 Found 661 hits of ic50 data for polymerid = 6207
Found 661 hits of ic50 data for polymerid = 6207 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM120095
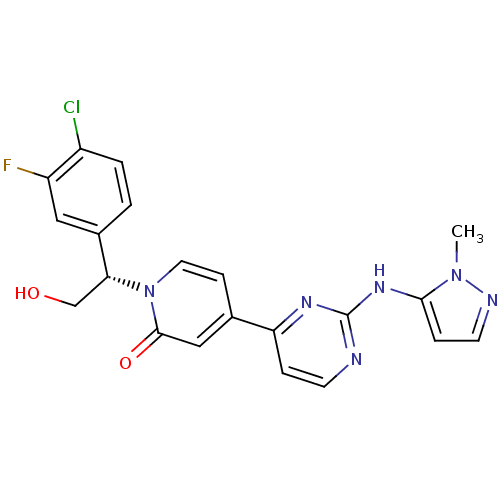
(US10525036, Example GDC-0994 | US10934304, Example...)Show SMILES Cn1nccc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-28-19(5-8-25-28)27-21-24-7-4-17(26-21)13-6-9-29(20(31)11-13)18(12-30)14-2-3-15(22)16(23)10-14/h2-11,18,30H,12H2,1H3,(H,24,26,27)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
Bioorg Med Chem Lett 25: 4047-56 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.093
BindingDB Entry DOI: 10.7270/Q2VX0JBS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50531622
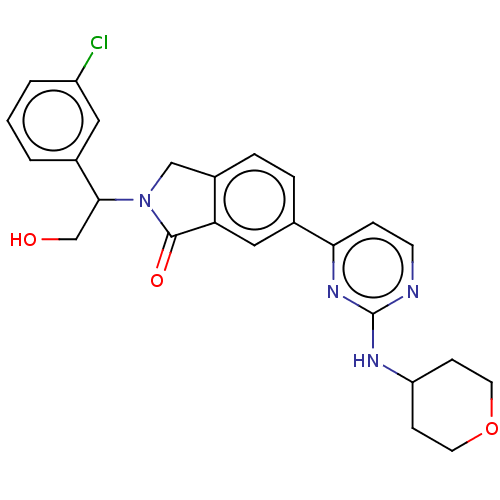
(CHEMBL4434915)Show SMILES OCC(N1Cc2ccc(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1cccc(Cl)c1 Show InChI InChI=1S/C25H25ClN4O3/c26-19-3-1-2-17(12-19)23(15-31)30-14-18-5-4-16(13-21(18)24(30)32)22-6-9-27-25(29-22)28-20-7-10-33-11-8-20/h1-6,9,12-13,20,23,31H,7-8,10-11,14-15H2,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using lipid as substrate after 40 mins by ADP-Glo luminescence assay |
Eur J Med Chem 164: 334-341 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.040
BindingDB Entry DOI: 10.7270/Q2ZS310S |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50601920

(ASN007 | Asn007 | Asn007 rd)Show SMILES Cc1cnc(NC2CCOCC2)nc1-n1cnc(c1)C(=O)N[C@H](CN)c1cc(F)cc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01244
BindingDB Entry DOI: 10.7270/Q2HT2TF2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM120086
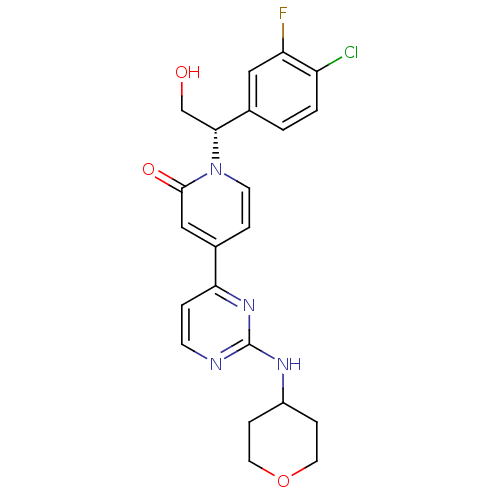
(US8697715, 1 | US9259470, 1)Show SMILES OC[C@H](c1ccc(Cl)c(F)c1)n1ccc(cc1=O)-c1ccnc(NC2CCOCC2)n1 |r| Show InChI InChI=1S/C22H22ClFN4O3/c23-17-2-1-15(11-18(17)24)20(13-29)28-8-4-14(12-21(28)30)19-3-7-25-22(27-19)26-16-5-9-31-10-6-16/h1-4,7-8,11-12,16,20,29H,5-6,9-10,13H2,(H,25,26,27)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM120086

(US8697715, 1 | US9259470, 1)Show SMILES OC[C@H](c1ccc(Cl)c(F)c1)n1ccc(cc1=O)-c1ccnc(NC2CCOCC2)n1 |r| Show InChI InChI=1S/C22H22ClFN4O3/c23-17-2-1-15(11-18(17)24)20(13-29)28-8-4-14(12-21(28)30)19-3-7-25-22(27-19)26-16-5-9-31-10-6-16/h1-4,7-8,11-12,16,20,29H,5-6,9-10,13H2,(H,25,26,27)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50586289

