

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM620080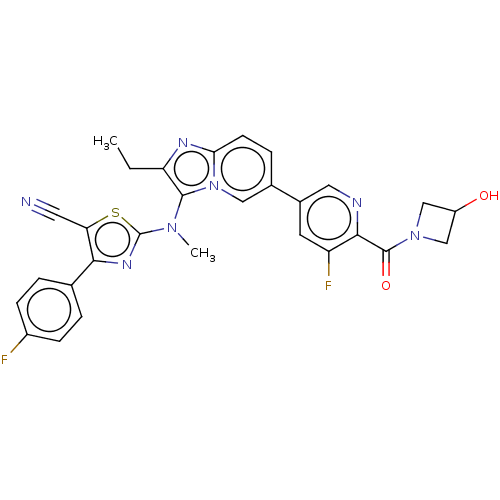 (2-((2-ethyl-6-(5-fluoro-6-(3-hydroxyazetidin-1-car...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50187686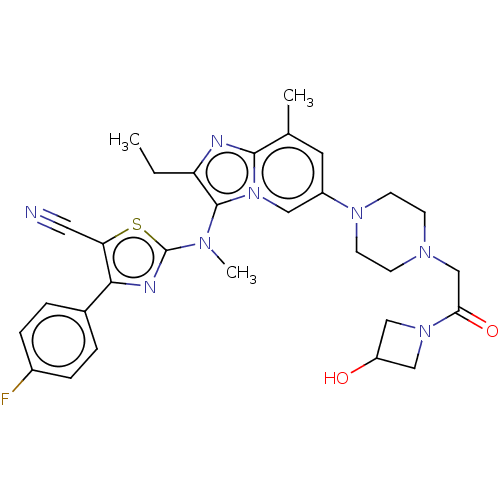 (CHEMBL3828074 | US10526329, Compound 2 | US1107261...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Inhibition of human ATX using FS3 as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by fluorescence assay | J Med Chem 59: 5604-21 (2016) Article DOI: 10.1021/acs.jmedchem.5b01599 BindingDB Entry DOI: 10.7270/Q2BZ681D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50596095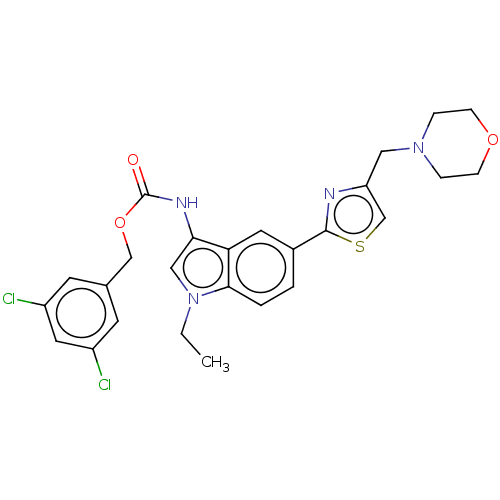 (CHEMBL5183599) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113951 BindingDB Entry DOI: 10.7270/Q2B85D5K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50540865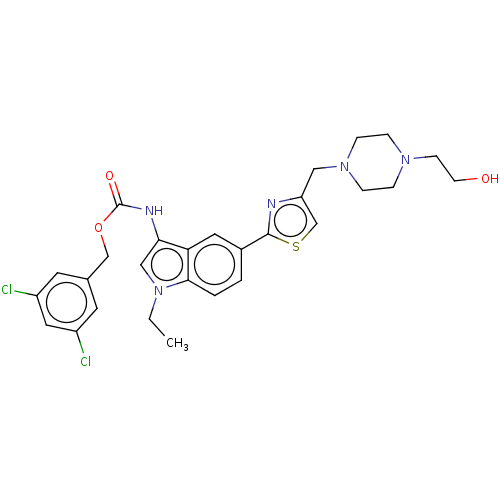 (CHEMBL4640012) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human ATX pre-incubated for 45 mins before fluorogenic substrate-3 addition and measured every minute for 30 mins by fluorescence based... | J Med Chem 63: 7326-7346 (2020) Article DOI: 10.1021/acs.jmedchem.0c00506 BindingDB Entry DOI: 10.7270/Q2GM8BW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM303716 ((3S)-N-[5-[2-(4- chlorophenyyl) ethyl]isoxazol- 3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description Measuring ATX activity using an enzyme coupled Quanta Red assay (Thermo Scientific Pierce Protein Research Products, Product #15159) was determined a... | US Patent US10138230 (2018) BindingDB Entry DOI: 10.7270/Q27S7QV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450552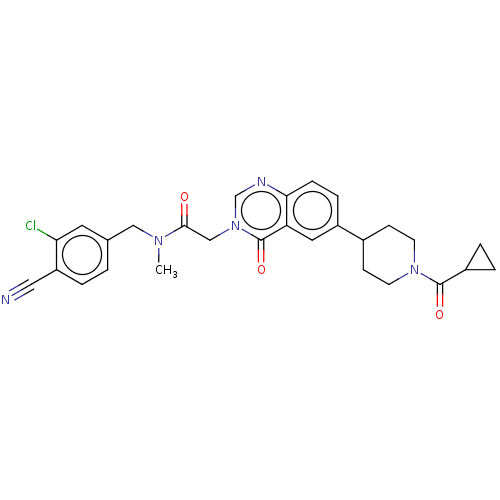 (N-[(3-Chloro-4- cyanophenyl)methyl]-N- methyl-2-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM303780 ((3S)-N-[5-[2-(4- chlorophenyl)ethyl]- 1,3,4-thiadi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description Measuring ATX activity using an enzyme coupled Quanta Red assay (Thermo Scientific Pierce Protein Research Products, Product #15159) was determined a... | US Patent US10138230 (2018) BindingDB Entry DOI: 10.7270/Q27S7QV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM303657 ((3S)-N-[5-[2-(4- chlorophenyl)ethyl]- 1,3,4-thiadi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description Measuring ATX activity using an enzyme coupled Quanta Red assay (Thermo Scientific Pierce Protein Research Products, Product #15159) was determined a... | US Patent US10138230 (2018) BindingDB Entry DOI: 10.7270/Q27S7QV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM303658 ((3S)-N-[5-[2-(4- chlorophenyl)ethyl]- 1,3,4-thiadi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description Measuring ATX activity using an enzyme coupled Quanta Red assay (Thermo Scientific Pierce Protein Research Products, Product #15159) was determined a... | US Patent US10138230 (2018) BindingDB Entry DOI: 10.7270/Q27S7QV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM303659 ((3S)-N-[5-[2-(4- chlorophenyl)ethyl]- 1,3,4-thiadi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description Measuring ATX activity using an enzyme coupled Quanta Red assay (Thermo Scientific Pierce Protein Research Products, Product #15159) was determined a... | US Patent US10138230 (2018) BindingDB Entry DOI: 