 Found 5645 hits of ic50 for UniProtKB: P21802
Found 5645 hits of ic50 for UniProtKB: P21802 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50166749
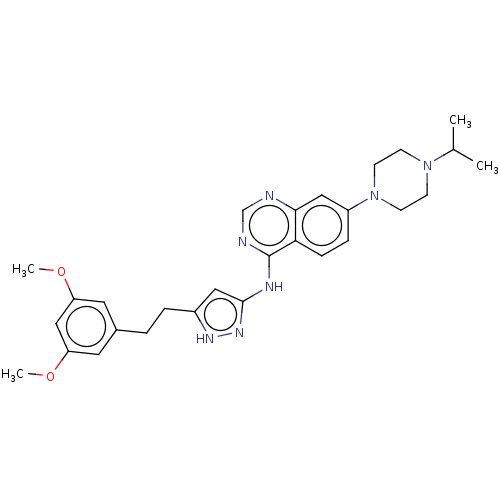
(CHEMBL3799403)Show SMILES COc1cc(CCc2cc(Nc3ncnc4cc(ccc34)N3CCN(CC3)C(C)C)n[nH]2)cc(OC)c1 Show InChI InChI=1S/C28H35N7O2/c1-19(2)34-9-11-35(12-10-34)22-7-8-25-26(16-22)29-18-30-28(25)31-27-15-21(32-33-27)6-5-20-13-23(36-3)17-24(14-20)37-4/h7-8,13-19H,5-6,9-12H2,1-4H3,(H2,29,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR2 in human SNU16 cells assessed as suppression of cell proliferation after 72 hrs by SRB/CCK-8 assay |
Bioorg Med Chem Lett 26: 2594-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.028
BindingDB Entry DOI: 10.7270/Q20C4XNM |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50166744
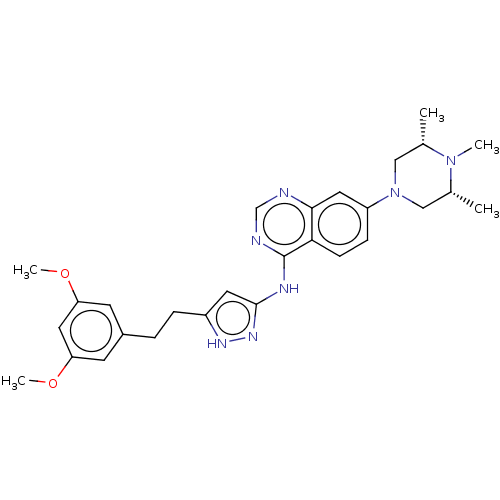
(CHEMBL3798625)Show SMILES COc1cc(CCc2cc(Nc3ncnc4cc(ccc34)N3C[C@H](C)N(C)[C@H](C)C3)n[nH]2)cc(OC)c1 |r| Show InChI InChI=1S/C28H35N7O2/c1-18-15-35(16-19(2)34(18)3)22-8-9-25-26(13-22)29-17-30-28(25)31-27-12-21(32-33-27)7-6-20-10-23(36-4)14-24(11-20)37-5/h8-14,17-19H,6-7,15-16H2,1-5H3,(H2,29,30,31,32,33)/t18-,19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR2 in human SNU16 cells assessed as suppression of cell proliferation after 72 hrs by SRB/CCK-8 assay |
Bioorg Med Chem Lett 26: 2594-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.028
BindingDB Entry DOI: 10.7270/Q20C4XNM |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50166748
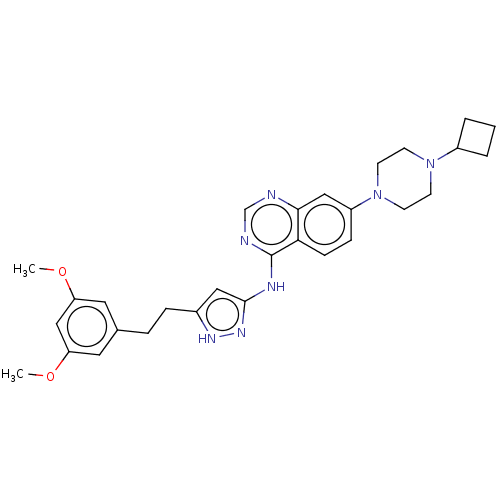
(CHEMBL3799426)Show SMILES COc1cc(CCc2cc(Nc3ncnc4cc(ccc34)N3CCN(CC3)C3CCC3)n[nH]2)cc(OC)c1 Show InChI InChI=1S/C29H35N7O2/c1-37-24-14-20(15-25(18-24)38-2)6-7-21-16-28(34-33-21)32-29-26-9-8-23(17-27(26)30-19-31-29)36-12-10-35(11-13-36)22-4-3-5-22/h8-9,14-19,22H,3-7,10-13H2,1-2H3,(H2,30,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR2 in human SNU16 cells assessed as suppression of cell proliferation after 72 hrs by SRB/CCK-8 assay |
Bioorg Med Chem Lett 26: 2594-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.028
BindingDB Entry DOI: 10.7270/Q20C4XNM |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50587623
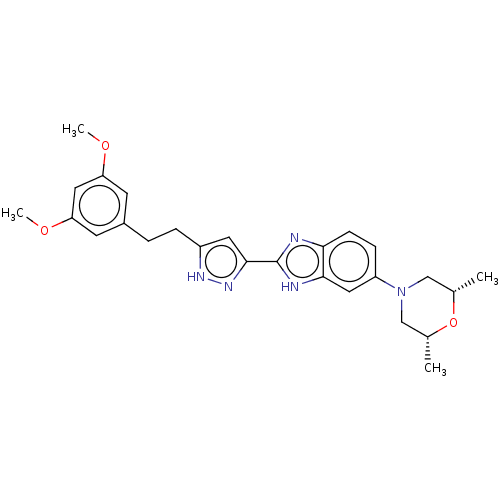
(CHEMBL5173892)Show SMILES COc1cc(CCc2cc(n[nH]2)-c2nc3ccc(cc3[nH]2)N2C[C@H](C)O[C@H](C)C2)cc(OC)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112990
BindingDB Entry DOI: 10.7270/Q2WD44HQ |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM339807
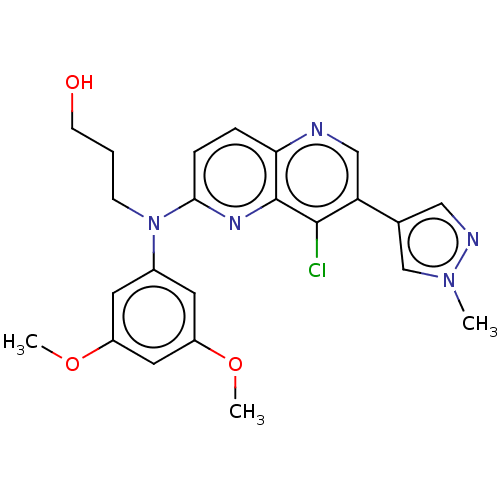
(US9757364, Compound 11)Show SMILES COc1cc(OC)cc(c1)N(CCCO)c1ccc2ncc(-c3cnn(C)c3)c(Cl)c2n1 Show InChI InChI=1S/C23H24ClN5O3/c1-28-14-15(12-26-28)19-13-25-20-5-6-21(27-23(20)22(19)24)29(7-4-8-30)16-9-17(31-2)11-18(10-16)32-3/h5-6,9-14,30H,4,7-8H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LTD
US Patent
| Assay Description
In a final reaction volume of 30 μL, FGFR2 (h) (150 ng/ml) was incubated with 50 mM HEPES pH 7.5, 6 mM MnCl2, 1 mM DTT, 0.1 mM Na3VO4, 0.01% Tri... |
US Patent US9757364 (2017)
BindingDB Entry DOI: 10.7270/Q2SB47VT |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM301217
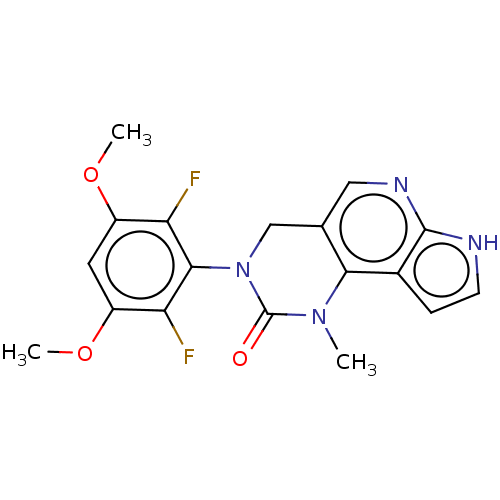
(3-(2,6-Difluoro-3,5-dimethoxyphenyl)-1-methyl-1,3,...)Show SMILES COc1cc(OC)c(F)c(N2Cc3cnc4[nH]ccc4c3N(C)C2=O)c1F Show InChI InChI=1S/C18H16F2N4O3/c1-23-15-9(7-22-17-10(15)4-5-21-17)8-24(18(23)25)16-13(19)11(26-2)6-12(27-3)14(16)20/h4-7H,8H2,1-3H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FGFR2 using biotinylated-EQEDEPEGDYFEWLE peptide as substrate incubated for 1 hr in presence of ATP by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00713
BindingDB Entry DOI: 10.7270/Q2S1867R |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50166610

