 Found 604 hits of ic50 for UniProtKB: P09237
Found 604 hits of ic50 for UniProtKB: P09237 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrilysin
(Homo sapiens (Human)) | BDBM50287379

(4-[(S)-2-((R)-2-Hydroxycarbamoylmethyl-4-methyl-pe...)Show SMILES COC(=O)c1ccc(NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)CC(=O)NO)cc1 Show InChI InChI=1S/C22H33N3O6/c1-13(2)10-16(12-19(26)25-30)20(27)24-18(11-14(3)4)21(28)23-17-8-6-15(7-9-17)22(29)31-5/h6-9,13-14,16,18,30H,10-12H2,1-5H3,(H,23,28)(H,24,27)(H,25,26)/t16-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrilysin, matrix metalloproteinase-7 |
Bioorg Med Chem Lett 6: 1601-1606 (1996)
Article DOI: 10.1016/S0960-894X(96)00283-1
BindingDB Entry DOI: 10.7270/Q2WD40JH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072583
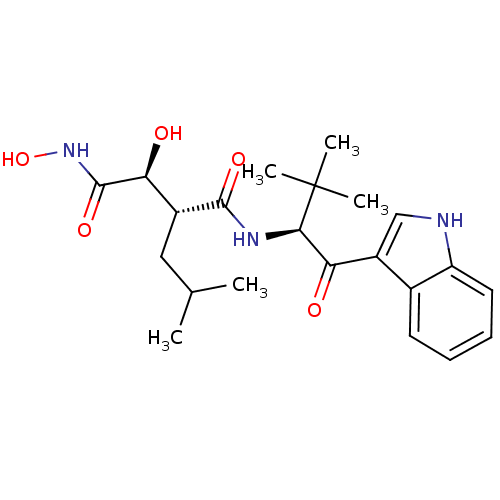
((2R,3S)-2,N*1*-Dihydroxy-N*4*-[(S)-1-(1H-indole-3-...)Show SMILES CC(C)C[C@H]([C@H](O)C(=O)NO)C(=O)N[C@H](C(=O)c1c[nH]c2ccccc12)C(C)(C)C Show InChI InChI=1S/C22H31N3O5/c1-12(2)10-14(18(27)21(29)25-30)20(28)24-19(22(3,4)5)17(26)15-11-23-16-9-7-6-8-13(15)16/h6-9,11-12,14,18-19,23,27,30H,10H2,1-5H3,(H,24,28)(H,25,29)/t14-,18+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072563
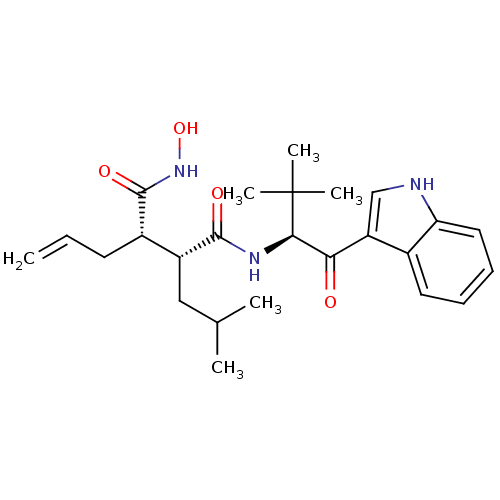
((2R,3S)-2-Allyl-N*1*-hydroxy-N*4*-[(S)-1-(1H-indol...)Show SMILES CC(C)C[C@H]([C@H](CC=C)C(=O)NO)C(=O)N[C@H](C(=O)c1c[nH]c2ccccc12)C(C)(C)C Show InChI InChI=1S/C25H35N3O4/c1-7-10-17(24(31)28-32)18(13-15(2)3)23(30)27-22(25(4,5)6)21(29)19-14-26-20-12-9-8-11-16(19)20/h7-9,11-12,14-15,17-18,22,26,32H,1,10,13H2,2-6H3,(H,27,30)(H,28,31)/t17-,18+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50287376

(2-[(S)-1-((R)-2-Hydroxycarbamoylmethyl-4-methyl-pe...)Show SMILES COC(=O)c1ccc2nc([nH]c2c1)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C22H32N4O5/c1-12(2)8-15(11-19(27)26-30)21(28)25-18(9-13(3)4)20-23-16-7-6-14(22(29)31-5)10-17(16)24-20/h6-7,10,12-13,15,18,30H,8-9,11H2,1-5H3,(H,23,24)(H,25,28)(H,26,27)/t15-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrilysin, matrix metalloproteinase-7 |
Bioorg Med Chem Lett 6: 1601-1606 (1996)
Article DOI: 10.1016/S0960-894X(96)00283-1
BindingDB Entry DOI: 10.7270/Q2WD40JH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50287383
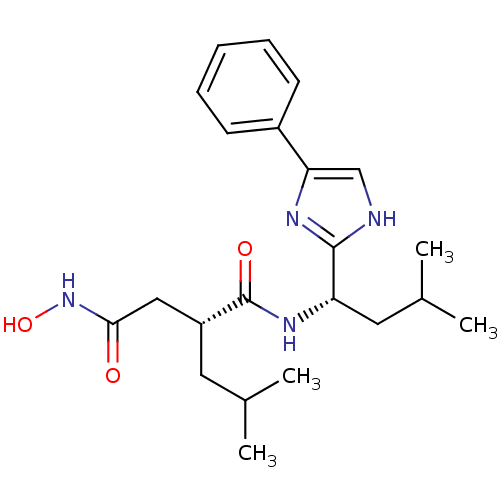
((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-3-methyl-1-(...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](CC(C)C)c1nc(c[nH]1)-c1ccccc1 Show InChI InChI=1S/C22H32N4O3/c1-14(2)10-17(12-20(27)26-29)22(28)25-18(11-15(3)4)21-23-13-19(24-21)16-8-6-5-7-9-16/h5-9,13-15,17-18,29H,10-12H2,1-4H3,(H,23,24)(H,25,28)(H,26,27)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrilysin, matrix metalloproteinase-7 |
Bioorg Med Chem Lett 6: 1601-1606 (1996)
Article DOI: 10.1016/S0960-894X(96)00283-1
BindingDB Entry DOI: 10.7270/Q2WD40JH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072564
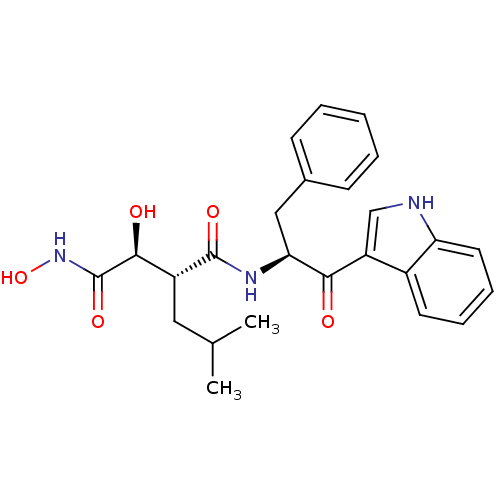
((2R,3S)-N*4*-[(S)-1-Benzyl-2-(1H-indol-3-yl)-2-oxo...)Show SMILES CC(C)C[C@H]([C@H](O)C(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)c1c[nH]c2ccccc12 Show InChI InChI=1S/C25H29N3O5/c1-15(2)12-18(23(30)25(32)28-33)24(31)27-21(13-16-8-4-3-5-9-16)22(29)19-14-26-20-11-7-6-10-17(19)20/h3-11,14-15,18,21,23,26,30,33H,12-13H2,1-2H3,(H,27,31)(H,28,32)/t18-,21+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072582