(CHEMBL5070887)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2CN([C@H](CO)c3cccc(Cl)c3)C(=O)n2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ERK1 (unknown origin) measured after 2 hr by ADP-glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00029
BindingDB Entry DOI: 10.7270/Q2C82F6M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50531640

(CHEMBL4588783)Show SMILES Clc1cccc(CN2Cc3ccc(cc3C2=O)-c2ccnc(NC3CCOCC3)n2)c1 Show InChI InChI=1S/C24H23ClN4O2/c25-19-3-1-2-16(12-19)14-29-15-18-5-4-17(13-21(18)23(29)30)22-6-9-26-24(28-22)27-20-7-10-31-11-8-20/h1-6,9,12-13,20H,7-8,10-11,14-15H2,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using lipid as substrate after 40 mins by ADP-Glo luminescence assay |
Eur J Med Chem 164: 334-341 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.040
BindingDB Entry DOI: 10.7270/Q2ZS310S |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50531627

(CHEMBL4567212)Show SMILES Cn1nccc1Nc1nccc(n1)-c1ccc2CN(C(CO)c3cccc(Cl)c3)C(=O)c2c1 Show InChI InChI=1S/C24H21ClN6O2/c1-30-22(8-10-27-30)29-24-26-9-7-20(28-24)15-5-6-17-13-31(23(33)19(17)12-15)21(14-32)16-3-2-4-18(25)11-16/h2-12,21,32H,13-14H2,1H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using lipid as substrate after 40 mins by ADP-Glo luminescence assay |
Eur J Med Chem 164: 334-341 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.040
BindingDB Entry DOI: 10.7270/Q2ZS310S |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532351

(CHEMBL4565958)Show SMILES Cc1n[nH]cc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-12-18(10-25-28-12)27-21-24-6-4-17(26-21)13-5-7-29(20(31)9-13)19(11-30)14-2-3-15(22)16(23)8-14/h2-10,19,30H,11H2,1H3,(H,25,28)(H,24,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532351

(CHEMBL4565958)Show SMILES Cc1n[nH]cc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-12-18(10-25-28-12)27-21-24-6-4-17(26-21)13-5-7-29(20(31)9-13)19(11-30)14-2-3-15(22)16(23)8-14/h2-10,19,30H,11H2,1H3,(H,25,28)(H,24,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532350

(CHEMBL4571067)Show SMILES Cc1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)ccn1 |r| Show InChI InChI=1S/C23H19ClFN5O2/c1-14-10-17(4-7-26-14)28-23-27-8-5-20(29-23)15-6-9-30(22(32)12-15)21(13-31)16-2-3-18(24)19(25)11-16/h2-12,21,31H,13H2,1H3,(H,26,27,28,29)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532350

(CHEMBL4571067)Show SMILES Cc1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)ccn1 |r| Show InChI InChI=1S/C23H19ClFN5O2/c1-14-10-17(4-7-26-14)28-23-27-8-5-20(29-23)15-6-9-30(22(32)12-15)21(13-31)16-2-3-18(24)19(25)11-16/h2-12,21,31H,13H2,1H3,(H,26,27,28,29)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01244
BindingDB Entry DOI: 10.7270/Q2HT2TF2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) after 45 mins by IMAP assay |
Bioorg Med Chem Lett 25: 4047-56 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.093
BindingDB Entry DOI: 10.7270/Q2VX0JBS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50531628

(CHEMBL4532652)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3CN(C(CO)c4cccc(Cl)c4)C(=O)c3c2)cn1 Show InChI InChI=1S/C24H21ClN6O2/c1-30-13-19(11-27-30)28-24-26-8-7-21(29-24)15-5-6-17-12-31(23(33)20(17)10-15)22(14-32)16-3-2-4-18(25)9-16/h2-11,13,22,32H,12,14H2,1H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using lipid as substrate after 40 mins by ADP-Glo luminescence assay |
Eur J Med Chem 164: 334-341 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.040
BindingDB Entry DOI: 10.7270/Q2ZS310S |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50601918
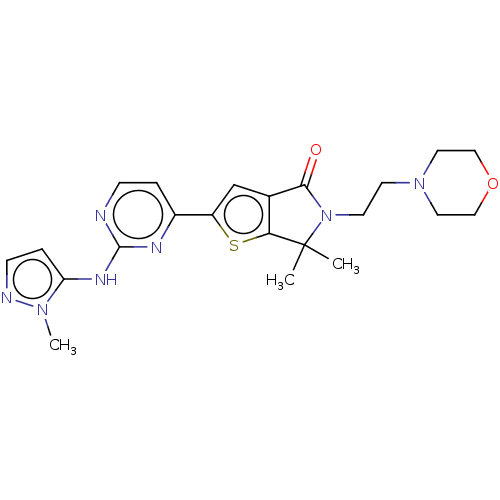
(LY-3214996 | LY3214996 | Ly 3214996 | Ly3214996 | ...)Show SMILES Cn1nccc1Nc1nccc(n1)-c1cc2C(=O)N(CCN3CCOCC3)C(C)(C)c2s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01244
BindingDB Entry DOI: 10.7270/Q2HT2TF2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532362

