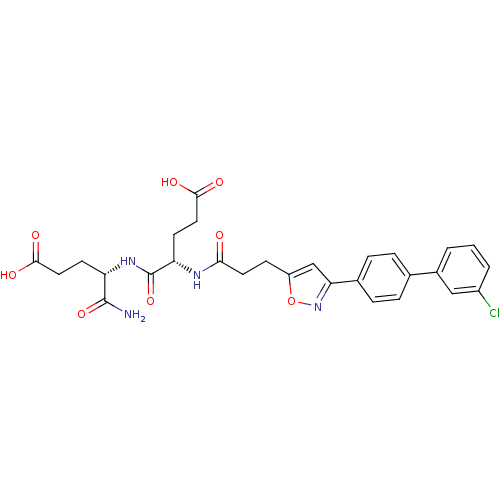 (US8691753, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120158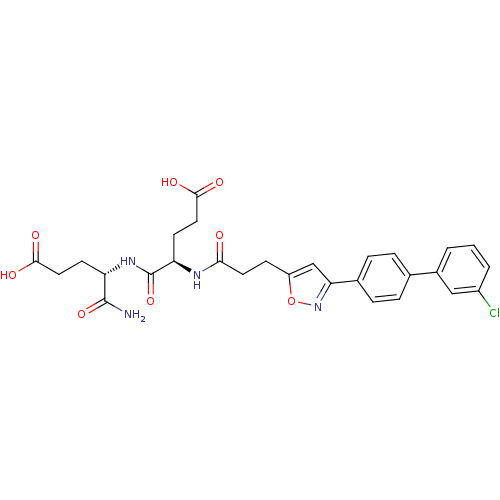 (US8691753, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01855 BindingDB Entry DOI: 10.7270/Q2GB281X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120164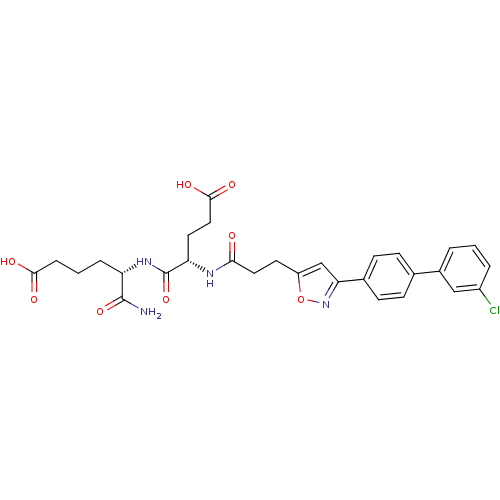 (US8691753, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM92448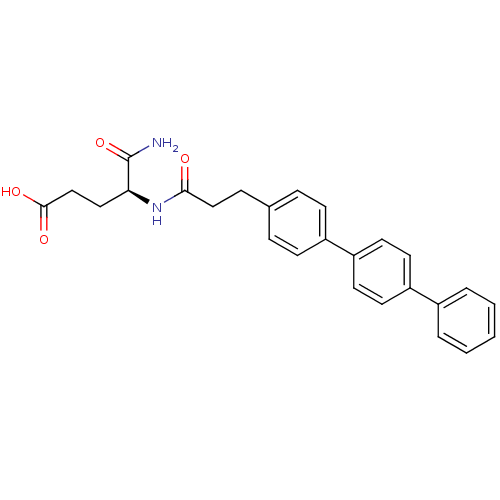 (Inhibitor, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 54 | -9.91 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
Commissariat á l'Energie Atomique | Assay Description Enzyme assay using human matrix metalloproteases or ADAMTS. | J Biol Chem 287: 26647-56 (2012) Article DOI: 10.1074/jbc.M112.380782 BindingDB Entry DOI: 10.7270/Q2H993SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120219 (US8691753, 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120212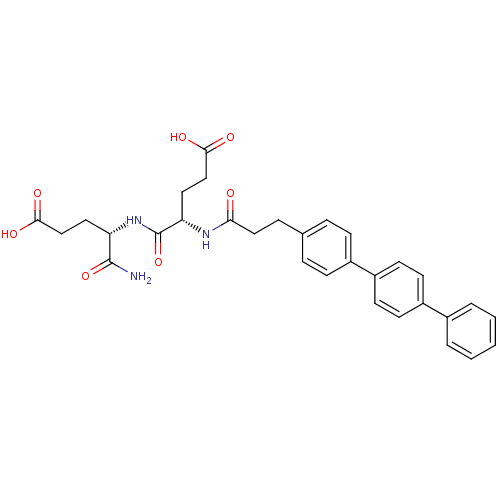 (US8691753, 61 | US8691753, 72) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120258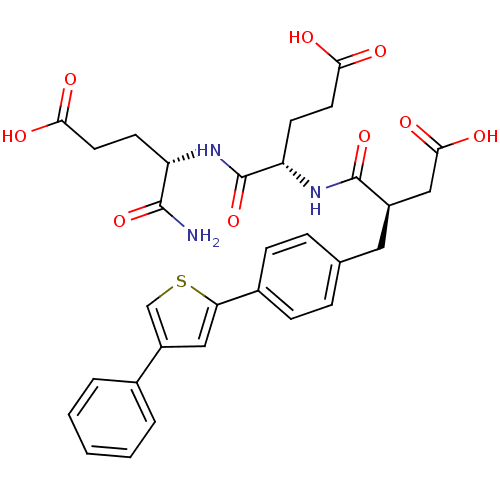 (US8691753, 95 bis) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120162 (US8691753, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120191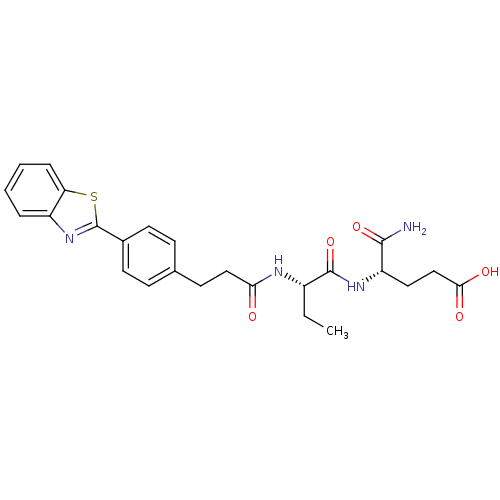 (US8691753, 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120213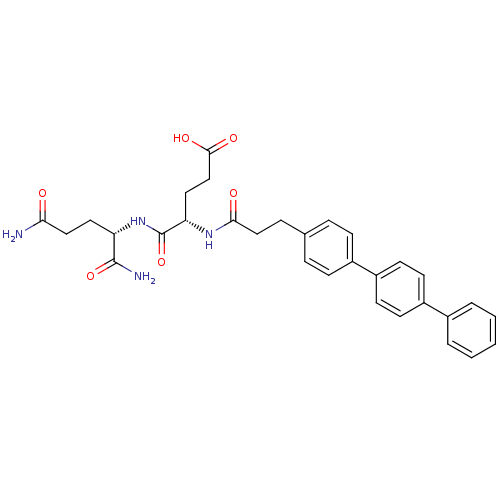 (US8691753, 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120159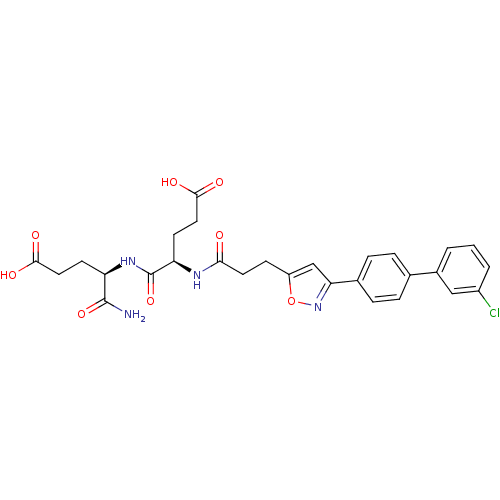 (US8691753, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120168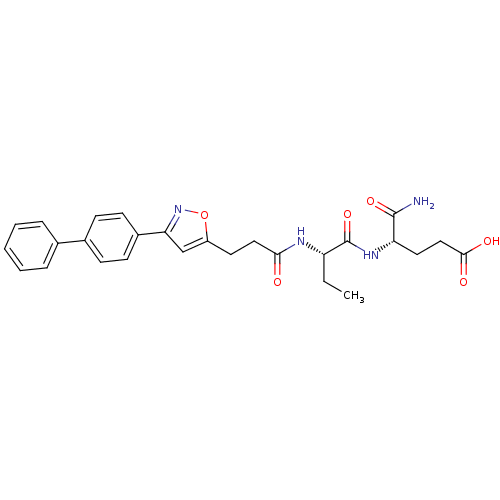 (US8691753, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120216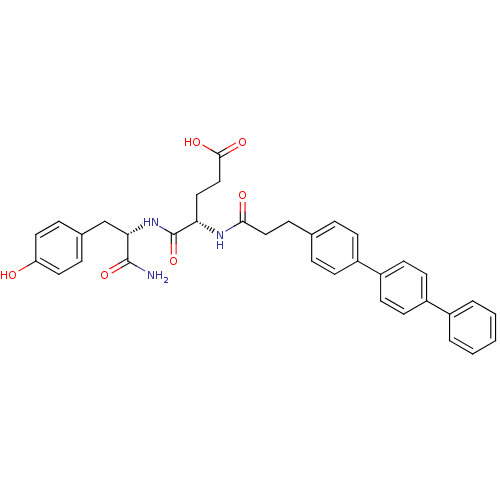 (US8691753, 65) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120156 (US8691753, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120217 (US8691753, 66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120241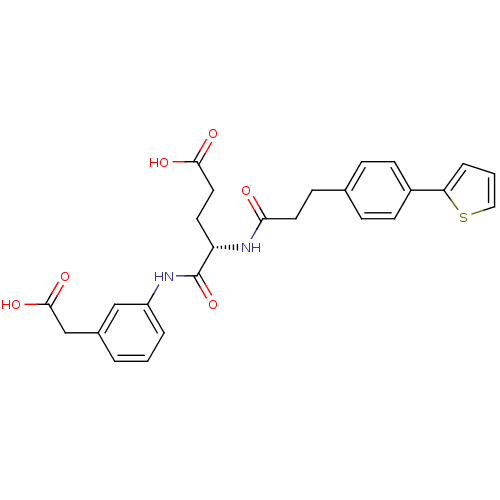 (US8691753, 90) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50265078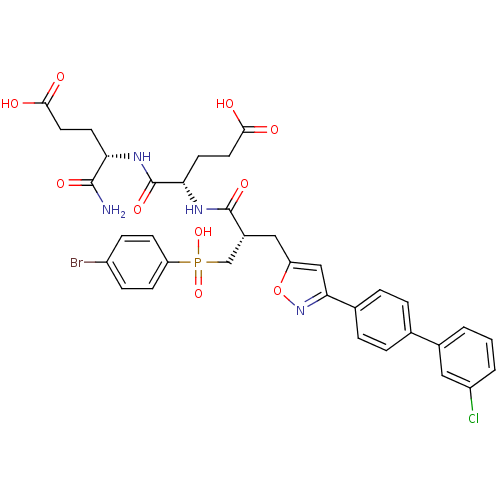 ((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Saclay Curated by ChEMBL | Assay Description Inhibition of human MMP10 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate by spectrophotometric analysis | J Med Chem 60: 403-414 (2017) Article DOI: 10.1021/acs.jmedchem.6b01420 BindingDB Entry DOI: 10.7270/Q24X5B7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120243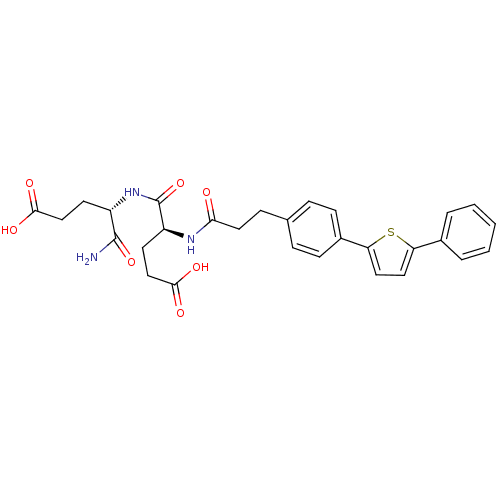 (US8691753, 92) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120218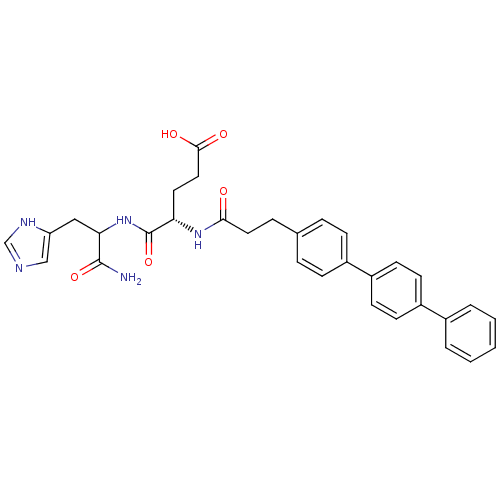 (US8691753, 67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50182403 ((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of MMP10 | Bioorg Med Chem Lett 16: 2357-63 (2006) Article DOI: 10.1016/j.bmcl.2006.01.055 BindingDB Entry DOI: 10.7270/Q2WD405K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120170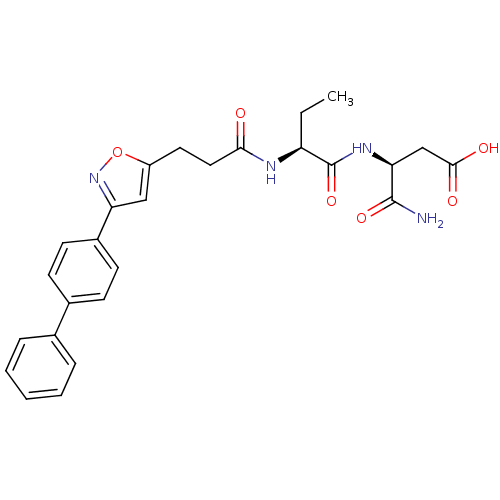 (US8691753, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50229322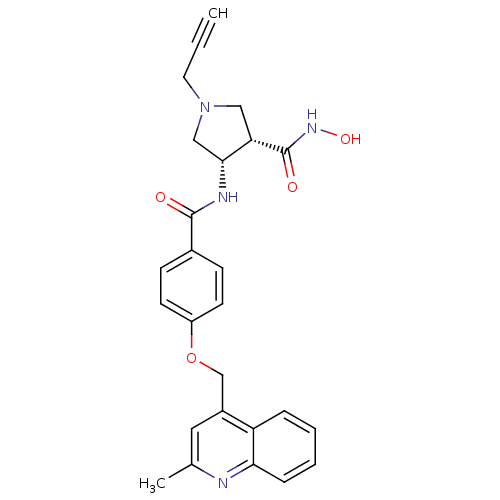 ((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP10 