(CHEMBL3800526)Show SMILES COc1cc(CCc2cc(Nc3ncnc4cc(ccc34)N3CCN4CCCCC4C3)n[nH]2)cc(OC)c1 Show InChI InChI=1S/C29H35N7O2/c1-37-24-13-20(14-25(17-24)38-2)6-7-21-15-28(34-33-21)32-29-26-9-8-22(16-27(26)30-19-31-29)36-12-11-35-10-4-3-5-23(35)18-36/h8-9,13-17,19,23H,3-7,10-12,18H2,1-2H3,(H2,30,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR2 (unknown origin) using (Glu,Tyr)4:1 as substrate incubated for 60 mins by ELISA |
Bioorg Med Chem Lett 26: 2594-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.028
BindingDB Entry DOI: 10.7270/Q20C4XNM |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50166744

(CHEMBL3798625)Show SMILES COc1cc(CCc2cc(Nc3ncnc4cc(ccc34)N3C[C@H](C)N(C)[C@H](C)C3)n[nH]2)cc(OC)c1 |r| Show InChI InChI=1S/C28H35N7O2/c1-18-15-35(16-19(2)34(18)3)22-8-9-25-26(13-22)29-17-30-28(25)31-27-12-21(32-33-27)7-6-20-10-23(36-4)14-24(11-20)37-5/h8-14,17-19H,6-7,15-16H2,1-5H3,(H2,29,30,31,32,33)/t18-,19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR2 (unknown origin) using (Glu,Tyr)4:1 as substrate incubated for 60 mins by ELISA |
Bioorg Med Chem Lett 26: 2594-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.028
BindingDB Entry DOI: 10.7270/Q20C4XNM |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50588768
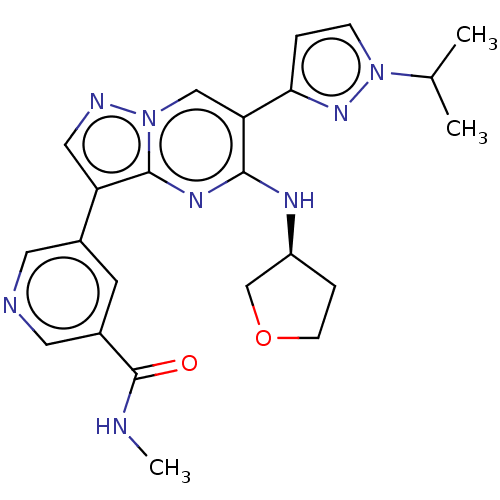
(CHEMBL5175256)Show SMILES [2H]C([2H])([2H])NC(=O)c1cncc(c1)-c1cnn2cc(-c3ccn(n3)C(C)C)c(N[C@H]3CCOC3)nc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01366
BindingDB Entry DOI: 10.7270/Q2PG1WPR |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50576988
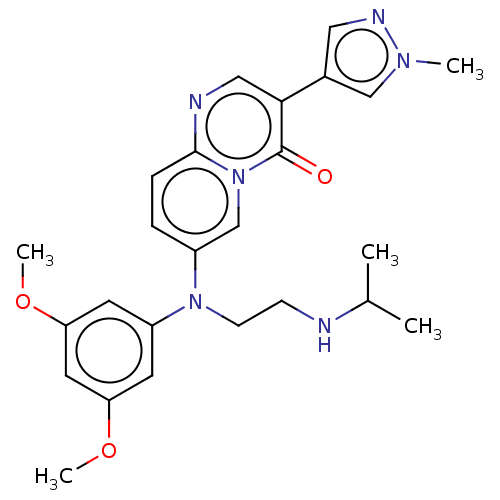
(CHEMBL4862576)Show SMILES COc1cc(OC)cc(c1)N(CCNC(C)C)c1ccc2ncc(-c3cnn(C)c3)c(=O)n2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human N-terminal GST fusion tagged FGFR2 cytoplasmic domain (399 to 821 end residues) expressed in baculovirus infected Sf21 insect cel... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113499
BindingDB Entry DOI: 10.7270/Q2HH6PWN |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50588744
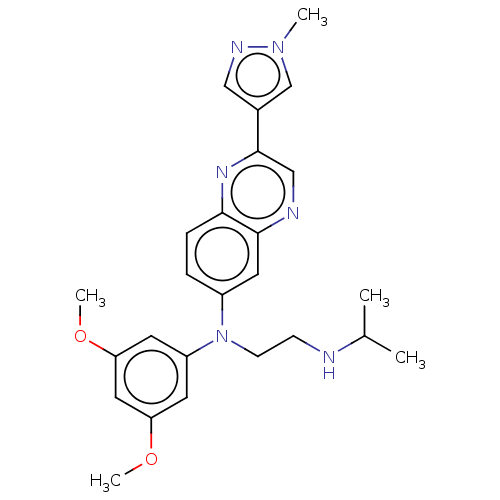
(CHEMBL5189383)Show SMILES COc1cc(OC)cc(c1)N(CCNC(C)C)c1ccc2nc(cnc2c1)-c1cnn(C)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01366
BindingDB Entry DOI: 10.7270/Q2PG1WPR |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50587622