(CHEMBL4436718)Show SMILES CCn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C22H20ClFN6O2/c1-2-29-12-16(11-26-29)27-22-25-7-5-19(28-22)14-6-8-30(21(32)10-14)20(13-31)15-3-4-17(23)18(24)9-15/h3-12,20,31H,2,13H2,1H3,(H,25,27,28)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532362

(CHEMBL4436718)Show SMILES CCn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C22H20ClFN6O2/c1-2-29-12-16(11-26-29)27-22-25-7-5-19(28-22)14-6-8-30(21(32)10-14)20(13-31)15-3-4-17(23)18(24)9-15/h3-12,20,31H,2,13H2,1H3,(H,25,27,28)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM120095

(US10525036, Example GDC-0994 | US10934304, Example...)Show SMILES Cn1nccc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-28-19(5-8-25-28)27-21-24-7-4-17(26-21)13-6-9-29(20(31)11-13)18(12-30)14-2-3-15(22)16(23)10-14/h2-11,18,30H,12H2,1H3,(H,24,26,27)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM120095

(US10525036, Example GDC-0994 | US10934304, Example...)Show SMILES Cn1nccc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-28-19(5-8-25-28)27-21-24-7-4-17(26-21)13-6-9-29(20(31)11-13)18(12-30)14-2-3-15(22)16(23)10-14/h2-11,18,30H,12H2,1H3,(H,24,26,27)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM570913
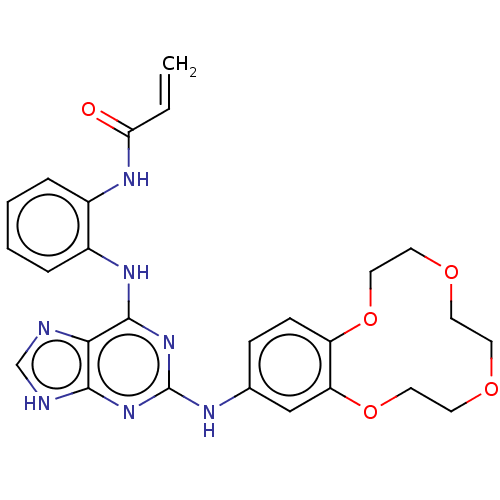
(US11440904, Compound 11)Show SMILES C=CC(=O)Nc1ccccc1Nc1nc(Nc2ccc3OCCOCCOCCOc3c2)nc2[nH]cnc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibiting activities of each of Compound-1 to Compound-24 against BTK wt, BTK(C481S), BMX, FAK, ITK, and EGFR wt kinases were determined using 3... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FB565Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532361

(CHEMBL4435650)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-28-11-15(10-25-28)26-21-24-6-4-18(27-21)13-5-7-29(20(31)9-13)19(12-30)14-2-3-16(22)17(23)8-14/h2-11,19,30H,12H2,1H3,(H,24,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50458811
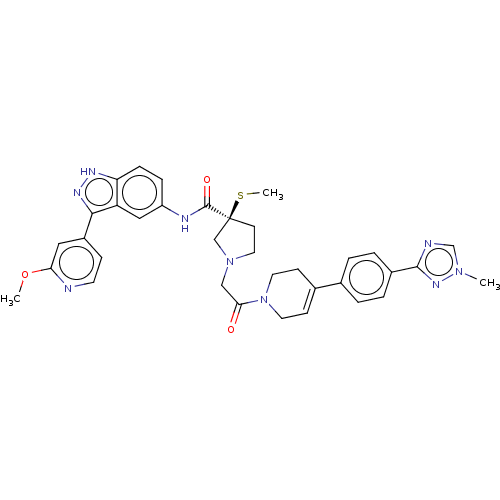
(CHEMBL4209570)Show SMILES COc1cc(ccn1)-c1n[nH]c2ccc(NC(=O)[C@@]3(CCN(CC(=O)N4CCC(=CC4)c4ccc(cc4)-c4ncn(C)n4)C3)SC)cc12 |r,c:29| Show InChI InChI=1S/C35H37N9O3S/c1-42-22-37-33(41-42)25-6-4-23(5-7-25)24-11-15-44(16-12-24)31(45)20-43-17-13-35(21-43,48-3)34(46)38-27-8-9-29-28(19-27)32(40-39-29)26-10-14-36-30(18-26)47-2/h4-11,14,18-19,22H,12-13,15-17,20-21H2,1-3H3,(H,38,46)(H,39,40)/t35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using peptide substrate measured after 45 mins by IMAP assay |
ACS Med Chem Lett 9: 761-767 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00220
BindingDB Entry DOI: 10.7270/Q2HX1G9S |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532361