((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-oxo-2-(1H-pyr...)Show SMILES CC(C)C[C@H]([C@H](CC=C)C(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)c1ccc[nH]1 Show InChI InChI=1S/C24H31N3O4/c1-4-9-18(24(30)27-31)19(14-16(2)3)23(29)26-21(15-17-10-6-5-7-11-17)22(28)20-12-8-13-25-20/h4-8,10-13,16,18-19,21,25,31H,1,9,14-15H2,2-3H3,(H,26,29)(H,27,30)/t18-,19+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072572
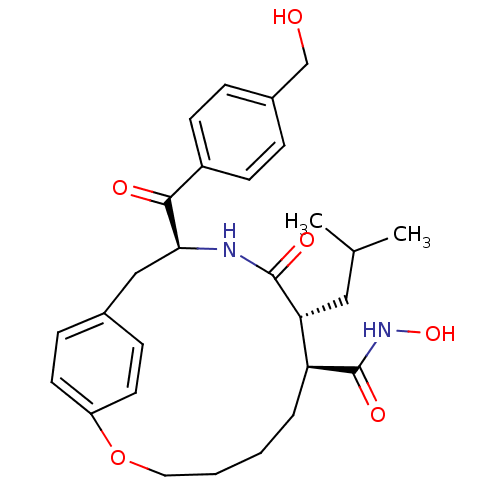
((7S,8R,11S)-11-(4-Hydroxymethyl-benzoyl)-8-isobuty...)Show SMILES CC(C)C[C@@H]1[C@H](CCCCOc2ccc(C[C@H](NC1=O)C(=O)c1ccc(CO)cc1)cc2)C(=O)NO Show InChI InChI=1S/C28H36N2O6/c1-18(2)15-24-23(28(34)30-35)5-3-4-14-36-22-12-8-19(9-13-22)16-25(29-27(24)33)26(32)21-10-6-20(17-31)7-11-21/h6-13,18,23-25,31,35H,3-5,14-17H2,1-2H3,(H,29,33)(H,30,34)/t23-,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50071263
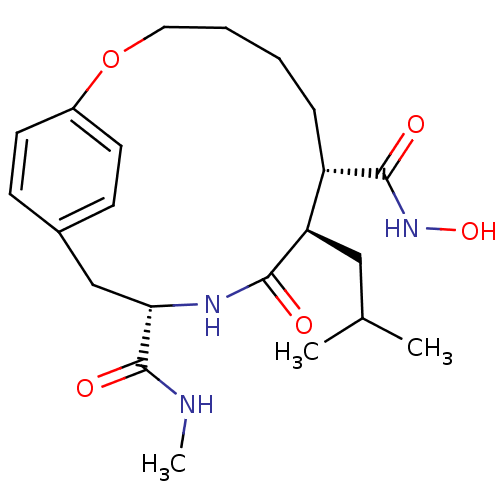
((7S,8R,11S)-8-Isobutyl-9-oxo-2-oxa-10-aza-bicyclo[...)Show SMILES CNC(=O)[C@@H]1Cc2ccc(OCCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C22H33N3O5/c1-14(2)12-18-17(21(27)25-29)6-4-5-11-30-16-9-7-15(8-10-16)13-19(22(28)23-3)24-20(18)26/h7-10,14,17-19,29H,4-6,11-13H2,1-3H3,(H,23,28)(H,24,26)(H,25,27)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of fibroblast matrilysin (MMP-7) |
Bioorg Med Chem Lett 8: 2087-92 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2G89 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50071268
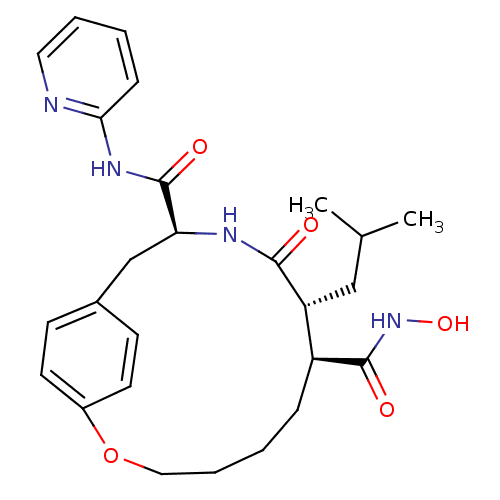
((7S,8R,11S)-8-Isobutyl-9-oxo-2-oxa-10-aza-bicyclo[...)Show SMILES CC(C)C[C@@H]1[C@H](CCCCOc2ccc(C[C@H](NC1=O)C(=O)Nc1ccccn1)cc2)C(=O)NO Show InChI InChI=1S/C26H34N4O5/c1-17(2)15-21-20(25(32)30-34)7-4-6-14-35-19-11-9-18(10-12-19)16-22(28-24(21)31)26(33)29-23-8-3-5-13-27-23/h3,5,8-13,17,20-22,34H,4,6-7,14-16H2,1-2H3,(H,28,31)(H,30,32)(H,27,29,33)/t20-,21+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of fibroblast matrilysin (MMP-7) |
Bioorg Med Chem Lett 8: 2087-92 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2G89 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50071263