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120173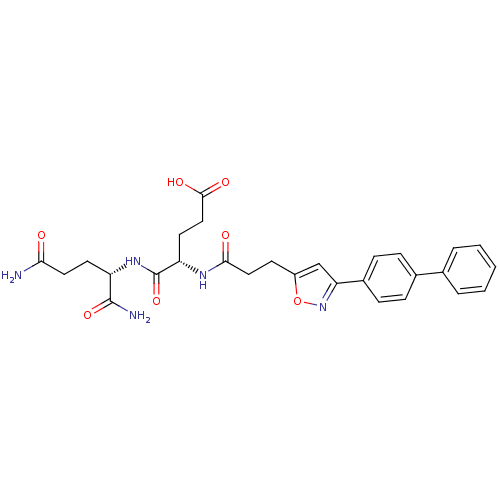 (US8691753, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120175 (US8691753, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 276 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM92446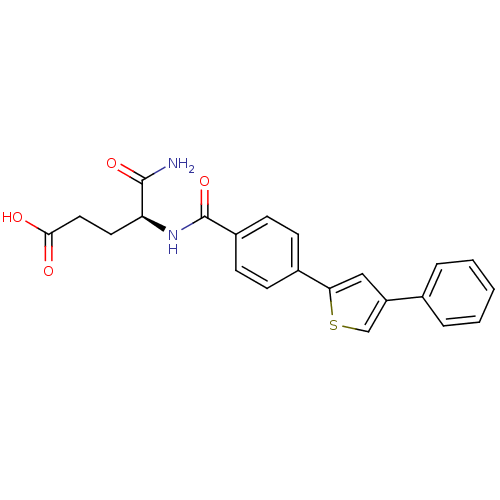 (Inhibitor, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | Article PubMed | 299 | -8.89 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
Commissariat á l'Energie Atomique | Assay Description Enzyme assay using human matrix metalloproteases or ADAMTS. | J Biol Chem 287: 26647-56 (2012) Article DOI: 10.1074/jbc.M112.380782 BindingDB Entry DOI: 10.7270/Q2H993SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120256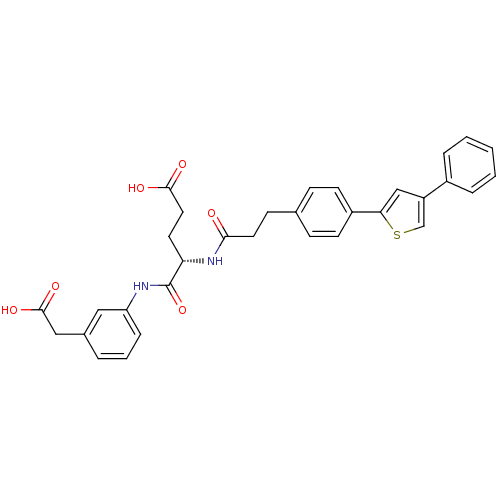 (US8691753, 106) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 318 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50260826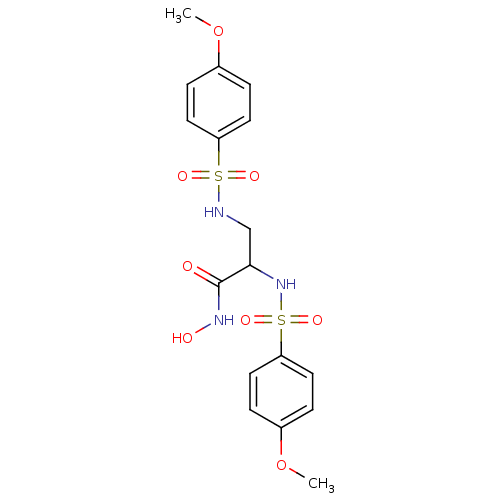 (CHEMBL497761 | N-hydroxy-2,3-bis(4-methoxyphenylsu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 