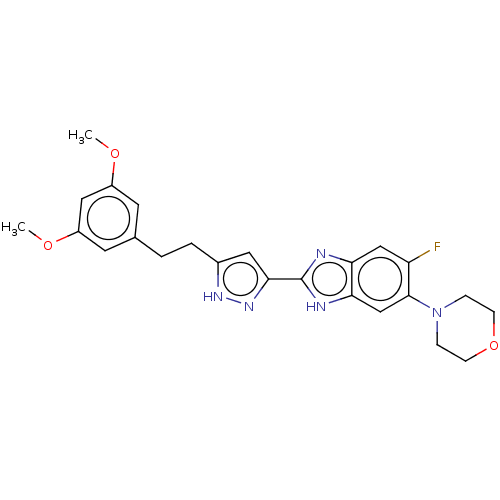
(CHEMBL5171314)Show SMILES COc1cc(CCc2cc(n[nH]2)-c2nc3cc(F)c(cc3[nH]2)N2CCOCC2)cc(OC)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112990
BindingDB Entry DOI: 10.7270/Q2WD44HQ |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50587621
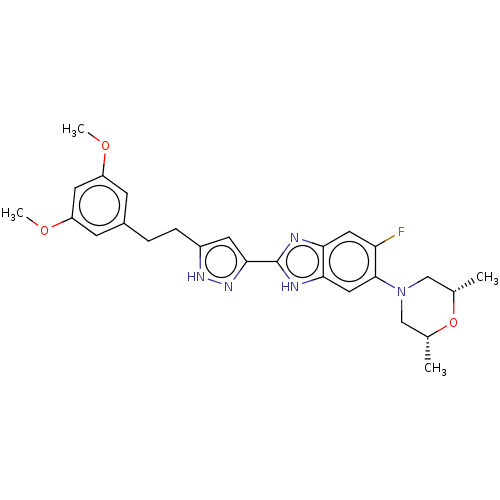
(CHEMBL5179660)Show SMILES COc1cc(CCc2cc(n[nH]2)-c2nc3cc(F)c(cc3[nH]2)N2C[C@H](C)O[C@H](C)C2)cc(OC)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112990
BindingDB Entry DOI: 10.7270/Q2WD44HQ |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM301220
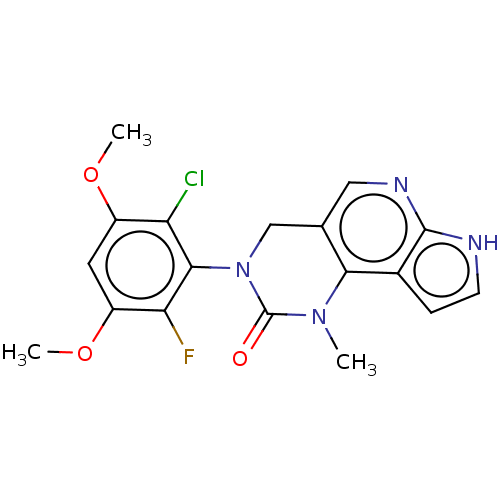
(3-(2-Chloro-6-fluoro-3,5-dimethoxyphenyl)-1-methyl...)Show SMILES COc1cc(OC)c(Cl)c(N2Cc3cnc4[nH]ccc4c3N(C)C2=O)c1F Show InChI InChI=1S/C18H16ClFN4O3/c1-23-15-9(7-22-17-10(15)4-5-21-17)8-24(18(23)25)16-13(19)11(26-2)6-12(27-3)14(16)20/h4-7H,8H2,1-3H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FGFR2 using biotinylated-EQEDEPEGDYFEWLE peptide as substrate incubated for 1 hr in presence of ATP by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00713
BindingDB Entry DOI: 10.7270/Q2S1867R |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM161438
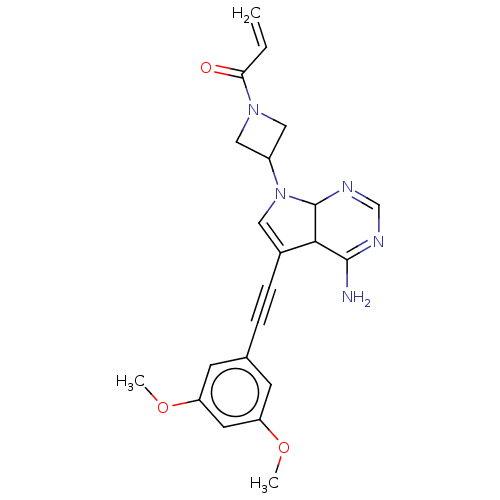
(US10124003, Ex. Compound 51 | US10835536, Ex. Comp...)Show SMILES COc1cc(OC)cc(c1)C#CC1=CN(C2CN(C2)C(=O)C=C)C2N=CN=C(N)C12 |c:26,t:13,28| Show InChI InChI=1S/C22H23N5O3/c1-4-19(28)26-11-16(12-26)27-10-15(20-21(23)24-13-25-22(20)27)6-5-14-7-17(29-2)9-18(8-14)30-3/h4,7-10,13,16,20,22H,1,11-12H2,2-3H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US9108973 (2015)
BindingDB Entry DOI: 10.7270/Q2XW4HJV |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM161453
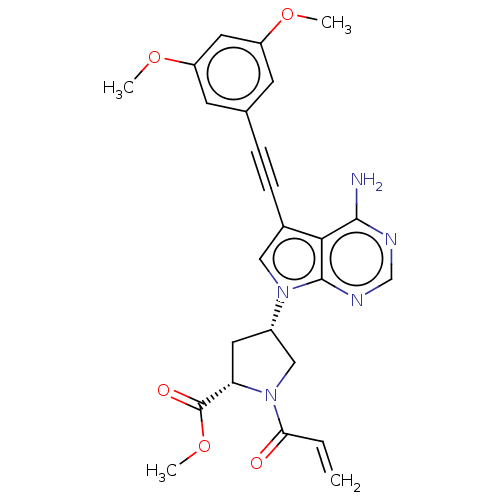
(US10124003, Ex. Compound 66 | US10835536, Ex. Comp...)Show SMILES COC(=O)[C@@H]1C[C@@H](CN1C(=O)C=C)n1cc(C#Cc2cc(OC)cc(OC)c2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H25N5O5/c1-5-21(31)30-13-17(10-20(30)25(32)35-4)29-12-16(22-23(26)27-14-28-24(22)29)7-6-15-8-18(33-2)11-19(9-15)34-3/h5,8-9,11-12,14,17,20H,1,10,13H2,2-4H3,(H2,26,27,28)/t17-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US9108973 (2015)
BindingDB Entry DOI: 10.7270/Q2XW4HJV |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50166738