10.7270/Q27S7QV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM303660 ((3S)-N-[5-[2-(4- chlorophenyl)ethyl]- 1,3,4-thiadi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description Measuring ATX activity using an enzyme coupled Quanta Red assay (Thermo Scientific Pierce Protein Research Products, Product #15159) was determined a... | US Patent US10138230 (2018) BindingDB Entry DOI: 10.7270/Q27S7QV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM303661 ((3S)-1-[4-(azepan-1- ylmethyl)phenyl]- N-[5-[2- (4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description Measuring ATX activity using an enzyme coupled Quanta Red assay (Thermo Scientific Pierce Protein Research Products, Product #15159) was determined a... | US Patent US10138230 (2018) BindingDB Entry DOI: 10.7270/Q27S7QV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM303662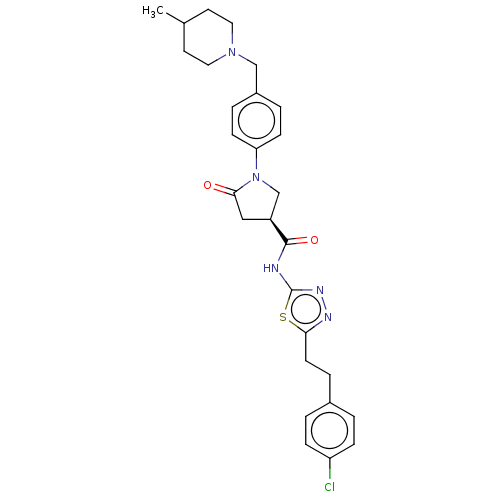 ((3S)-N-[5-[2-(4- chlorophenyl)ethyl]- 1,3,4-thiadi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description Measuring ATX activity using an enzyme coupled Quanta Red assay (Thermo Scientific Pierce Protein Research Products, Product #15159) was determined a... | US Patent US10138230 (2018) BindingDB Entry DOI: 10.7270/Q27S7QV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM303663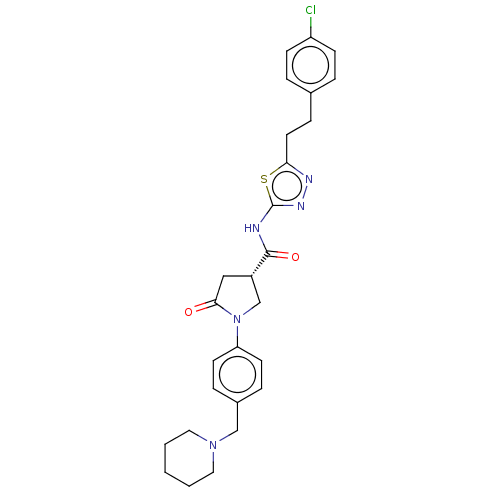 ((3S)-N-[5-[2-(4- chlorophenyl)ethyl]- 1,3,4-thiadi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description Measuring ATX activity using an enzyme coupled Quanta Red assay (Thermo Scientific Pierce Protein Research Products, Product #15159) was determined a... | US Patent US10138230 (2018) BindingDB Entry DOI: 