335 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP10 | Bioorg Med Chem Lett 18: 3333-7 (2008) Article DOI: 10.1016/j.bmcl.2008.04.035 BindingDB Entry DOI: 10.7270/Q2SX6D1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM92449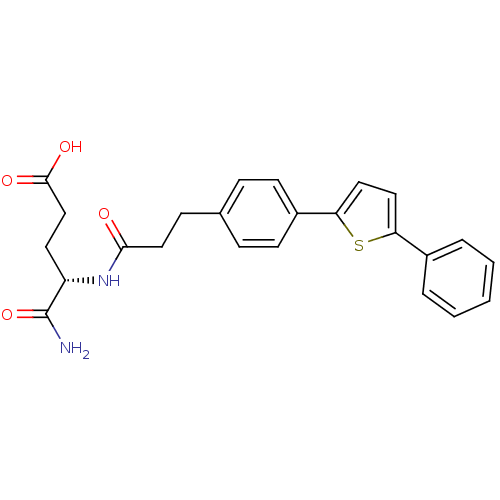 (Inhibitor, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 352 | -8.80 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
Commissariat á l'Energie Atomique | Assay Description Enzyme assay using human matrix metalloproteases or ADAMTS. | J Biol Chem 287: 26647-56 (2012) Article DOI: 10.1074/jbc.M112.380782 BindingDB Entry DOI: 10.7270/Q2H993SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120242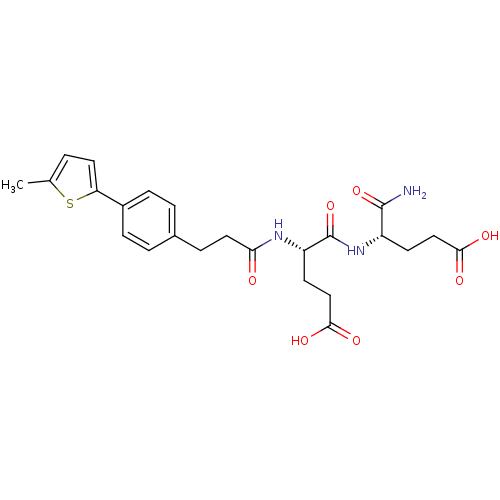 (US8691753, 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 353 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50260802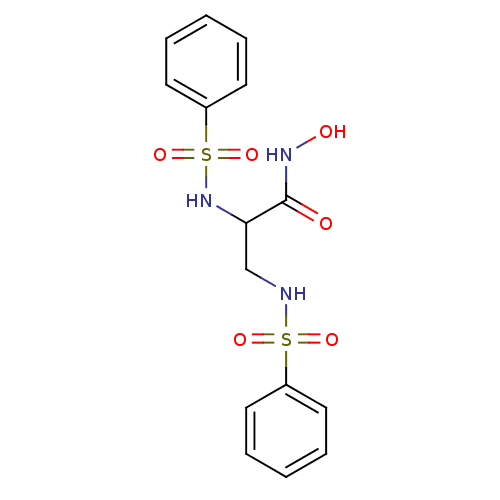 (CHEMBL496717 | N-hydroxy-2,3-bis(phenylsulfonamido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 356 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP10 | Bioorg Med Chem Lett 18: 3333-7 (2008) Article DOI: 10.1016/j.bmcl.2008.04.035 BindingDB Entry DOI: 10.7270/Q2SX6D1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120231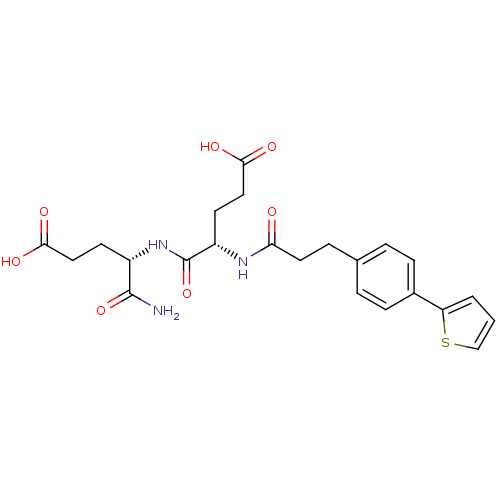 (US8691753, 80) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 373 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120220 (US8691753, 69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 382 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM92447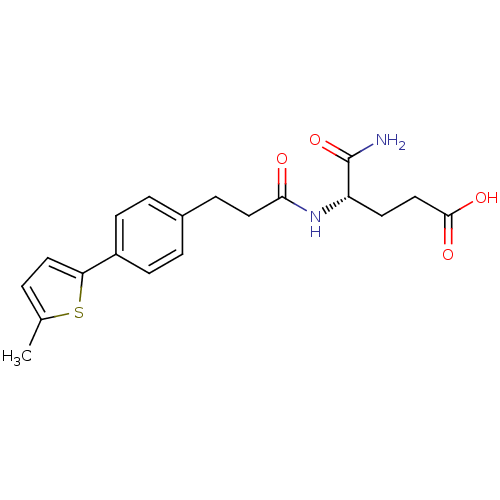 (Inhibitor, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 390 | -8.74 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
Commissariat á l'Energie Atomique | Assay Description Enzyme assay using human matrix metalloproteases or ADAMTS. | J Biol Chem 287: 26647-56 (2012) Article DOI: 10.1074/jbc.M112.380782 BindingDB Entry DOI: 10.7270/Q2H993SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120221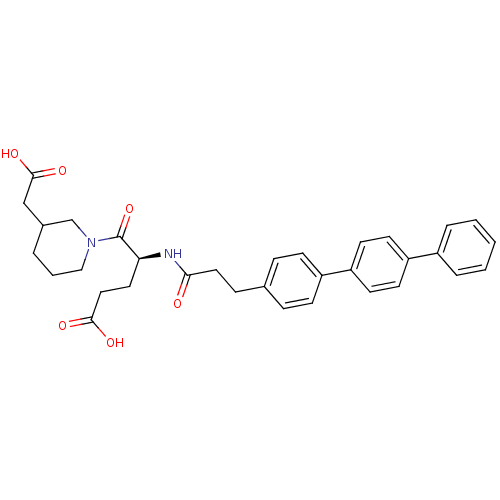 (US8691753, 70) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 421 