(CHEMBL3797845)Show SMILES COc1cc(CCc2cc(Nc3ncnc4cc(ccc34)N3CCC(CC3)N(C)C)n[nH]2)cc(OC)c1 Show InChI InChI=1S/C28H35N7O2/c1-34(2)21-9-11-35(12-10-21)22-7-8-25-26(16-22)29-18-30-28(25)31-27-15-20(32-33-27)6-5-19-13-23(36-3)17-24(14-19)37-4/h7-8,13-18,21H,5-6,9-12H2,1-4H3,(H2,29,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR2 (unknown origin) using (Glu,Tyr)4:1 as substrate incubated for 60 mins by ELISA |
Bioorg Med Chem Lett 26: 2594-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.028
BindingDB Entry DOI: 10.7270/Q20C4XNM |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50166608
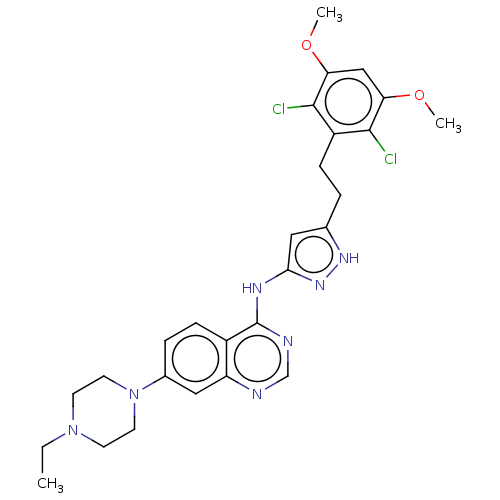
(CHEMBL3797507)Show SMILES CCN1CCN(CC1)c1ccc2c(Nc3cc(CCc4c(Cl)c(OC)cc(OC)c4Cl)[nH]n3)ncnc2c1 Show InChI InChI=1S/C27H31Cl2N7O2/c1-4-35-9-11-36(12-10-35)18-6-8-19-21(14-18)30-16-31-27(19)32-24-13-17(33-34-24)5-7-20-25(28)22(37-2)15-23(38-3)26(20)29/h6,8,13-16H,4-5,7,9-12H2,1-3H3,(H2,30,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR2 (unknown origin) using (Glu,Tyr)4:1 as substrate incubated for 60 mins by ELISA |
Bioorg Med Chem Lett 26: 2594-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.028
BindingDB Entry DOI: 10.7270/Q20C4XNM |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM161438

(US10124003, Ex. Compound 51 | US10835536, Ex. Comp...)Show SMILES COc1cc(OC)cc(c1)C#CC1=CN(C2CN(C2)C(=O)C=C)C2N=CN=C(N)C12 |c:26,t:13,28| Show InChI InChI=1S/C22H23N5O3/c1-4-19(28)26-11-16(12-26)27-10-15(20-21(23)24-13-25-22(20)27)6-5-14-7-17(29-2)9-18(8-14)30-3/h4,7-10,13,16,20,22H,1,11-12H2,2-3H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US10124003 (2018)
BindingDB Entry DOI: 10.7270/Q2B27XBQ |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM161453

(US10124003, Ex. Compound 66 | US10835536, Ex. Comp...)Show SMILES COC(=O)[C@@H]1C[C@@H](CN1C(=O)C=C)n1cc(C#Cc2cc(OC)cc(OC)c2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H25N5O5/c1-5-21(31)30-13-17(10-20(30)25(32)35-4)29-12-16(22-23(26)27-14-28-24(22)29)7-6-15-8-18(33-2)11-19(9-15)34-3/h5,8-9,11-12,14,17,20H,1,10,13H2,2-4H3,(H2,26,27,28)/t17-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US10124003 (2018)
BindingDB Entry DOI: 10.7270/Q2B27XBQ |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50234570
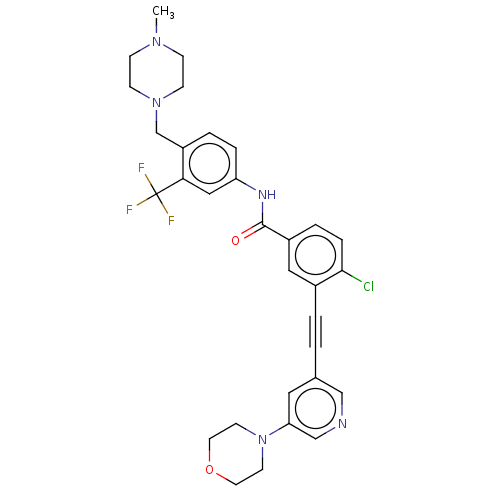
(CHEMBL4091628)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(Cl)c(c3)C#Cc3cncc(c3)N3CCOCC3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C31H31ClF3N5O2/c1-38-8-10-39(11-9-38)21-25-4-6-26(18-28(25)31(33,34)35)37-30(41)24-5-7-29(32)23(17-24)3-2-22-16-27(20-36-19-22)40-12-14-42-15-13-40/h4-7,16-20H,8-15,21H2,1H3,(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR2 (unknown origin) using poly (Glu,Tyr) 4:1 as substrate after 60 mins by ELISA |
Eur J Med Chem 126: 122-132 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.003
BindingDB Entry DOI: 10.7270/Q2FX7CQK |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM161438

(US10124003, Ex. Compound 51 | US10835536, Ex. Comp...)Show SMILES COc1cc(OC)cc(c1)C#CC1=CN(C2CN(C2)C(=O)C=C)C2N=CN=C(N)C12 |c:26,t:13,28| Show InChI InChI=1S/C22H23N5O3/c1-4-19(28)26-11-16(12-26)27-10-15(20-21(23)24-13-25-22(20)27)6-5-14-7-17(29-2)9-18(8-14)30-3/h4,7-10,13,16,20,22H,1,11-12H2,2-3H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US10835536 (2020)
BindingDB Entry DOI: 10.7270/Q23N26F5 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM161453