(CHEMBL4435650)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-28-11-15(10-25-28)26-21-24-6-4-18(27-21)13-5-7-29(20(31)9-13)19(12-30)14-2-3-16(22)17(23)8-14/h2-11,19,30H,12H2,1H3,(H,24,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using peptide substrate measured after 45 mins by IMAP assay |
ACS Med Chem Lett 9: 761-767 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00220
BindingDB Entry DOI: 10.7270/Q2HX1G9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM103302
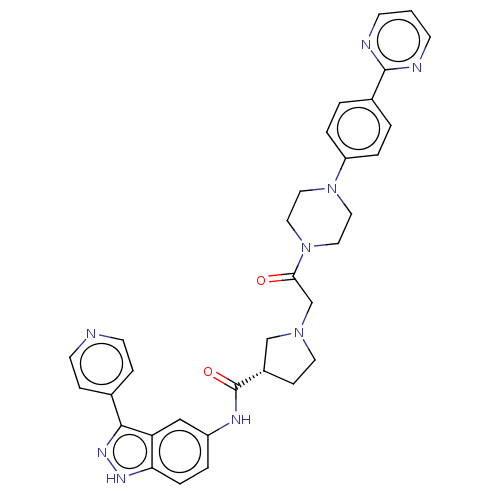
(SCH772984 | US8546404, 6)Show SMILES O=C(CN1CC[C@@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C33H33N9O2/c43-30(42-18-16-41(17-19-42)27-5-2-24(3-6-27)32-35-11-1-12-36-32)22-40-15-10-25(21-40)33(44)37-26-4-7-29-28(20-26)31(39-38-29)23-8-13-34-14-9-23/h1-9,11-14,20,25H,10,15-19,21-22H2,(H,37,44)(H,38,39)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
George Washington University
| Assay Description
The IC50 values of selected kinases were determined using either Z'LYTE, Adapta or LanthaScreen assays. |
Nat Chem Biol 10: 853-860 (2014)
Article DOI: 10.1038/nchembio.1629
BindingDB Entry DOI: 10.7270/Q28G8JCH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM318045

(US10301317, Example 1 | US9624228, Example 1)Show SMILES O=C1Nc2cc3[nH]nc(-c4ccncc4)c3cc2CN1Cc1ccccc1 Show InChI InChI=1S/C21H17N5O/c27-21-23-18-11-19-17(20(25-24-19)15-6-8-22-9-7-15)10-16(18)13-26(21)12-14-4-2-1-3-5-14/h1-11H,12-13H2,(H,23,27)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01244
BindingDB Entry DOI: 10.7270/Q2HT2TF2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM351000
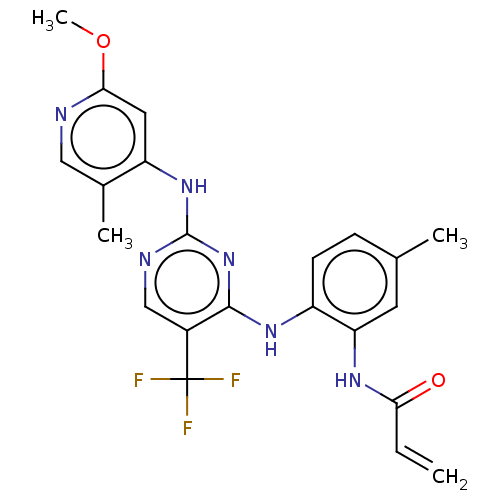
(US9796700, Compound I-90)Show SMILES COc1cc(Nc2ncc(c(Nc3ccc(C)cc3NC(=O)C=C)n2)C(F)(F)F)c(C)cn1 Show InChI InChI=1S/C22H21F3N6O2/c1-5-18(32)28-17-8-12(2)6-7-15(17)29-20-14(22(23,24)25)11-27-21(31-20)30-16-9-19(33-4)26-10-13(16)3/h5-11H,1H2,2-4H3,(H,28,32)(H2,26,27,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01244
BindingDB Entry DOI: 10.7270/Q2HT2TF2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM206343
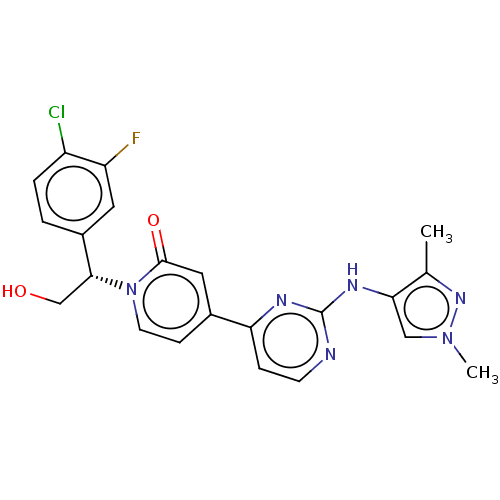
(US9259470, 167)Show SMILES Cc1nn(C)cc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C22H20ClFN6O2/c1-13-19(11-29(2)28-13)27-22-25-7-5-18(26-22)14-6-8-30(21(32)10-14)20(12-31)15-3-4-16(23)17(24)9-15/h3-11,20,31H,12H2,1-2H3,(H,25,26,27)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM206343