((7S,8R,11S)-8-Isobutyl-9-oxo-2-oxa-10-aza-bicyclo[...)Show SMILES CNC(=O)[C@@H]1Cc2ccc(OCCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C22H33N3O5/c1-14(2)12-18-17(21(27)25-29)6-4-5-11-30-16-9-7-15(8-10-16)13-19(22(28)23-3)24-20(18)26/h7-10,14,17-19,29H,4-6,11-13H2,1-3H3,(H,23,28)(H,24,26)(H,25,27)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50287378

((R)-N*1*-[(S)-1-(1H-Benzoimidazol-2-yl)-3-methyl-b...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](CC(C)C)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C20H30N4O3/c1-12(2)9-14(11-18(25)24-27)20(26)23-17(10-13(3)4)19-21-15-7-5-6-8-16(15)22-19/h5-8,12-14,17,27H,9-11H2,1-4H3,(H,21,22)(H,23,26)(H,24,25)/t14-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrilysin, matrix metalloproteinase-7 |
Bioorg Med Chem Lett 6: 1601-1606 (1996)
Article DOI: 10.1016/S0960-894X(96)00283-1
BindingDB Entry DOI: 10.7270/Q2WD40JH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072566

((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-(1H-indol-3-y...)Show SMILES CC(C)C[C@H]([C@H](CC=C)C(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)c1c[nH]c2ccccc12 Show InChI InChI=1S/C28H33N3O4/c1-4-10-21(28(34)31-35)22(15-18(2)3)27(33)30-25(16-19-11-6-5-7-12-19)26(32)23-17-29-24-14-9-8-13-20(23)24/h4-9,11-14,17-18,21-22,25,29,35H,1,10,15-16H2,2-3H3,(H,30,33)(H,31,34)/t21-,22+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072579
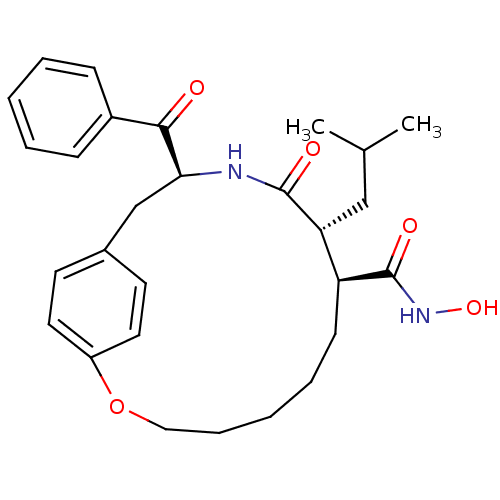
((8S,9R,12S)-12-Benzoyl-9-isobutyl-10-oxo-2-oxa-11-...)Show SMILES CC(C)C[C@@H]1[C@H](CCCCCOc2ccc(C[C@H](NC1=O)C(=O)c1ccccc1)cc2)C(=O)NO Show InChI InChI=1S/C28H36N2O5/c1-19(2)17-24-23(28(33)30-34)11-7-4-8-16-35-22-14-12-20(13-15-22)18-25(29-27(24)32)26(31)21-9-5-3-6-10-21/h3,5-6,9-10,12-15,19,23-25,34H,4,7-8,11,16-18H2,1-2H3,(H,29,32)(H,30,33)/t23-,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072566

((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-(1H-indol-3-y...)Show SMILES CC(C)C[C@H]([C@H](CC=C)C(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)c1c[nH]c2ccccc12 Show InChI InChI=1S/C28H33N3O4/c1-4-10-21(28(34)31-35)22(15-18(2)3)27(33)30-25(16-19-11-6-5-7-12-19)26(32)23-17-29-24-14-9-8-13-20(23)24/h4-9,11-14,17-18,21-22,25,29,35H,1,10,15-16H2,2-3H3,(H,30,33)(H,31,34)/t21-,22+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MMP7 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50071269
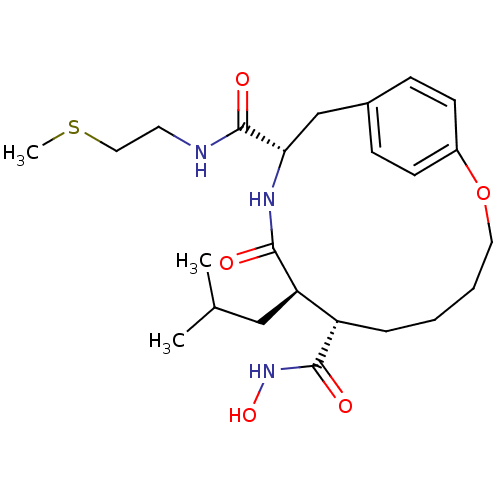
((7S,8R,11S)-8-Isobutyl-9-oxo-2-oxa-10-aza-bicyclo[...)Show SMILES CSCCNC(=O)[C@@H]1Cc2ccc(OCCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C24H37N3O5S/c1-16(2)14-20-19(23(29)27-31)6-4-5-12-32-18-9-7-17(8-10-18)15-21(26-22(20)28)24(30)25-11-13-33-3/h7-10,16,19-21,31H,4-6,11-15H2,1-3H3,(H,25,30)(H,26,28)(H,27,29)/t19-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of fibroblast matrilysin (MMP-7) |
Bioorg Med Chem Lett 8: 2087-92 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2G89 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50071262
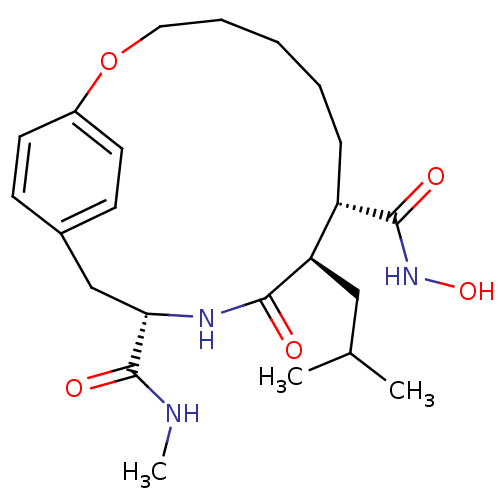
((8S,9R,12S)-9-Isobutyl-10-oxo-2-oxa-11-aza-bicyclo...)Show SMILES CNC(=O)[C@@H]1Cc2ccc(OCCCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H35N3O5/c1-15(2)13-19-18(22(28)26-30)7-5-4-6-12-31-17-10-8-16(9-11-17)14-20(23(29)24-3)25-21(19)27/h8-11,15,18-20,30H,4-7,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t18-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of fibroblast matrilysin (MMP-7) |
Bioorg Med Chem Lett 8: 2087-92 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2G89 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50071265