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120225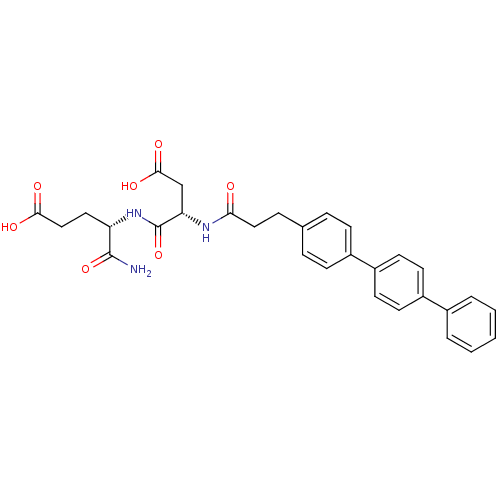 (US8691753, 74) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120203 (US8691753, 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 433 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120234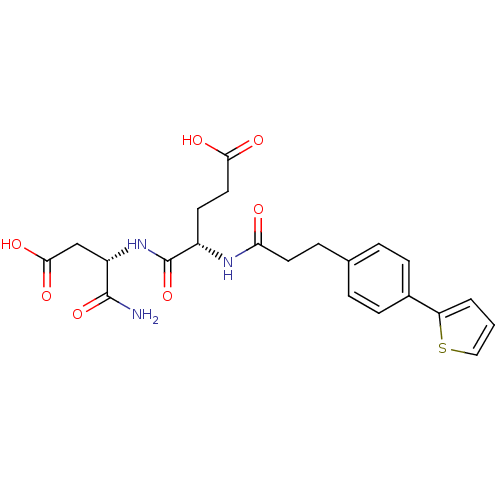 (US8691753, 83) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 435 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120190 (US8691753, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 446 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120214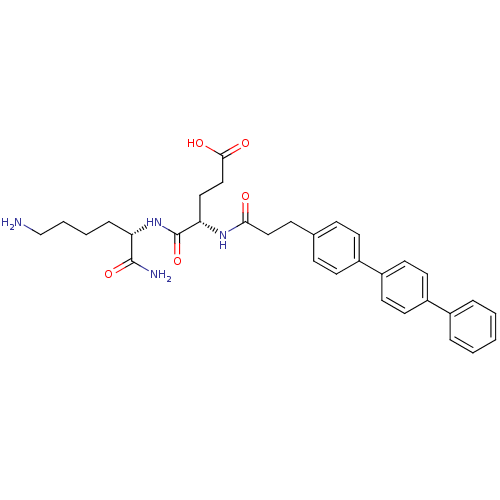 (US8691753, 63) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120158 (US8691753, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 489 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120235 (US8691753, 84) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 514 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120161 (US8691753, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 139 total ) | Next | Last >> |