(US10124003, Ex. Compound 66 | US10835536, Ex. Comp...)Show SMILES COC(=O)[C@@H]1C[C@@H](CN1C(=O)C=C)n1cc(C#Cc2cc(OC)cc(OC)c2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H25N5O5/c1-5-21(31)30-13-17(10-20(30)25(32)35-4)29-12-16(22-23(26)27-14-28-24(22)29)7-6-15-8-18(33-2)11-19(9-15)34-3/h5,8-9,11-12,14,17,20H,1,10,13H2,2-4H3,(H2,26,27,28)/t17-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US10835536 (2020)
BindingDB Entry DOI: 10.7270/Q23N26F5 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM161396
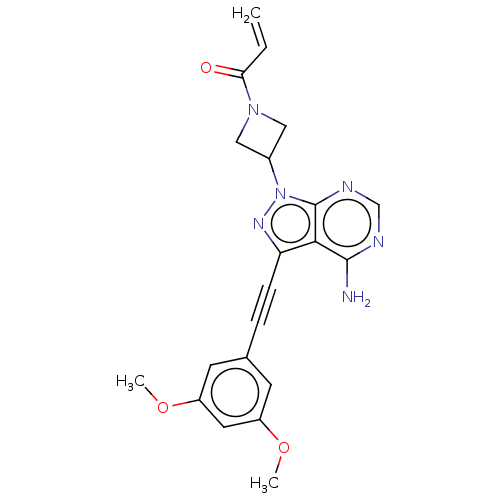
(US10124003, Ex. Compound 9 | US10835536, Ex. Comp ...)Show SMILES COc1cc(OC)cc(c1)C#Cc1nn(C2CN(C2)C(=O)C=C)c2ncnc(N)c12 Show InChI InChI=1S/C21H20N6O3/c1-4-18(28)26-10-14(11-26)27-21-19(20(22)23-12-24-21)17(25-27)6-5-13-7-15(29-2)9-16(8-13)30-3/h4,7-9,12,14H,1,10-11H2,2-3H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US10894048 (2021)
BindingDB Entry DOI: 10.7270/Q2319ZZF |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM478146
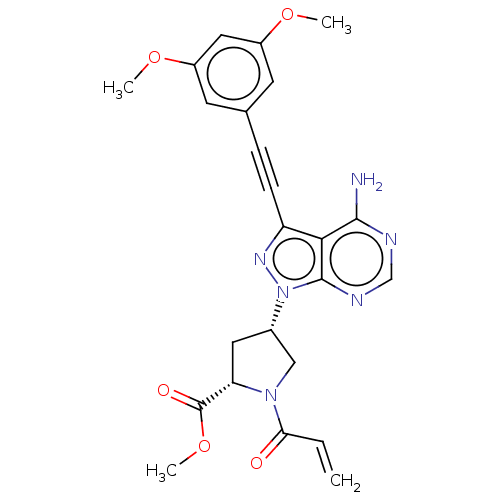
(US10894048, Ex Comp 66)Show SMILES COC(=O)[C@@H]1C[C@@H](CN1C(=O)C=C)n1nc(C#Cc2cc(OC)cc(OC)c2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24N6O5/c1-5-20(31)29-12-15(10-19(29)24(32)35-4)30-23-21(22(25)26-13-27-23)18(28-30)7-6-14-8-16(33-2)11-17(9-14)34-3/h5,8-9,11,13,15,19H,1,10,12H2,2-4H3,(H2,25,26,27)/t15-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US10894048 (2021)
BindingDB Entry DOI: 10.7270/Q2319ZZF |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM301310
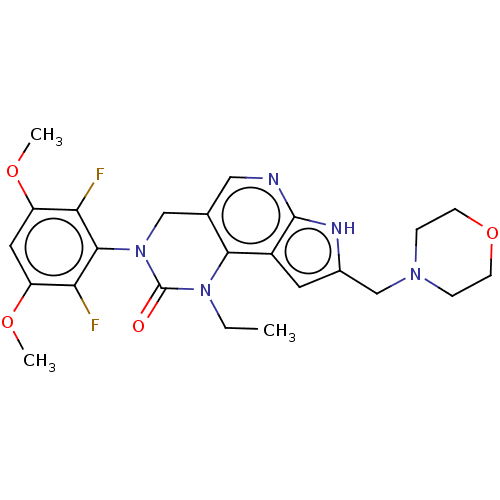
(3-(2,6-difluoro-3,5-dimethoxyphenyl)-1-ethyl-8-(mo...)Show SMILES CCN1C(=O)N(Cc2cnc3[nH]c(CN4CCOCC4)cc3c12)c1c(F)c(OC)cc(OC)c1F Show InChI InChI=1S/C24H27F2N5O4/c1-4-30-21-14(11-27-23-16(21)9-15(28-23)13-29-5-7-35-8-6-29)12-31(24(30)32)22-19(25)17(33-2)10-18(34-3)20(22)26/h9-11H,4-8,12-13H2,1-3H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01366
BindingDB Entry DOI: 10.7270/Q2PG1WPR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM161426
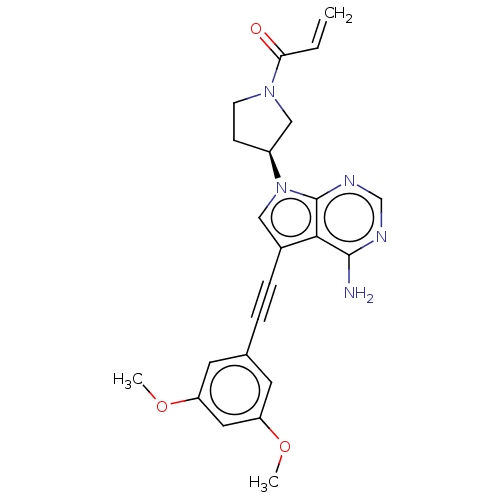
(US10124003, Ex. Compound 39 | US10894048, Ex Comp ...)Show SMILES COc1cc(OC)cc(c1)C#Cc1cn([C@H]2CCN(C2)C(=O)C=C)c2ncnc(N)c12 |r| Show InChI InChI=1S/C23H23N5O3/c1-4-20(29)27-8-7-17(13-27)28-12-16(21-22(24)25-14-26-23(21)28)6-5-15-9-18(30-2)11-19(10-15)31-3/h4,9-12,14,17H,1,7-8,13H2,2-3H3,(H2,24,25,26)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US10894048 (2021)
BindingDB Entry DOI: 10.7270/Q2319ZZF |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM161426

(US10124003, Ex. Compound 39 | US10894048, Ex Comp ...)Show SMILES COc1cc(OC)cc(c1)C#Cc1cn([C@H]2CCN(C2)C(=O)C=C)c2ncnc(N)c12 |r| Show InChI InChI=1S/C23H23N5O3/c1-4-20(29)27-8-7-17(13-27)28-12-16(21-22(24)25-14-26-23(21)28)6-5-15-9-18(30-2)11-19(10-15)31-3/h4,9-12,14,17H,1,7-8,13H2,2-3H3,(H2,24,25,26)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US9108973 (2015)
BindingDB Entry DOI: 10.7270/Q2XW4HJV |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50166610