((7S,8R,11S)-9-Oxo-8-(3-p-tolyl-propyl)-2-oxa-10-az...)Show SMILES CNC(=O)[C@@H]1Cc2ccc(OCCCC[C@@H]([C@@H](CCCc3ccc(C)cc3)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C28H37N3O5/c1-19-9-11-20(12-10-19)6-5-8-23-24(27(33)31-35)7-3-4-17-36-22-15-13-21(14-16-22)18-25(28(34)29-2)30-26(23)32/h9-16,23-25,35H,3-8,17-18H2,1-2H3,(H,29,34)(H,30,32)(H,31,33)/t23-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of fibroblast matrilysin (MMP-7) |
Bioorg Med Chem Lett 8: 2087-92 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2G89 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50064341
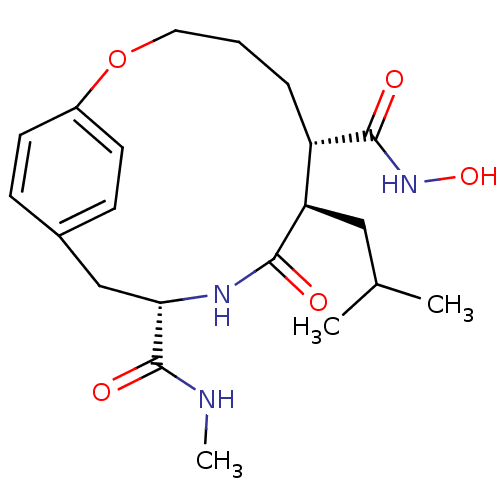
((6S,7R,10S)-7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[1...)Show SMILES CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C21H31N3O5/c1-13(2)11-17-16(20(26)24-28)5-4-10-29-15-8-6-14(7-9-15)12-18(21(27)22-3)23-19(17)25/h6-9,13,16-18,28H,4-5,10-12H2,1-3H3,(H,22,27)(H,23,25)(H,24,26)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of fibroblast matrilysin (MMP-7) |
Bioorg Med Chem Lett 8: 2087-92 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2G89 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50287381

((S)-2-Cyclohexyl-N*4*-hydroxy-N*1*-[(S)-3-methyl-1...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](CC(=O)NO)C1CCCCC1)c1nc(c[nH]1)-c1ccccc1 Show InChI InChI=1S/C24H34N4O3/c1-16(2)13-20(23-25-15-21(26-23)18-11-7-4-8-12-18)27-24(30)19(14-22(29)28-31)17-9-5-3-6-10-17/h4,7-8,11-12,15-17,19-20,31H,3,5-6,9-10,13-14H2,1-2H3,(H,25,26)(H,27,30)(H,28,29)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrilysin, matrix metalloproteinase-7 |
Bioorg Med Chem Lett 6: 1601-1606 (1996)
Article DOI: 10.1016/S0960-894X(96)00283-1
BindingDB Entry DOI: 10.7270/Q2WD40JH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072568
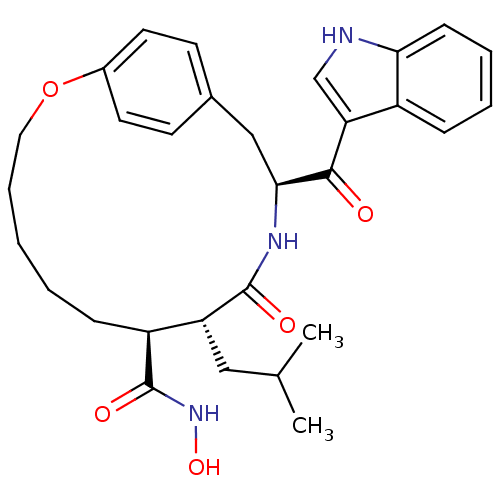
((8S,9R,12S)-12-(1H-Indole-3-carbonyl)-9-isobutyl-1...)Show SMILES CC(C)C[C@@H]1[C@H](CCCCCOc2ccc(C[C@H](NC1=O)C(=O)c1c[nH]c3ccccc13)cc2)C(=O)NO Show InChI InChI=1S/C30H37N3O5/c1-19(2)16-24-23(30(36)33-37)9-4-3-7-15-38-21-13-11-20(12-14-21)17-27(32-29(24)35)28(34)25-18-31-26-10-6-5-8-22(25)26/h5-6,8,10-14,18-19,23-24,27,31,37H,3-4,7,9,15-17H2,1-2H3,(H,32,35)(H,33,36)/t23-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072567
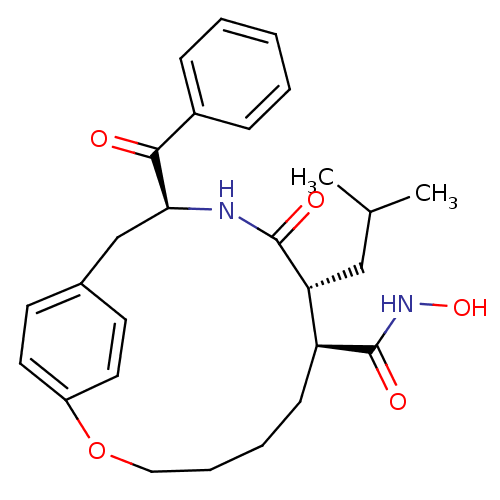
((7S,8R,11S)-11-Benzoyl-8-isobutyl-9-oxo-2-oxa-10-a...)Show SMILES CC(C)C[C@@H]1[C@H](CCCCOc2ccc(C[C@H](NC1=O)C(=O)c1ccccc1)cc2)C(=O)NO Show InChI InChI=1S/C27H34N2O5/c1-18(2)16-23-22(27(32)29-33)10-6-7-15-34-21-13-11-19(12-14-21)17-24(28-26(23)31)25(30)20-8-4-3-5-9-20/h3-5,8-9,11-14,18,22-24,33H,6-7,10,15-17H2,1-2H3,(H,28,31)(H,29,32)/t22-,23+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072578

