 Found 11 hits of ki data for polymerid = 7572
Found 11 hits of ki data for polymerid = 7572 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50004774
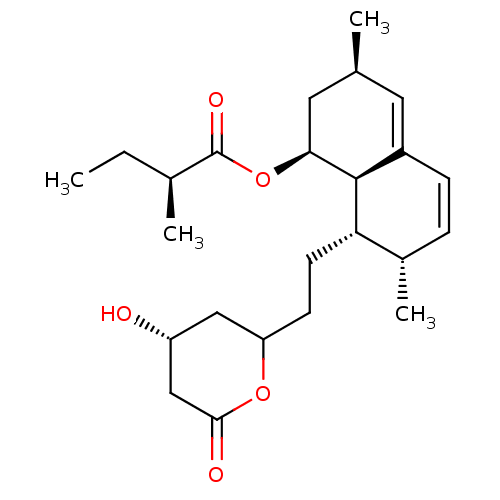
((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human HMGCoA reductase |
J Nat Prod 52: 153-161 (1989)
Article DOI: 10.1021/np50061a020
BindingDB Entry DOI: 10.7270/Q28052MW |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM18372
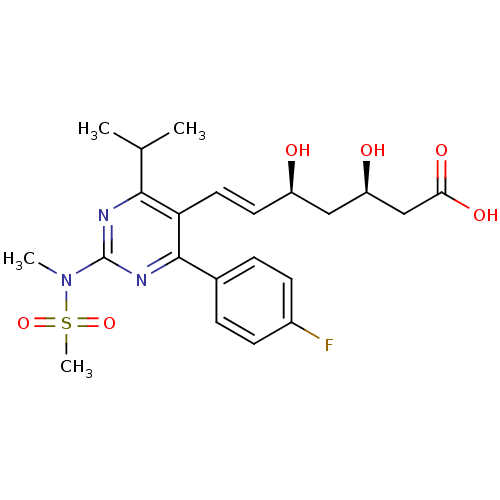
((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50279858
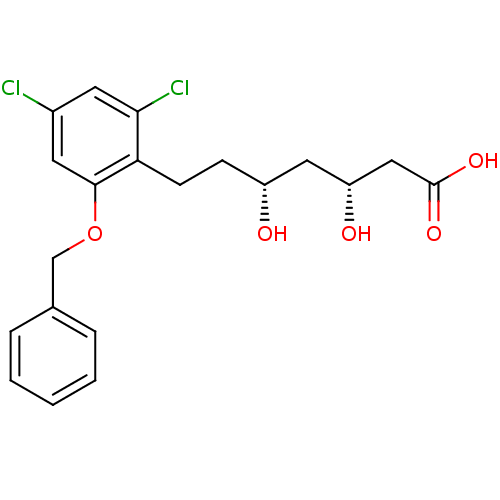
((3R,5R)-7-(2-Benzyloxy-4,6-dichloro-phenyl)-3,5-di...)Show SMILES O[C@H](CCc1c(Cl)cc(Cl)cc1OCc1ccccc1)C[C@@H](O)CC(O)=O Show InChI InChI=1S/C20H22Cl2O5/c21-14-8-18(22)17(7-6-15(23)10-16(24)11-20(25)26)19(9-14)27-12-13-4-2-1-3-5-13/h1-5,8-9,15-16,23-24H,6-7,10-12H2,(H,25,26)/t15-,16-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The inhibitory activity of the compound against purified recombinant human HMG-CoA reductase was evaluated |
Bioorg Med Chem Lett 1: 151-154 (1991)
Article DOI: 10.1016/S0960-894X(01)80788-5
BindingDB Entry DOI: 10.7270/Q21836DP |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM22164
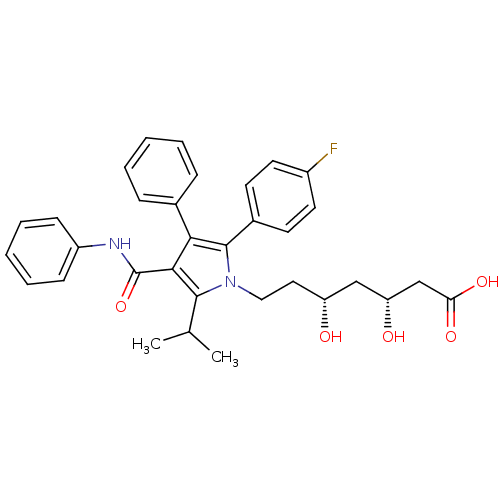
((3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylca...)Show SMILES CC(C)c1c(C(=O)Nc2ccccc2)c(c(-c2ccc(F)cc2)n1CC[C@@H](O)C[C@@H](O)CC(O)=O)-c1ccccc1 |r| Show InChI InChI=1S/C33H35FN2O5/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40)/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA Reductase (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50279859
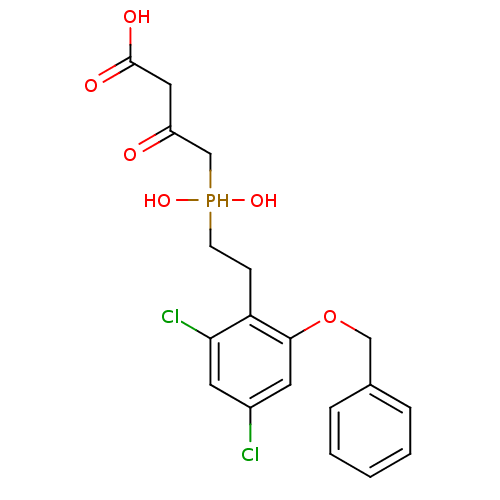
((S)-4-{[2-(2-Benzyloxy-4,6-dichloro-phenyl)-ethyl]...)Show SMILES OC(=O)CC(=O)CP(O)(O)CCc1c(Cl)cc(Cl)cc1OCc1ccccc1 Show InChI InChI=1S/C19H21Cl2O6P/c20-14-8-17(21)16(6-7-28(25,26)12-15(22)10-19(23)24)18(9-14)27-11-13-4-2-1-3-5-13/h1-5,8-9,25-26,28H,6-7,10-12H2,(H,23,24) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The inhibitory activity of the compound against purified recombinant human HMG-CoA reductase was evaluated |