10.7270/Q27S7QV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM303664 ((3S)-N-[5-[2-(4- chlorophenyl)ethyl]- 1,3,4-thiadi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description Measuring ATX activity using an enzyme coupled Quanta Red assay (Thermo Scientific Pierce Protein Research Products, Product #15159) was determined a... | US Patent US10138230 (2018) BindingDB Entry DOI: 10.7270/Q27S7QV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM303698 (5-[2-(4- chlorophenyl)ethyl]-N-[1- [4-(morpholino-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description Measuring ATX activity using an enzyme coupled Quanta Red assay (Thermo Scientific Pierce Protein Research Products, Product #15159) was determined a... | US Patent US10138230 (2018) BindingDB Entry DOI: 10.7270/Q27S7QV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50187688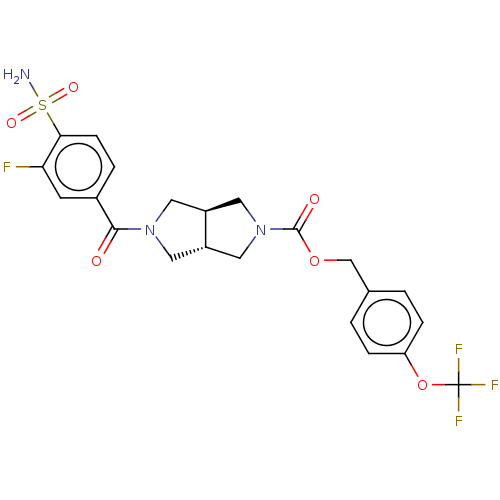 (CHEMBL3828650 | US10913745, Example 1.11) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged ATX expressed in HEK293E cells using lysophosphatidylcholine as substrate preincubated with enz... | J Med Chem 59: 5604-21 (2016) Article DOI: 10.1021/acs.jmedchem.5b01599 BindingDB Entry DOI: 10.7270/Q2BZ681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50200622 (CHEMBL3917975) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of human recombinant ATX using Rac-1-Palmitoyl-glycero-3-phosphocholine as substrate incubated for 2 hrs using ADHP fluorogenic peroxidase... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258501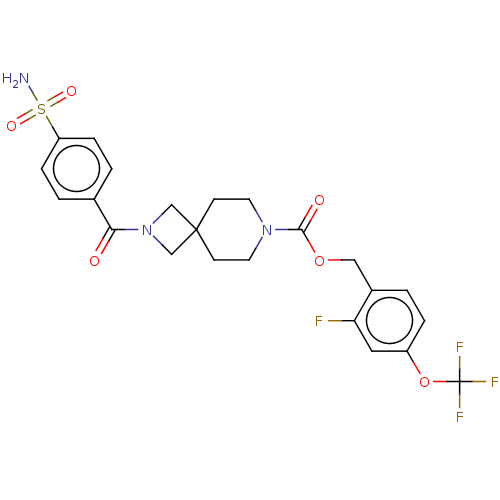 (US10633384, Example 1.23 | US9493486, 1.23) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258574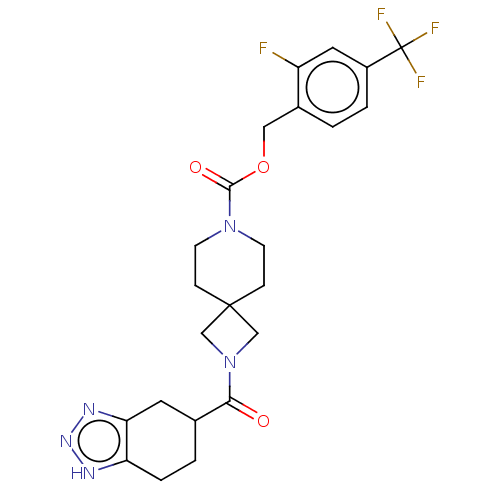 (US10633384, Example 12.36 | US9493486, 12.36) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258575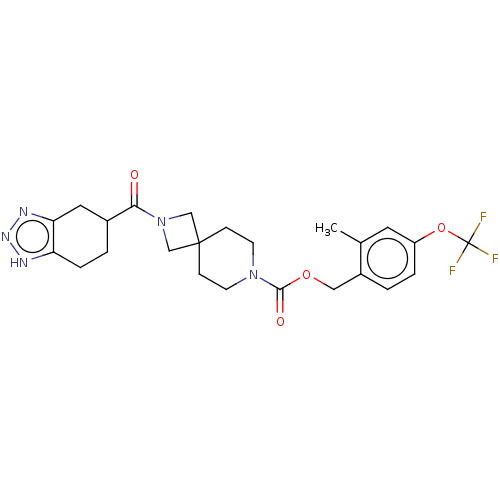 (US10633384, Example 12.37 | US9493486, 12.37) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258580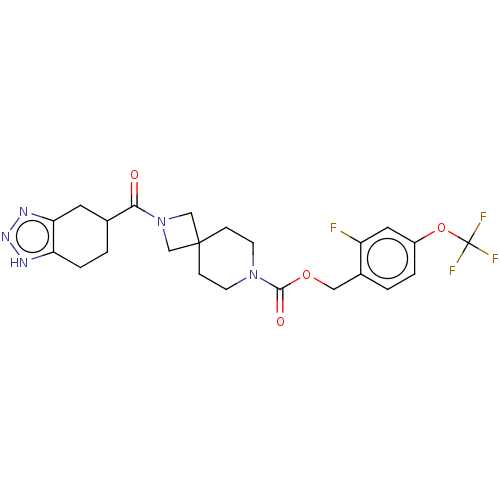 (US10633384, Example 12.42 | US9493486, 12.42) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258581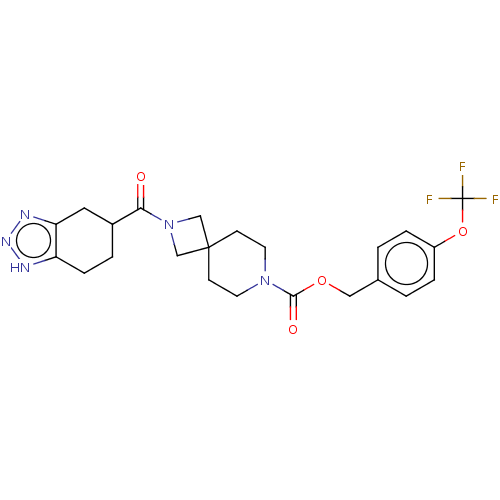 (US10633384, Example 12.43 | US9493486, 12.43) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258585 (US10633384, 13.01 | US9493486, 13.01) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM258590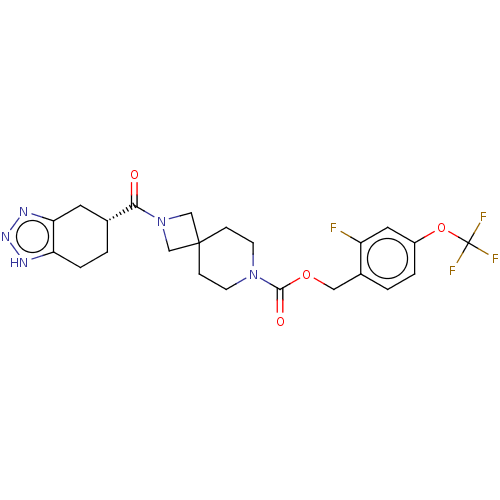 (US9493486, 15B) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Hoffmann-La Roches Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US9493486 (2016) BindingDB Entry DOI: 10.7270/Q2VT1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556734 (US11352330, Example D50) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556656 (US11352330, Example B88) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556661 (US11352330, Example B92) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556672 (US11352330, Example C5) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556687 (US11352330, Example D5) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556694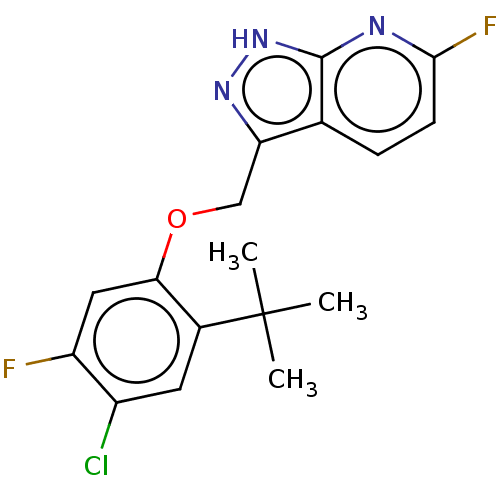 (US11352330, Example D12) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50540872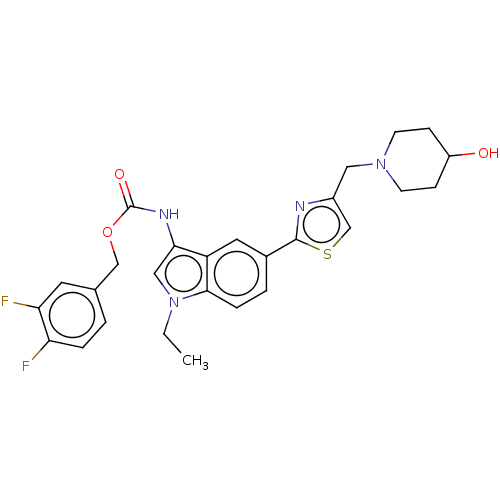 (CHEMBL4647222) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ATX using FS-3 as substrate preincubated for 45 mins followed by substrate addition and measured every 1 min for 30 mins by fluor... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116362 BindingDB Entry DOI: 10.7270/Q2XK8K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50572947 (CHEMBL4871014) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ATX assessed as reduction in LPA formation using FS-3 as substrate preincubated for 30 mins followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00211 BindingDB Entry DOI: 10.7270/Q2Q81HVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556539 (US11352330, Example A12) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556564 (US11352330, Example B2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM556565 (US11352330, Example B3) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM477058 (US10882857, Example 2.06 | US11673888, Example 2.0...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10882857 (2021) BindingDB Entry DOI: 10.7270/Q2SQ93GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM477062 (US10882857, Example 4 | US11673888, Example 4 | [3...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10882857 (2021) BindingDB Entry DOI: 10.7270/Q2SQ93GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM477064 (US10882857, Example 4.02 | US11673888, Example 4.0...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0;ATX solution: ATX (human His-tagged) stock so... | US Patent US10882857 (2021) BindingDB Entry DOI: 10.7270/Q2SQ93GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50540872 (CHEMBL4647222) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human ATX pre-incubated for 45 mins before fluorogenic substrate-3 addition and measured every minute for 30 mins by fluorescence based... | J Med Chem 63: 7326-7346 (2020) Article DOI: 10.1021/acs.jmedchem.0c00506 BindingDB Entry DOI: 10.7270/Q2GM8BW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450562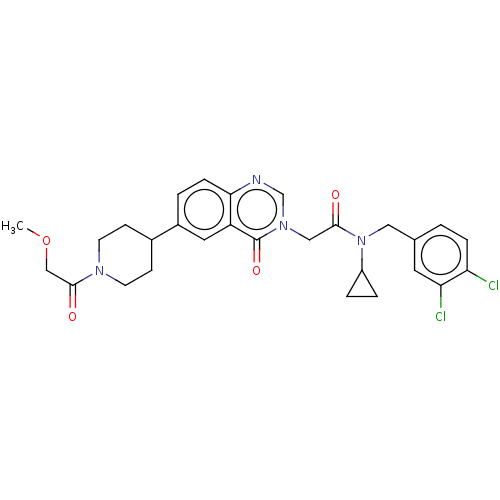 (N-Cyclopropyl-N-[(3,4-dichlorophenyl)methyl]-2-[6-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450575 (N-[(3-Chloro-4- cyanophenyl)methyl]-N- methyl-2-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450579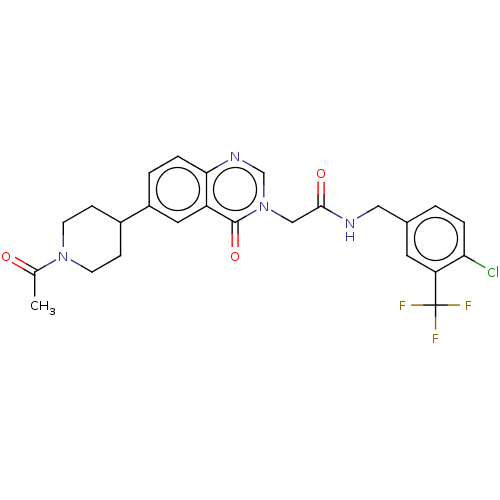 (2-[6-(1-tert-Butoxycarbonyl-4- piperidyl)-4-oxoqui...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450603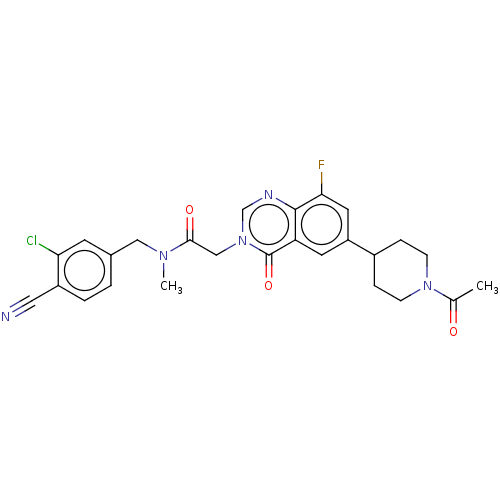 (2-[6-(1-Acetylpiperidin-4-yl)-8-fluoro-4-oxoquinaz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450604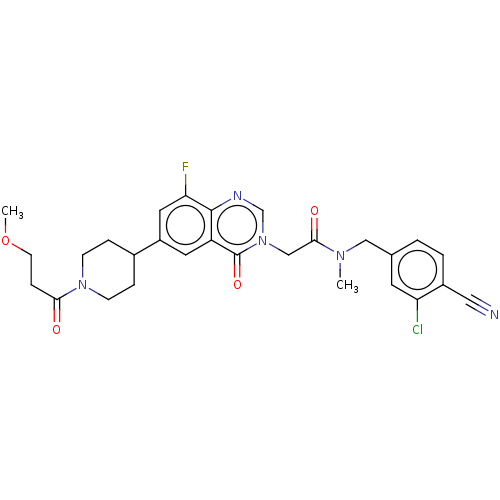 (N-[(3-Chloro-4-cyanophenyl)methyl]-2-[8-fluoro-6-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450605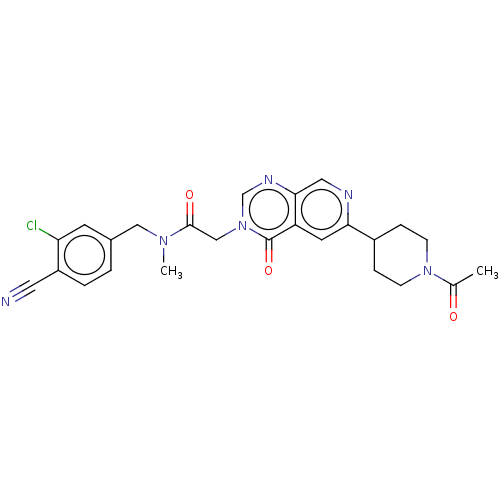 (2-[6-(1-Acetylpiperidin-4-yl)-4-oxopyrido[3,4-d]py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450606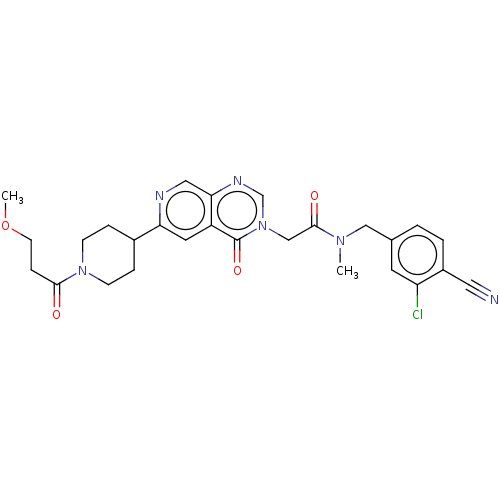 (N-[(3-Chloro-4-cyanophenyl)methyl]-2-[6-[1-(3-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450546 (N-[(3-Chloro-4-cyanophenyl)methyl]-2-[6-[1-(2-hydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450548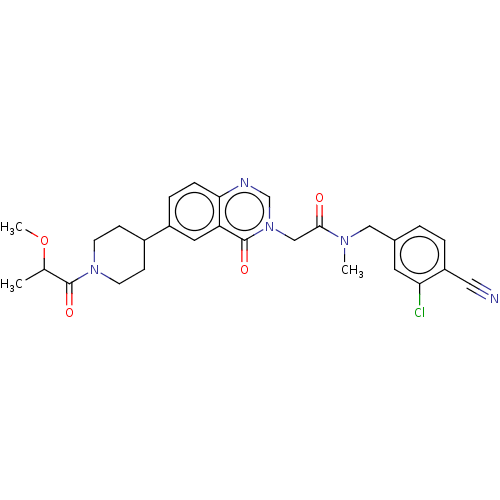 (N-[(3-Chloro-4- cyanophenyl)methyl]-N- methyl-2-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450549 (N-[(3-Chloro-4- cyanophenyl)methyl]-N- methyl-2-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3910 total ) | Next | Last >> |