(CHEMBL3800526)Show SMILES COc1cc(CCc2cc(Nc3ncnc4cc(ccc34)N3CCN4CCCCC4C3)n[nH]2)cc(OC)c1 Show InChI InChI=1S/C29H35N7O2/c1-37-24-13-20(14-25(17-24)38-2)6-7-21-15-28(34-33-21)32-29-26-9-8-22(16-27(26)30-19-31-29)36-12-11-35-10-4-3-5-23(35)18-36/h8-9,13-17,19,23H,3-7,10-12,18H2,1-2H3,(H2,30,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS)
Curated by ChEMBL
| Assay Description
Inhibition of FGFR2 in human KATO III cells assessed as suppression of cell proliferation after 72 hrs by SRB/CCK-8 assay |
Bioorg Med Chem Lett 26: 2594-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.028
BindingDB Entry DOI: 10.7270/Q20C4XNM |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50166655
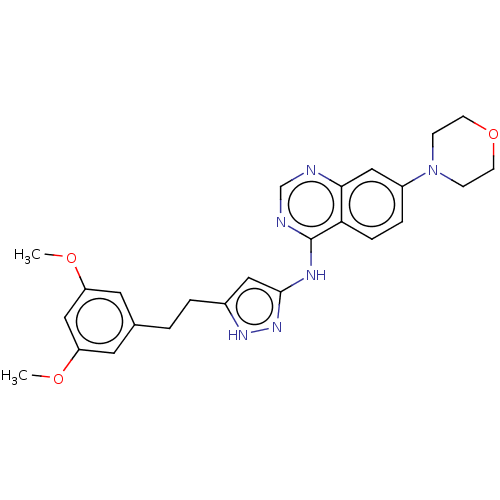
(CHEMBL3798342)Show SMILES COc1cc(CCc2cc(Nc3ncnc4cc(ccc34)N3CCOCC3)n[nH]2)cc(OC)c1 Show InChI InChI=1S/C25H28N6O3/c1-32-20-11-17(12-21(15-20)33-2)3-4-18-13-24(30-29-18)28-25-22-6-5-19(14-23(22)26-16-27-25)31-7-9-34-10-8-31/h5-6,11-16H,3-4,7-10H2,1-2H3,(H2,26,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR2 (unknown origin) using (Glu,Tyr)4:1 as substrate incubated for 60 mins by ELISA |
Bioorg Med Chem Lett 26: 2594-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.028
BindingDB Entry DOI: 10.7270/Q20C4XNM |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50166736
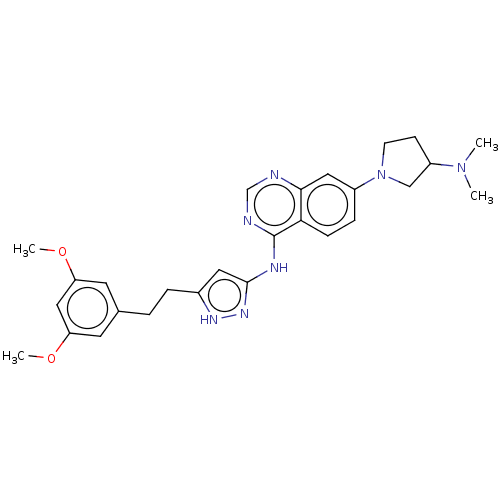
(CHEMBL3797594)Show SMILES COc1cc(CCc2cc(Nc3ncnc4cc(ccc34)N3CCC(C3)N(C)C)n[nH]2)cc(OC)c1 Show InChI InChI=1S/C27H33N7O2/c1-33(2)21-9-10-34(16-21)20-7-8-24-25(14-20)28-17-29-27(24)30-26-13-19(31-32-26)6-5-18-11-22(35-3)15-23(12-18)36-4/h7-8,11-15,17,21H,5-6,9-10,16H2,1-4H3,(H2,28,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences (CAS)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR2 (unknown origin) using (Glu,Tyr)4:1 as substrate incubated for 60 mins by ELISA |
Bioorg Med Chem Lett 26: 2594-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.028
BindingDB Entry DOI: 10.7270/Q20C4XNM |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM161419
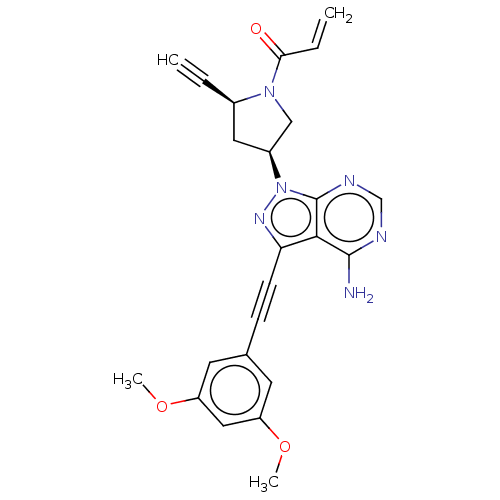
(US10124003, Ex. Compound 32 | US10835536, Ex. Comp...)Show SMILES COc1cc(OC)cc(c1)C#Cc1nn([C@H]2C[C@@H](C#C)N(C2)C(=O)C=C)c2ncnc(N)c12 |r| Show InChI InChI=1S/C24H22N6O3/c1-5-16-11-17(13-29(16)21(31)6-2)30-24-22(23(25)26-14-27-24)20(28-30)8-7-15-9-18(32-3)12-19(10-15)33-4/h1,6,9-10,12,14,16-17H,2,11,13H2,3-4H3,(H2,25,26,27)/t16-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US10124003 (2018)
BindingDB Entry DOI: 10.7270/Q2B27XBQ |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM161426

