

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 3A5 | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50318484 (2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human recombinant CYP3A5 expressed in baculovirus-infected insect cells using diltiazem as substrate incubated for 15 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00724 BindingDB Entry DOI: 10.7270/Q2697778 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 3A5 measured by testosterone hydroxylation | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50061306 ((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 3A5 measured by testosterone hydroxylation | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 829 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A5 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 855 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A5 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50088503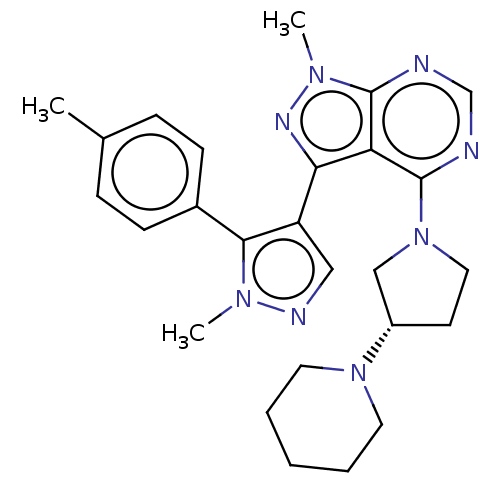 (CHEMBL3527048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of testosterone 6beta-hydroxylase activity of human recombinant CYP3A5 in presence of human P450 oxidoreductase and b5 assessed as decreas... | Drug Metab Dispos 40: 1686-97 (2012) Article DOI: 10.1124/dmd.112.045302 BindingDB Entry DOI: 10.7270/Q2GT5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50088503 (CHEMBL3527048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A5 harboring human P450 oxidoreductase and b5 assessed as decrease in enzyme... | Drug Metab Dispos 40: 1686-97 (2012) Article DOI: 10.1124/dmd.112.045302 BindingDB Entry DOI: 10.7270/Q2GT5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50030448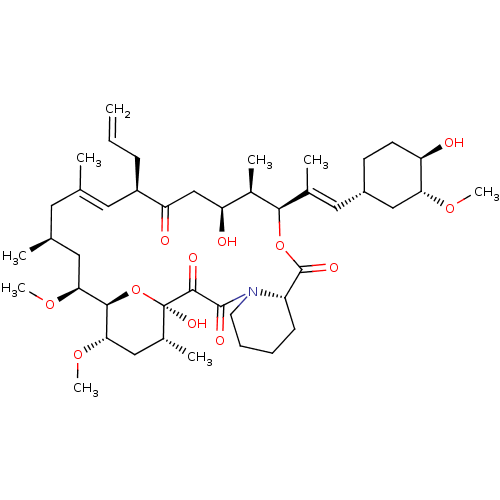 (8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo Curated by ChEMBL | Assay Description Reversible competitive inhibition of human CYP3A5-mediated 1'-OH midazolam formation in human liver microsomes after 7.5 mins by nonlinear regression... | Drug Metab Dispos 40: 655-61 (2012) Article DOI: 10.1124/dmd.111.043018 BindingDB Entry DOI: 10.7270/Q22Z1796 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50121977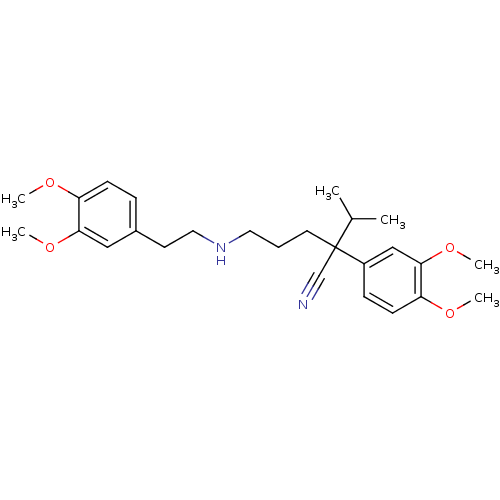 (2-(3,4-Dimethoxy-phenyl)-5-[2-(3,4-dimethoxy-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 4.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 3A5 measured by testosterone hydroxylation | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50310823 (CHEMBL1078442 | bergamottin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 3A5 | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM5445 (CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A5 using testosterone as substrate assessed as testosterone 6-beta-hydroxylation preincubated up to 30 mins with ... | Drug Metab Dispos 40: 1414-22 (2012) Article DOI: 10.1124/dmd.112.044958 BindingDB Entry DOI: 10.7270/Q2SB47HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||