(US9259470, 167)Show SMILES Cc1nn(C)cc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C22H20ClFN6O2/c1-13-19(11-29(2)28-13)27-22-25-7-5-18(26-22)14-6-8-30(21(32)10-14)20(12-31)15-3-4-16(23)17(24)9-15/h3-11,20,31H,12H2,1-2H3,(H,25,26,27)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50458806

(CHEMBL4205192)Show SMILES CCNc1nnc(o1)-c1ccc(cc1)C1=CCN(CC1)C(=O)CN1CC[C@@](C1)(SC)C(=O)Nc1ccc2[nH]nc(-c3ccc(F)cc3)c2c1 |r,t:16| Show InChI InChI=1S/C36H37FN8O3S/c1-3-38-35-43-42-33(48-35)26-6-4-23(5-7-26)24-14-17-45(18-15-24)31(46)21-44-19-16-36(22-44,49-2)34(47)39-28-12-13-30-29(20-28)32(41-40-30)25-8-10-27(37)11-9-25/h4-14,20H,3,15-19,21-22H2,1-2H3,(H,38,43)(H,39,47)(H,40,41)/t36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using peptide substrate measured after 45 mins by IMAP assay |
ACS Med Chem Lett 9: 761-767 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00220
BindingDB Entry DOI: 10.7270/Q2HX1G9S |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50166412
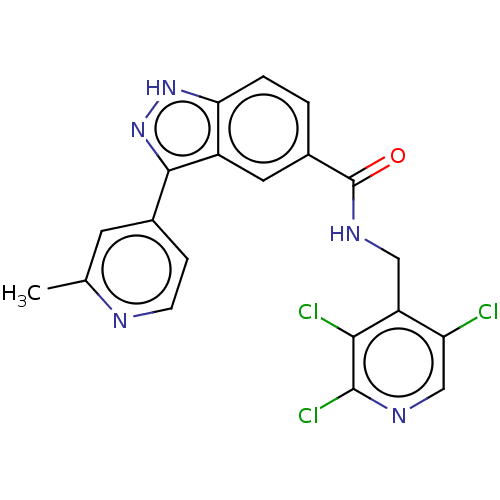
(CHEMBL3797917)Show SMILES Cc1cc(ccn1)-c1n[nH]c2ccc(cc12)C(=O)NCc1c(Cl)cnc(Cl)c1Cl Show InChI InChI=1S/C20H14Cl3N5O/c1-10-6-11(4-5-24-10)18-13-7-12(2-3-16(13)27-28-18)20(29)26-8-14-15(21)9-25-19(23)17(14)22/h2-7,9H,8H2,1H3,(H,26,29)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Valley Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged ERK1 expressed in Escherichia coli using ser/thr 3 Peptide as substrate preincubated for 1 hr measured aft... |
Bioorg Med Chem Lett 26: 2600-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.029
BindingDB Entry DOI: 10.7270/Q2H99731 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM120114
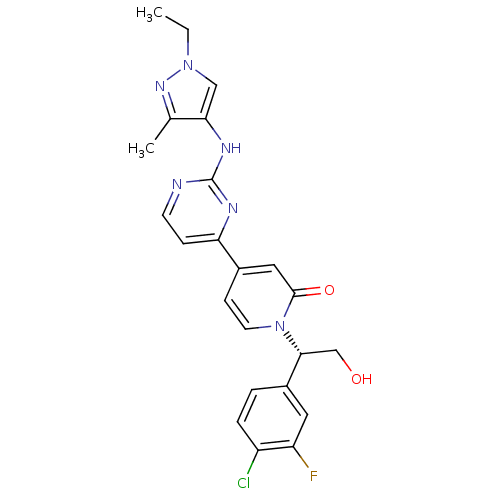
(US8697715, 173 | US9259470, 173)Show SMILES CCn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)c(C)n1 |r| Show InChI InChI=1S/C23H22ClFN6O2/c1-3-30-12-20(14(2)29-30)28-23-26-8-6-19(27-23)15-7-9-31(22(33)11-15)21(13-32)16-4-5-17(24)18(25)10-16/h4-12,21,32H,3,13H2,1-2H3,(H,26,27,28)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM120114