((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-phenyl-...)Show SMILES CC(C)C[C@H]([C@H](CC=C)C(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)c1ccccc1 Show InChI InChI=1S/C26H32N2O4/c1-4-11-21(26(31)28-32)22(16-18(2)3)25(30)27-23(17-19-12-7-5-8-13-19)24(29)20-14-9-6-10-15-20/h4-10,12-15,18,21-23,32H,1,11,16-17H2,2-3H3,(H,27,30)(H,28,31)/t21-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-7 (MMP-7) in fluorimetric assay |
J Med Chem 45: 219-32 (2001)
BindingDB Entry DOI: 10.7270/Q2XP747B |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50071259
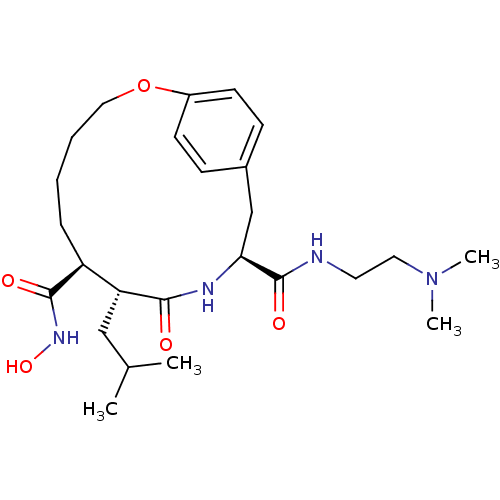
((7S,8R,11S)-8-Isobutyl-9-oxo-2-oxa-10-aza-bicyclo[...)Show SMILES CC(C)C[C@@H]1[C@H](CCCCOc2ccc(C[C@H](NC1=O)C(=O)NCCN(C)C)cc2)C(=O)NO Show InChI InChI=1S/C25H40N4O5/c1-17(2)15-21-20(24(31)28-33)7-5-6-14-34-19-10-8-18(9-11-19)16-22(27-23(21)30)25(32)26-12-13-29(3)4/h8-11,17,20-22,33H,5-7,12-16H2,1-4H3,(H,26,32)(H,27,30)(H,28,31)/t20-,21+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of fibroblast matrilysin (MMP-7) |
Bioorg Med Chem Lett 8: 2087-92 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2G89 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072569

((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxazol-2-yl-2...)Show SMILES CC(C)C[C@H]([C@H](CC=C)C(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)c1ncco1 Show InChI InChI=1S/C23H29N3O5/c1-4-8-17(22(29)26-30)18(13-15(2)3)21(28)25-19(14-16-9-6-5-7-10-16)20(27)23-24-11-12-31-23/h4-7,9-12,15,17-19,30H,1,8,13-14H2,2-3H3,(H,25,28)(H,26,29)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem 16: 1562-95 (2008)
Article DOI: 10.1016/j.bmc.2007.11.015
BindingDB Entry DOI: 10.7270/Q21J9BNH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072574
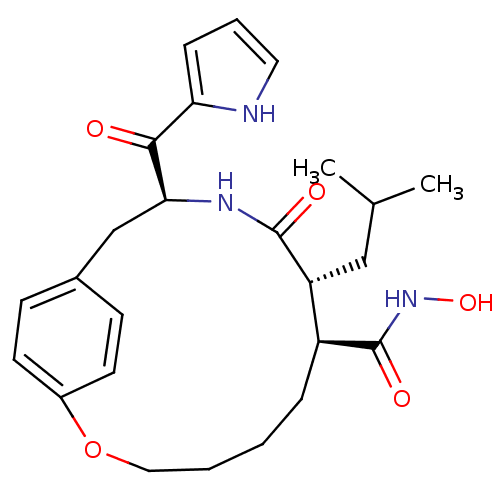
((7S,8R,11S)-8-Isobutyl-9-oxo-11-(1H-pyrrole-2-carb...)Show SMILES CC(C)C[C@@H]1[C@H](CCCCOc2ccc(C[C@H](NC1=O)C(=O)c1ccc[nH]1)cc2)C(=O)NO Show InChI InChI=1S/C25H33N3O5/c1-16(2)14-20-19(25(31)28-32)6-3-4-13-33-18-10-8-17(9-11-18)15-22(27-24(20)30)23(29)21-7-5-12-26-21/h5,7-12,16,19-20,22,26,32H,3-4,6,13-15H2,1-2H3,(H,27,30)(H,28,31)/t19-,20+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072569