(US10124003, Ex. Compound 39 | US10894048, Ex Comp ...)Show SMILES COc1cc(OC)cc(c1)C#Cc1cn([C@H]2CCN(C2)C(=O)C=C)c2ncnc(N)c12 |r| Show InChI InChI=1S/C23H23N5O3/c1-4-20(29)27-8-7-17(13-27)28-12-16(21-22(24)25-14-26-23(21)28)6-5-15-9-18(30-2)11-19(10-15)31-3/h4,9-12,14,17H,1,7-8,13H2,2-3H3,(H2,24,25,26)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US10124003 (2018)
BindingDB Entry DOI: 10.7270/Q2B27XBQ |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50065454
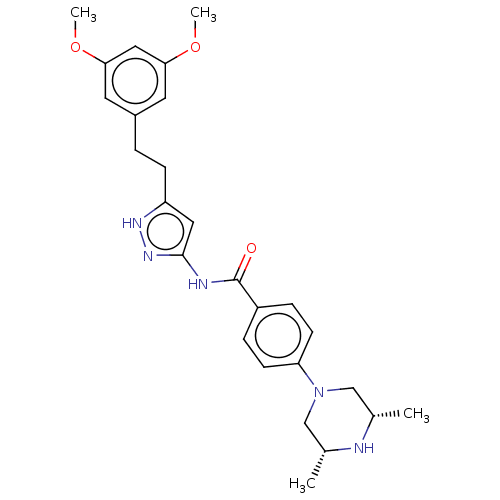
(CHEBI:63453 | CHEMBL3348846)Show SMILES COc1cc(CCc2cc(NC(=O)c3ccc(cc3)N3C[C@H](C)N[C@H](C)C3)n[nH]2)cc(OC)c1 Show InChI InChI=1S/C26H33N5O3/c1-17-15-31(16-18(2)27-17)22-9-6-20(7-10-22)26(32)28-25-13-21(29-30-25)8-5-19-11-23(33-3)14-24(12-19)34-4/h6-7,9-14,17-18,27H,5,8,15-16H2,1-4H3,(H2,28,29,30,32)/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR2 using poly (Glu, Tyr)4:1 as substrate measured after 60 mins by ELISA |
J Med Chem 62: 7473-7488 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00510
BindingDB Entry DOI: 10.7270/Q2KP85D8 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50065454

(CHEBI:63453 | CHEMBL3348846)Show SMILES COc1cc(CCc2cc(NC(=O)c3ccc(cc3)N3C[C@H](C)N[C@H](C)C3)n[nH]2)cc(OC)c1 Show InChI InChI=1S/C26H33N5O3/c1-17-15-31(16-18(2)27-17)22-9-6-20(7-10-22)26(32)28-25-13-21(29-30-25)8-5-19-11-23(33-3)14-24(12-19)34-4/h6-7,9-14,17-18,27H,5,8,15-16H2,1-4H3,(H2,28,29,30,32)/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR2 using poly (Glu,Tyr)4:1 as substrate incubated for 60 mins by ELISA |
ACS Med Chem Lett 7: 629-34 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00066
BindingDB Entry DOI: 10.7270/Q2P272M2 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50065454

(CHEBI:63453 | CHEMBL3348846)Show SMILES COc1cc(CCc2cc(NC(=O)c3ccc(cc3)N3C[C@H](C)N[C@H](C)C3)n[nH]2)cc(OC)c1 Show InChI InChI=1S/C26H33N5O3/c1-17-15-31(16-18(2)27-17)22-9-6-20(7-10-22)26(32)28-25-13-21(29-30-25)8-5-19-11-23(33-3)14-24(12-19)34-4/h6-7,9-14,17-18,27H,5,8,15-16H2,1-4H3,(H2,28,29,30,32)/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR2 using poly (Glu,Tyr)4:1 as substrate incubated for 60 mins by ELISA |
ACS Med Chem Lett 7: 629-34 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00066
BindingDB Entry DOI: 10.7270/Q2P272M2 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM161419

(US10124003, Ex. Compound 32 | US10835536, Ex. Comp...)Show SMILES COc1cc(OC)cc(c1)C#Cc1nn([C@H]2C[C@@H](C#C)N(C2)C(=O)C=C)c2ncnc(N)c12 |r| Show InChI InChI=1S/C24H22N6O3/c1-5-16-11-17(13-29(16)21(31)6-2)30-24-22(23(25)26-14-27-24)20(28-30)8-7-15-9-18(32-3)12-19(10-15)33-4/h1,6,9-10,12,14,16-17H,2,11,13H2,3-4H3,(H2,25,26,27)/t16-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US10835536 (2020)
BindingDB Entry DOI: 10.7270/Q23N26F5 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
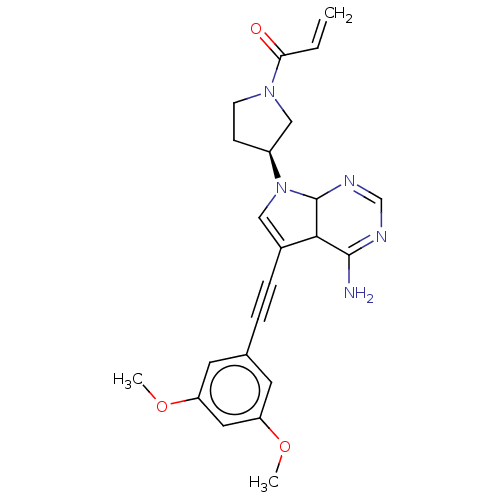
(Homo sapiens (Human)) | BDBM472833
(US10835536, Ex. Comp 39)Show SMILES COc1cc(OC)cc(c1)C#CC1=CN([C@H]2CCN(C2)C(=O)C=C)C2N=CN=C(N)C12 |r,c:27,t:13,29| Show InChI InChI=1S/C23H25N5O3/c1-4-20(29)27-8-7-17(13-27)28-12-16(21-22(24)25-14-26-23(21)28)6-5-15-9-18(30-2)11-19(10-15)31-3/h4,9-12,14,17,21,23H,1,7-8,13H2,2-3H3,(H2,24,25,26)/t17-,21?,23?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US10835536 (2020)
BindingDB Entry DOI: 10.7270/Q23N26F5 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM161419

(US10124003, Ex. Compound 32 | US10835536, Ex. Comp...)Show SMILES COc1cc(OC)cc(c1)C#Cc1nn([C@H]2C[C@@H](C#C)N(C2)C(=O)C=C)c2ncnc(N)c12 |r| Show InChI InChI=1S/C24H22N6O3/c1-5-16-11-17(13-29(16)21(31)6-2)30-24-22(23(25)26-14-27-24)20(28-30)8-7-15-9-18(32-3)12-19(10-15)33-4/h1,6,9-10,12,14,16-17H,2,11,13H2,3-4H3,(H2,25,26,27)/t16-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US10894048 (2021)
BindingDB Entry DOI: 10.7270/Q2319ZZF |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM161419