(US8697715, 173 | US9259470, 173)Show SMILES CCn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)c(C)n1 |r| Show InChI InChI=1S/C23H22ClFN6O2/c1-3-30-12-20(14(2)29-30)28-23-26-8-6-19(27-23)15-7-9-31(22(33)11-15)21(13-32)16-4-5-17(24)18(25)10-16/h4-12,21,32H,3,13H2,1-2H3,(H,26,27,28)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532349
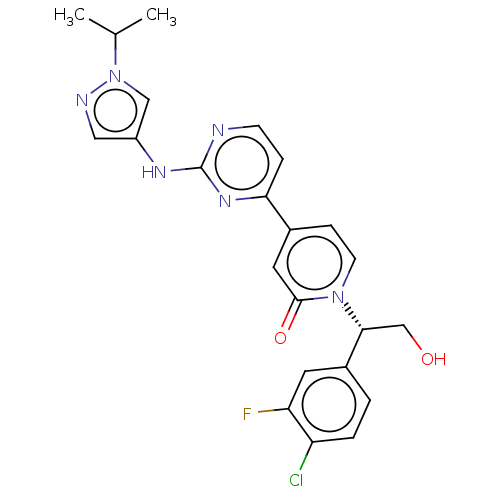
(CHEMBL4474917)Show SMILES CC(C)n1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C23H22ClFN6O2/c1-14(2)31-12-17(11-27-31)28-23-26-7-5-20(29-23)15-6-8-30(22(33)10-15)21(13-32)16-3-4-18(24)19(25)9-16/h3-12,14,21,32H,13H2,1-2H3,(H,26,28,29)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50458805
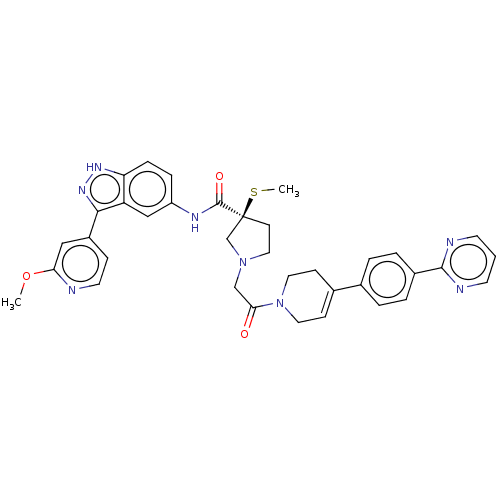
(CHEMBL4206960)Show SMILES COc1cc(ccn1)-c1n[nH]c2ccc(NC(=O)[C@@]3(CCN(CC(=O)N4CCC(=CC4)c4ccc(cc4)-c4ncccn4)C3)SC)cc12 |r,c:29| Show InChI InChI=1S/C36H36N8O3S/c1-47-31-20-27(10-16-37-31)33-29-21-28(8-9-30(29)41-42-33)40-35(46)36(48-2)13-19-43(23-36)22-32(45)44-17-11-25(12-18-44)24-4-6-26(7-5-24)34-38-14-3-15-39-34/h3-11,14-16,20-21H,12-13,17-19,22-23H2,1-2H3,(H,40,46)(H,41,42)/t36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using peptide substrate measured after 45 mins by IMAP assay |
ACS Med Chem Lett 9: 761-767 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00220
BindingDB Entry DOI: 10.7270/Q2HX1G9S |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50166572

(CHEMBL3798624)Show SMILES C[C@H](NC(=O)c1ccc2[nH]nc(-c3ccnc(C)c3)c2c1)c1c(Cl)cccc1Cl |r| Show InChI InChI=1S/C22H18Cl2N4O/c1-12-10-14(8-9-25-12)21-16-11-15(6-7-19(16)27-28-21)22(29)26-13(2)20-17(23)4-3-5-18(20)24/h3-11,13H,1-2H3,(H,26,29)(H,27,28)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Valley Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged ERK1 expressed in Escherichia coli using ser/thr 3 Peptide as substrate preincubated for 1 hr measured aft... |
Bioorg Med Chem Lett 26: 2600-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.029
BindingDB Entry DOI: 10.7270/Q2H99731 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532349

(CHEMBL4474917)Show SMILES CC(C)n1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C23H22ClFN6O2/c1-14(2)31-12-17(11-27-31)28-23-26-7-5-20(29-23)15-6-8-30(22(33)10-15)21(13-32)16-3-4-18(24)19(25)9-16/h3-12,14,21,32H,13H2,1-2H3,(H,26,28,29)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM7266