((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxazol-2-yl-2...)Show SMILES CC(C)C[C@H]([C@H](CC=C)C(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)c1ncco1 Show InChI InChI=1S/C23H29N3O5/c1-4-8-17(22(29)26-30)18(13-15(2)3)21(28)25-19(14-16-9-6-5-7-10-16)20(27)23-24-11-12-31-23/h4-7,9-12,15,17-19,30H,1,8,13-14H2,2-3H3,(H,25,28)(H,26,29)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072575
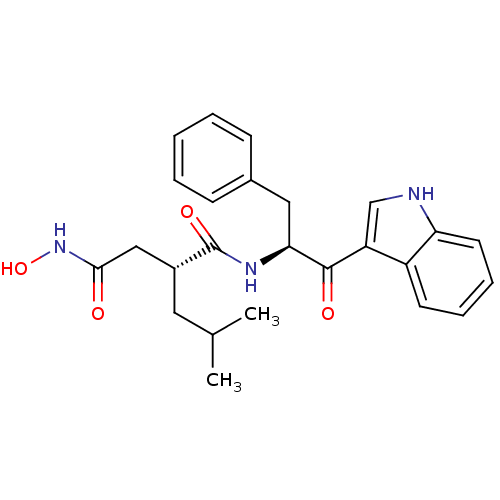
((R)-N*1*-[(S)-1-Benzyl-2-(1H-indol-3-yl)-2-oxo-eth...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)c1c[nH]c2ccccc12 Show InChI InChI=1S/C25H29N3O4/c1-16(2)12-18(14-23(29)28-32)25(31)27-22(13-17-8-4-3-5-9-17)24(30)20-15-26-21-11-7-6-10-19(20)21/h3-11,15-16,18,22,26,32H,12-14H2,1-2H3,(H,27,31)(H,28,29)/t18-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50592899

(BB-3644 | SOLIMASTAT | Solimastat)Show SMILES CO[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1ccccn1)C(C)(C)C)C(=O)NO | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072576
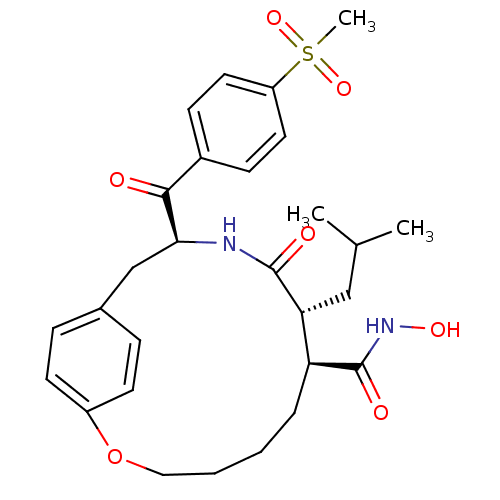
((7S,8R,11S)-8-Isobutyl-11-(4-methanesulfonyl-benzo...)Show SMILES CC(C)C[C@@H]1[C@H](CCCCOc2ccc(C[C@H](NC1=O)C(=O)c1ccc(cc1)S(C)(=O)=O)cc2)C(=O)NO Show InChI InChI=1S/C28H36N2O7S/c1-18(2)16-24-23(28(33)30-34)6-4-5-15-37-21-11-7-19(8-12-21)17-25(29-27(24)32)26(31)20-9-13-22(14-10-20)38(3,35)36/h7-14,18,23-25,34H,4-6,15-17H2,1-3H3,(H,29,32)(H,30,33)/t23-,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50071264
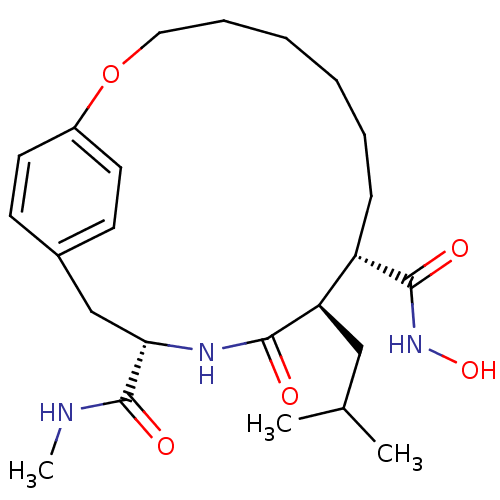
((9S,10R,13S)-10-Isobutyl-11-oxo-2-oxa-12-aza-bicyc...)Show SMILES CNC(=O)[C@@H]1Cc2ccc(OCCCCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C24H37N3O5/c1-16(2)14-20-19(23(29)27-31)8-6-4-5-7-13-32-18-11-9-17(10-12-18)15-21(24(30)25-3)26-22(20)28/h9-12,16,19-21,31H,4-8,13-15H2,1-3H3,(H,25,30)(H,26,28)(H,27,29)/t19-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of fibroblast matrilysin (MMP-7) |
Bioorg Med Chem Lett 8: 2087-92 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2G89 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50071267
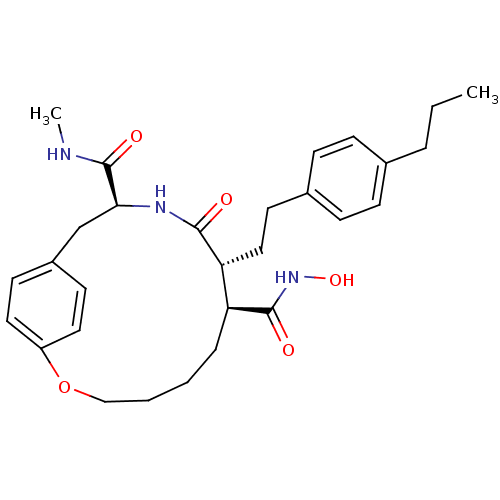
((7S,8R,11S)-9-Oxo-8-[2-(4-propyl-phenyl)-ethyl]-2-...)Show SMILES CCCc1ccc(CC[C@@H]2[C@H](CCCCOc3ccc(C[C@H](NC2=O)C(=O)NC)cc3)C(=O)NO)cc1 Show InChI InChI=1S/C29H39N3O5/c1-3-6-20-8-10-21(11-9-20)14-17-25-24(28(34)32-36)7-4-5-18-37-23-15-12-22(13-16-23)19-26(29(35)30-2)31-27(25)33/h8-13,15-16,24-26,36H,3-7,14,17-19H2,1-2H3,(H,30,35)(H,31,33)(H,32,34)/t24-,25+,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of fibroblast matrilysin (MMP-7) |
Bioorg Med Chem Lett 8: 2087-92 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2G89 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50592893
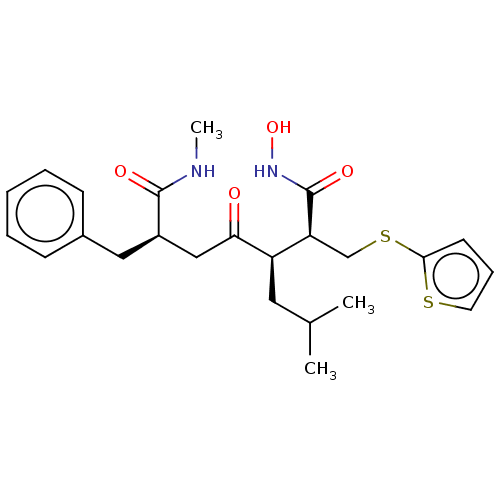
(CHEMBL5205063)Show SMILES CNC(=O)[C@@H](CC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO)Cc1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
50% inhibition of human recombinant Matrilysin |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50071262