Bioorg Med Chem Lett 1: 151-154 (1991)
Article DOI: 10.1016/S0960-894X(01)80788-5
BindingDB Entry DOI: 10.7270/Q21836DP |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50160720
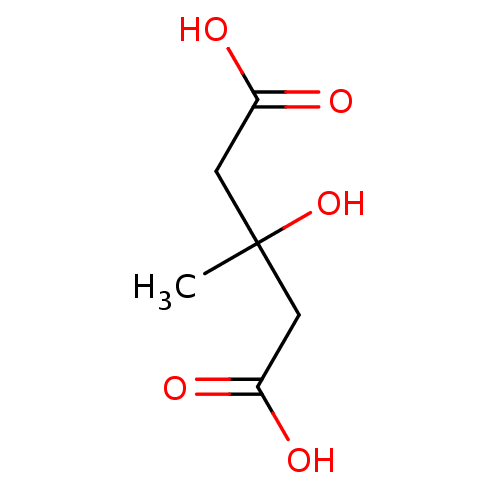
(3-hydroxy-3-methylglutaric acid | 3-hydroxy-3-meth...)Show InChI InChI=1S/C6H10O5/c1-6(11,2-4(7)8)3-5(9)10/h11H,2-3H2,1H3,(H,7,8)(H,9,10) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 23.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321104
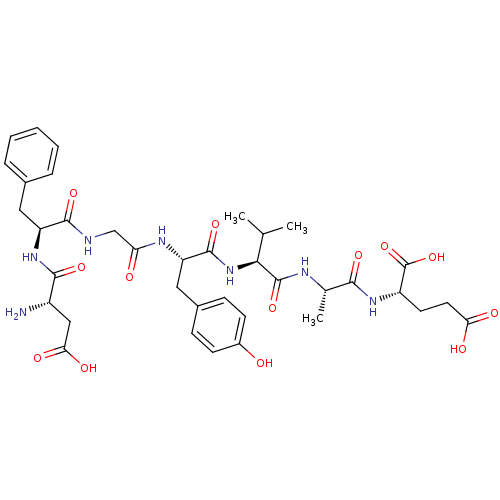
(CHEMBL1163804 | DFGYVAE)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C37H49N7O13/c1-19(2)31(36(55)40-20(3)32(51)42-25(37(56)57)13-14-29(47)48)44-35(54)27(16-22-9-11-23(45)12-10-22)41-28(46)18-39-34(53)26(15-21-7-5-4-6-8-21)43-33(52)24(38)17-30(49)50/h4-12,19-20,24-27,31,45H,13-18,38H2,1-3H3,(H,39,53)(H,40,55)(H,41,46)(H,42,51)(H,43,52)(H,44,54)(H,47,48)(H,49,50)(H,56,57)/t20-,24-,25-,26-,27-,31-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Competitive inhibition of HMG-CoA reductase by Dixon plot analysis |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50321100
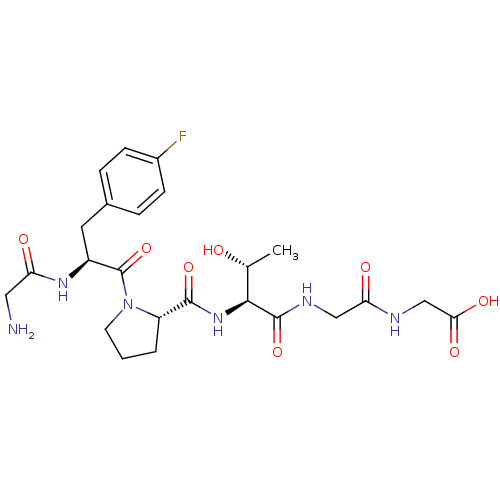
(CHEMBL1164546 | GF(4-fluro)PTGG)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)CN)C(=O)NCC(=O)NCC(O)=O |r| Show InChI InChI=1S/C24H33FN6O8/c1-13(32)21(23(38)28-11-19(34)27-12-20(35)36)30-22(37)17-3-2-8-31(17)24(39)16(29-18(33)10-26)9-14-4-6-15(25)7-5-14/h4-7,13,16-17,21,32H,2-3,8-12,26H2,1H3,(H,27,34)(H,28,38)(H,29,33)(H,30,37)(H,35,36)/t13-,16+,17+,21+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of the Chemistry of Plant Substances
Curated by ChEMBL
| Assay Description
Competitive inhibition of HMG-CoA reductase by Dixon plot analysis |
Bioorg Med Chem 18: 4300-9 (2010)
Article DOI: 10.1016/j.bmc.2010.04.090
BindingDB Entry DOI: 10.7270/Q2VM4CFG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase with alpha asarone |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM18372

((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase with alpha asarone |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data