(14-bromo-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...)Show InChI InChI=1S/C16H11BrN2O/c17-9-5-6-14-11(7-9)12-8-15(20)18-13-4-2-1-3-10(13)16(12)19-14/h1-7,19H,8H2,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency at human bradykinin B2 receptor assessed as effect on inositol monophosphate accumulation in CHOdhfr- cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM120112
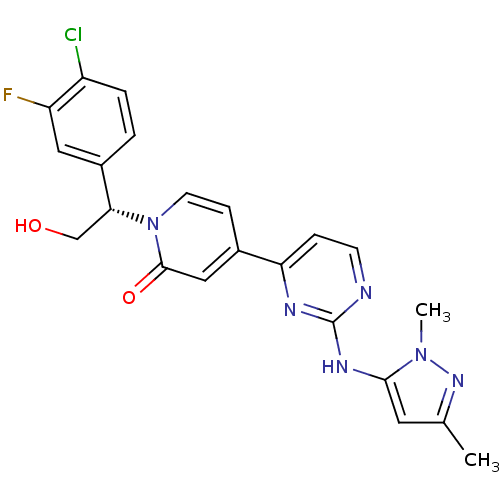
(US8697715, 167)Show SMILES Cc1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)n(C)n1 |r| Show InChI InChI=1S/C22H20ClFN6O2/c1-13-9-20(29(2)28-13)27-22-25-7-5-18(26-22)14-6-8-30(21(32)11-14)19(12-31)15-3-4-16(23)17(24)10-15/h3-11,19,31H,12H2,1-2H3,(H,25,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50458803

(CHEMBL4213970)Show SMILES CS[C@]1(CCN(CC(=O)N2CCC(=CC2)c2ccc(cc2)-c2ncn(C)n2)C1)C(=O)Nc1ccc2[nH]nc(-c3ccc(OC(C)C)nc3)c2c1 |r,c:12| Show InChI InChI=1S/C37H41N9O3S/c1-24(2)49-32-12-9-28(20-38-32)34-30-19-29(10-11-31(30)41-42-34)40-36(48)37(50-4)15-18-45(22-37)21-33(47)46-16-13-26(14-17-46)25-5-7-27(8-6-25)35-39-23-44(3)43-35/h5-13,19-20,23-24H,14-18,21-22H2,1-4H3,(H,40,48)(H,41,42)/t37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using peptide substrate measured after 45 mins by IMAP assay |
ACS Med Chem Lett 9: 761-767 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00220
BindingDB Entry DOI: 10.7270/Q2HX1G9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50088859
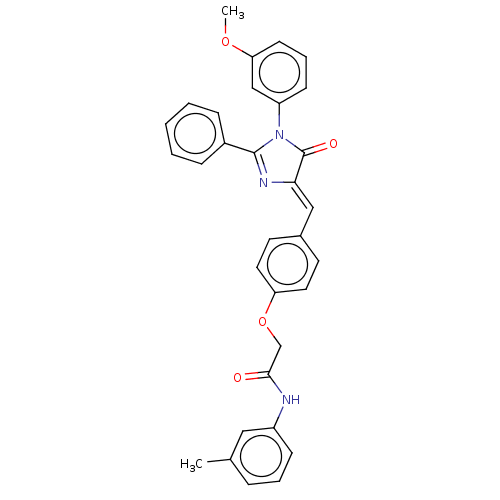
(CHEMBL3577565)Show SMILES COc1cccc(c1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3cccc(C)c3)cc2)N=C1c1ccccc1 |c:34| Show InChI InChI=1S/C32H27N3O4/c1-22-8-6-11-25(18-22)33-30(36)21-39-27-16-14-23(15-17-27)19-29-32(37)35(26-12-7-13-28(20-26)38-2)31(34-29)24-9-4-3-5-10-24/h3-20H,21H2,1-2H3,(H,33,36)/b29-19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM120112

(US8697715, 167)Show SMILES Cc1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)n(C)n1 |r| Show InChI InChI=1S/C22H20ClFN6O2/c1-13-9-20(29(2)28-13)27-22-25-7-5-18(26-22)14-6-8-30(21(32)11-14)19(12-31)15-3-4-16(23)17(24)10-15/h3-11,19,31H,12H2,1-2H3,(H,25,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50089077
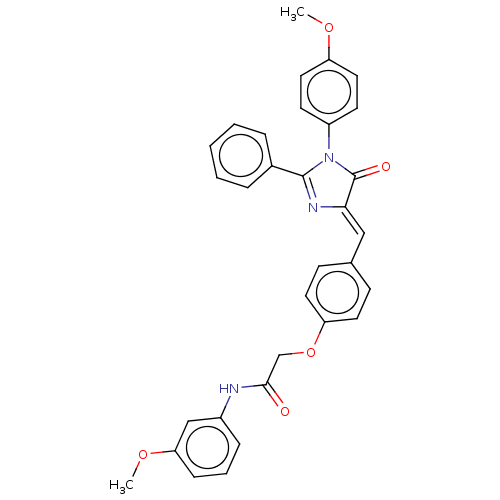
(CHEMBL3577641)Show SMILES COc1ccc(cc1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3cccc(OC)c3)cc2)N=C1c1ccccc1 |c:35| Show InChI InChI=1S/C32H27N3O5/c1-38-26-17-13-25(14-18-26)35-31(23-7-4-3-5-8-23)34-29(32(35)37)19-22-11-15-27(16-12-22)40-21-30(36)33-24-9-6-10-28(20-24)39-2/h3-20H,21H2,1-2H3,(H,33,36)/b29-19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50531646