((8S,9R,12S)-9-Isobutyl-10-oxo-2-oxa-11-aza-bicyclo...)Show SMILES CNC(=O)[C@@H]1Cc2ccc(OCCCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H35N3O5/c1-15(2)13-19-18(22(28)26-30)7-5-4-6-12-31-17-10-8-16(9-11-17)14-20(23(29)24-3)25-21(19)27/h8-11,15,18-20,30H,4-7,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t18-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50203953
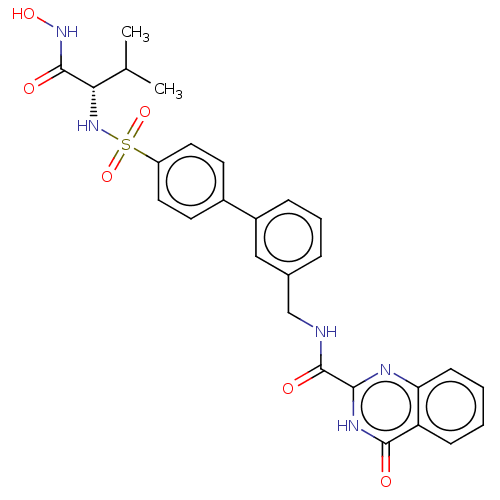
(CHEMBL3932562)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1cccc(CNC(=O)c2nc3ccccc3c(=O)[nH]2)c1)C(=O)NO |r| Show InChI InChI=1S/C27H27N5O6S/c1-16(2)23(26(34)31-36)32-39(37,38)20-12-10-18(11-13-20)19-7-5-6-17(14-19)15-28-27(35)24-29-22-9-4-3-8-21(22)25(33)30-24/h3-14,16,23,32,36H,15H2,1-2H3,(H,28,35)(H,31,34)(H,29,30,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AMPA-activated MMP7 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate measured after 40 mins by spectrofluorimetric method |
Bioorg Med Chem 24: 6149-6165 (2016)
Article DOI: 10.1016/j.bmc.2016.09.009
BindingDB Entry DOI: 10.7270/Q2H1341V |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072580
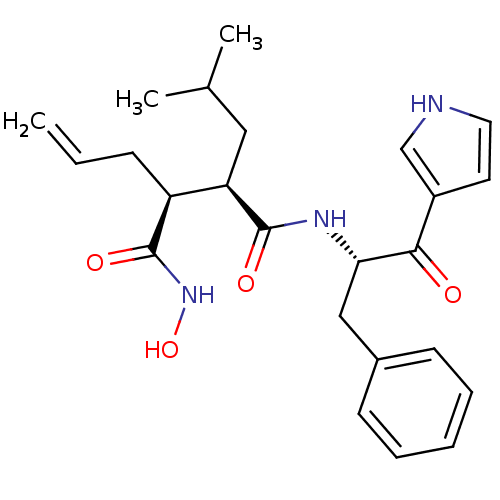
((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-oxo-2-(1H-pyr...)Show SMILES CC(C)C[C@H]([C@H](CC=C)C(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)c1cc[nH]c1 Show InChI InChI=1S/C24H31N3O4/c1-4-8-19(24(30)27-31)20(13-16(2)3)23(29)26-21(14-17-9-6-5-7-10-17)22(28)18-11-12-25-15-18/h4-7,9-12,15-16,19-21,25,31H,1,8,13-14H2,2-3H3,(H,26,29)(H,27,30)/t19-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50042944
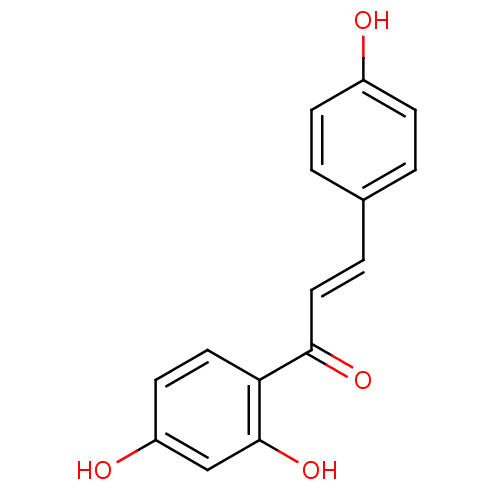
((E)-1-(2,4-Dihydroxy-phenyl)-3-(4-hydroxy-phenyl)-...)Show InChI InChI=1S/C15H12O4/c16-11-4-1-10(2-5-11)3-8-14(18)13-7-6-12(17)9-15(13)19/h1-9,16-17,19H/b8-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00626
BindingDB Entry DOI: 10.7270/Q2057KZK |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50031795
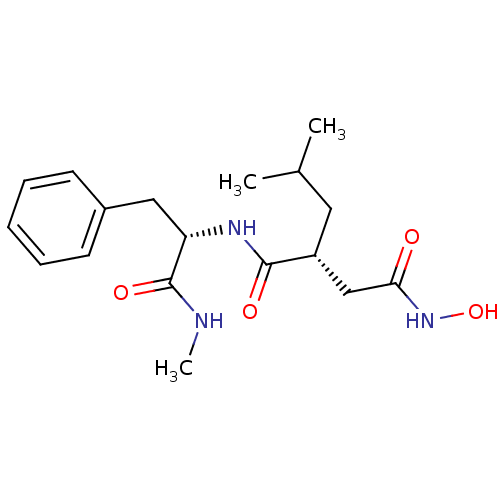
((N-(2-HYDROXAMATEMETHYLENE-4-METHYL-PENTOYL)PHENYL...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C18H27N3O4/c1-12(2)9-14(11-16(22)21-25)17(23)20-15(18(24)19-3)10-13-7-5-4-6-8-13/h4-8,12,14-15,25H,9-11H2,1-3H3,(H,19,24)(H,20,23)(H,21,22)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Università G. d'Annunzio
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP7 |
Eur J Med Chem 43: 1008-14 (2008)
Article DOI: 10.1016/j.ejmech.2007.07.002
BindingDB Entry DOI: 10.7270/Q23T9H1W |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072577
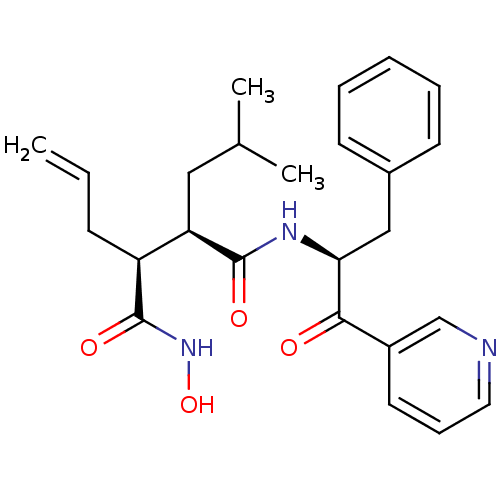
((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-pyridin...)Show SMILES CC(C)C[C@H]([C@H](CC=C)C(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)c1cccnc1 Show InChI InChI=1S/C25H31N3O4/c1-4-9-20(25(31)28-32)21(14-17(2)3)24(30)27-22(15-18-10-6-5-7-11-18)23(29)19-12-8-13-26-16-19/h4-8,10-13,16-17,20-22,32H,1,9,14-15H2,2-3H3,(H,27,30)(H,28,31)/t20-,21+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50287377
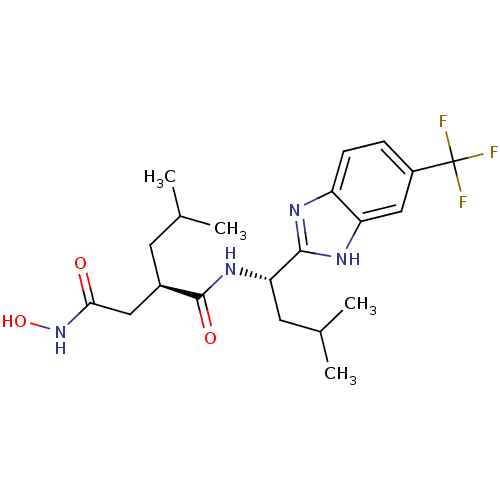
((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-3-methyl-1-(...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](CC(C)C)c1nc2ccc(cc2[nH]1)C(F)(F)F Show InChI InChI=1S/C21H29F3N4O3/c1-11(2)7-13(9-18(29)28-31)20(30)27-17(8-12(3)4)19-25-15-6-5-14(21(22,23)24)10-16(15)26-19/h5-6,10-13,17,31H,7-9H2,1-4H3,(H,25,26)(H,27,30)(H,28,29)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrilysin, matrix metalloproteinase-7 |
Bioorg Med Chem Lett 6: 1601-1606 (1996)
Article DOI: 10.1016/S0960-894X(96)00283-1
BindingDB Entry DOI: 10.7270/Q2WD40JH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50589859
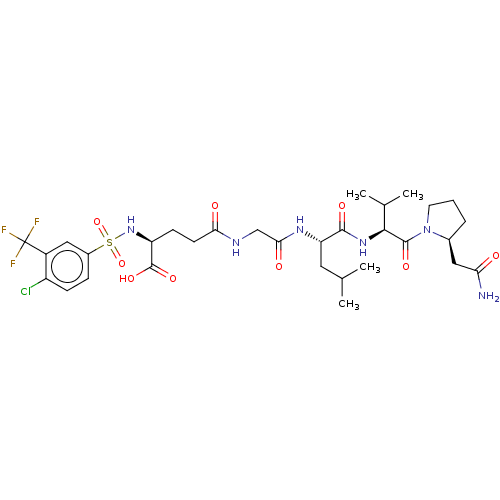
(CHEMBL5204457)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)CC[C@H](NS(=O)(=O)c1ccc(Cl)c(c1)C(F)(F)F)C(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01088
BindingDB Entry DOI: 10.7270/Q2542SJ0 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50287380

((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-1-(6-methoxy...)Show SMILES COc1ccc2nc([nH]c2c1)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C21H32N4O4/c1-12(2)8-14(10-19(26)25-28)21(27)24-18(9-13(3)4)20-22-16-7-6-15(29-5)11-17(16)23-20/h6-7,11-14,18,28H,8-10H2,1-5H3,(H,22,23)(H,24,27)(H,25,26)/t14-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrilysin, matrix metalloproteinase-7 |
Bioorg Med Chem Lett 6: 1601-1606 (1996)
Article DOI: 10.1016/S0960-894X(96)00283-1
BindingDB Entry DOI: 10.7270/Q2WD40JH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00626
BindingDB Entry DOI: 10.7270/Q2057KZK |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50287385

((R)-N*1*-[1-(1H-Benzoimidazol-2-yl)-2-(1H-indol-3-...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C25H29N5O3/c1-15(2)11-16(13-23(31)30-33)25(32)29-22(24-27-20-9-5-6-10-21(20)28-24)12-17-14-26-19-8-4-3-7-18(17)19/h3-10,14-16,22,26,33H,11-13H2,1-2H3,(H,27,28)(H,29,32)(H,30,31)/t16-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrilysin, matrix metalloproteinase-7 |
Bioorg Med Chem Lett 6: 1601-1606 (1996)
Article DOI: 10.1016/S0960-894X(96)00283-1
BindingDB Entry DOI: 10.7270/Q2WD40JH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data