(CHEMBL4476178)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3CN(Cc4cccc(Cl)c4)C(=O)c3c2)cn1 Show InChI InChI=1S/C23H19ClN6O/c1-29-14-19(11-26-29)27-23-25-8-7-21(28-23)16-5-6-17-13-30(22(31)20(17)10-16)12-15-3-2-4-18(24)9-15/h2-11,14H,12-13H2,1H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using lipid as substrate after 40 mins by ADP-Glo luminescence assay |
Eur J Med Chem 164: 334-341 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.040
BindingDB Entry DOI: 10.7270/Q2ZS310S |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50166413

(CHEMBL3799117)Show SMILES CC(NC(=O)c1ccc2[nH]nc(-c3ccnc(C)c3)c2c1)c1c(Cl)cnc(Cl)c1Cl Show InChI InChI=1S/C21H16Cl3N5O/c1-10-7-12(5-6-25-10)19-14-8-13(3-4-16(14)28-29-19)21(30)27-11(2)17-15(22)9-26-20(24)18(17)23/h3-9,11H,1-2H3,(H,27,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Valley Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged ERK1 expressed in Escherichia coli using ser/thr 3 Peptide as substrate preincubated for 1 hr measured aft... |
Bioorg Med Chem Lett 26: 2600-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.029
BindingDB Entry DOI: 10.7270/Q2H99731 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM7262
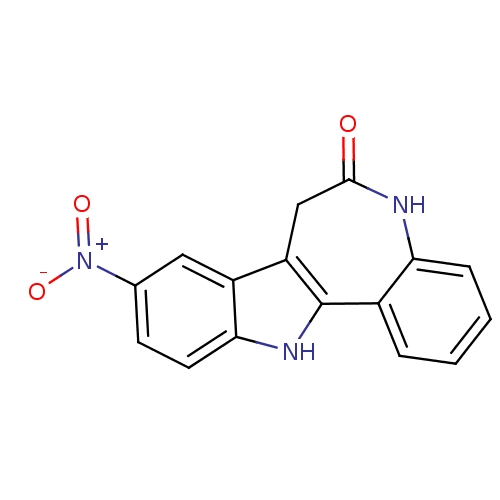
(14-nitro-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...)Show SMILES [O-][N+](=O)c1ccc2[nH]c-3c(CC(=O)Nc4ccccc-34)c2c1 Show InChI InChI=1S/C16H11N3O3/c20-15-8-12-11-7-9(19(21)22)5-6-14(11)18-16(12)10-3-1-2-4-13(10)17-15/h1-7,18H,8H2,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency at human bradykinin B2 receptor assessed as effect on inositol monophosphate accumulation in CHOdhfr- cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50089076

(CHEMBL3577642)Show SMILES COc1ccc(NC(=O)COc2ccc(\C=C3/N=C(N(C3=O)c3ccc(OC)cc3)c3ccccc3)cc2)cc1 |c:17| Show InChI InChI=1S/C32H27N3O5/c1-38-26-16-10-24(11-17-26)33-30(36)21-40-28-14-8-22(9-15-28)20-29-32(37)35(25-12-18-27(39-2)19-13-25)31(34-29)23-6-4-3-5-7-23/h3-20H,21H2,1-2H3,(H,33,36)/b29-20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50089082
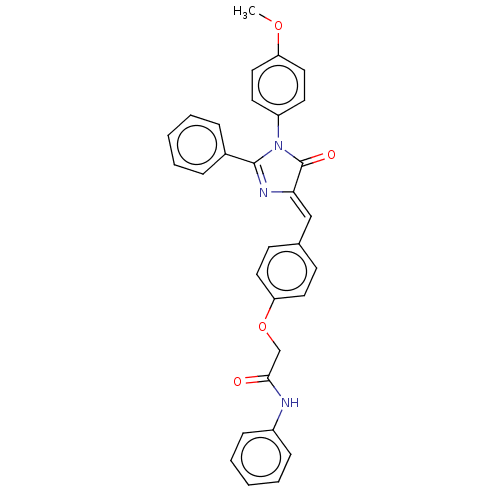
(CHEMBL3577636)Show SMILES COc1ccc(cc1)N1C(=O)\C(=C\c2ccc(OCC(=O)Nc3ccccc3)cc2)N=C1c1ccccc1 |c:33| Show InChI InChI=1S/C31H25N3O4/c1-37-26-18-14-25(15-19-26)34-30(23-8-4-2-5-9-23)33-28(31(34)36)20-22-12-16-27(17-13-22)38-21-29(35)32-24-10-6-3-7-11-24/h2-20H,21H2,1H3,(H,32,35)/b28-20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) using GFP-ATF2 as substrate after 1 hr by TR-FRET assay |
Eur J Med Chem 94: 397-404 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.008
BindingDB Entry DOI: 10.7270/Q2BZ67SV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data