(US10124003, Ex. Compound 32 | US10835536, Ex. Comp...)Show SMILES COc1cc(OC)cc(c1)C#Cc1nn([C@H]2C[C@@H](C#C)N(C2)C(=O)C=C)c2ncnc(N)c12 |r| Show InChI InChI=1S/C24H22N6O3/c1-5-16-11-17(13-29(16)21(31)6-2)30-24-22(23(25)26-14-27-24)20(28-30)8-7-15-9-18(32-3)12-19(10-15)33-4/h1,6,9-10,12,14,16-17H,2,11,13H2,3-4H3,(H2,25,26,27)/t16-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... |
US Patent US9108973 (2015)
BindingDB Entry DOI: 10.7270/Q2XW4HJV |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
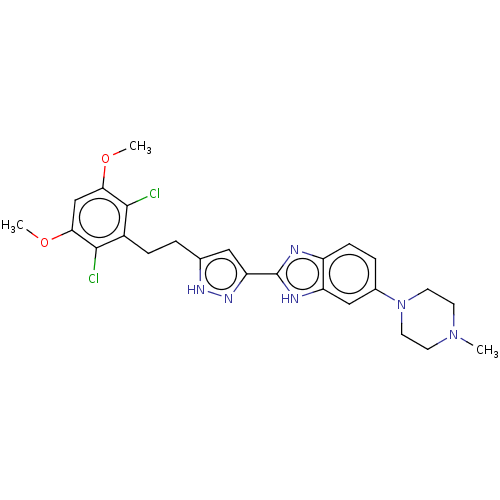
(Homo sapiens (Human)) | BDBM50587619
(CHEMBL5205436)Show SMILES COc1cc(OC)c(Cl)c(CCc2cc(n[nH]2)-c2nc3ccc(cc3[nH]2)N2CCN(C)CC2)c1Cl | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112990
BindingDB Entry DOI: 10.7270/Q2WD44HQ |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
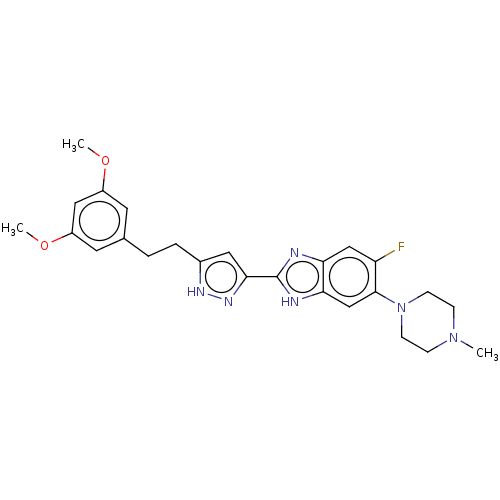
(Homo sapiens (Human)) | BDBM50587620
(CHEMBL5189958)Show SMILES COc1cc(CCc2cc(n[nH]2)-c2nc3cc(F)c(cc3[nH]2)N2CCN(C)CC2)cc(OC)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.437 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112990
BindingDB Entry DOI: 10.7270/Q2WD44HQ |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
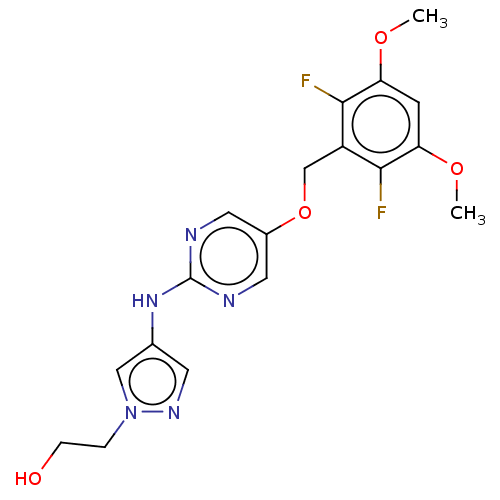
(Homo sapiens (Human)) | BDBM50593531
(ASP-5878 | ASP5878 | Asp 5878 | Asp-5878 | Asp5878)Show SMILES COc1cc(OC)c(F)c(COc2cnc(Nc3cnn(CCO)c3)nc2)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01977
BindingDB Entry DOI: 10.7270/Q2R49VVS |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM301310

(3-(2,6-difluoro-3,5-dimethoxyphenyl)-1-ethyl-8-(mo...)Show SMILES CCN1C(=O)N(Cc2cnc3[nH]c(CN4CCOCC4)cc3c12)c1c(F)c(OC)cc(OC)c1F Show InChI InChI=1S/C24H27F2N5O4/c1-4-30-21-14(11-27-23-16(21)9-15(28-23)13-29-5-7-35-8-6-29)12-31(24(30)32)22-19(25)17(33-2)10-18(34-3)20(22)26/h9-11H,4-8,12-13H2,1-3H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FGFR2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113499
BindingDB Entry DOI: 10.7270/Q2HH6PWN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50468395
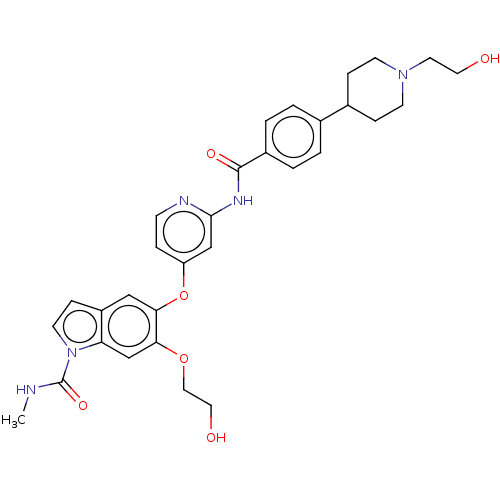
(CHEMBL4294625)Show SMILES CNC(=O)n1ccc2cc(Oc3ccnc(NC(=O)c4ccc(cc4)C4CCN(CCO)CC4)c3)c(OCCO)cc12 Show InChI InChI=1S/C31H35N5O6/c1-32-31(40)36-13-9-24-18-28(27(20-26(24)36)41-17-16-38)42-25-6-10-33-29(19-25)34-30(39)23-4-2-21(3-5-23)22-7-11-35(12-8-22)14-15-37/h2-6,9-10,13,18-20,22,37-38H,7-8,11-12,14-17H2,1H3,(H,32,40)(H,33,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal GST-tagged FGFR2 (399 to 821 residues) cytoplasmic domain expressed in baculovirus expression system after 60 mins by ... |
Eur J Med Chem 154: 9-28 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.005
BindingDB Entry DOI: 10.7270/Q2736TM3 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50596830
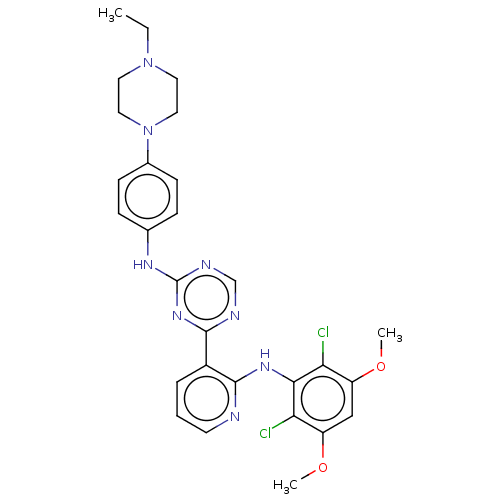
(CHEMBL5179166)Show SMILES CCN1CCN(CC1)c1ccc(Nc2ncnc(n2)-c2cccnc2Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01776
BindingDB Entry DOI: 10.7270/Q2765KCQ |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50525939
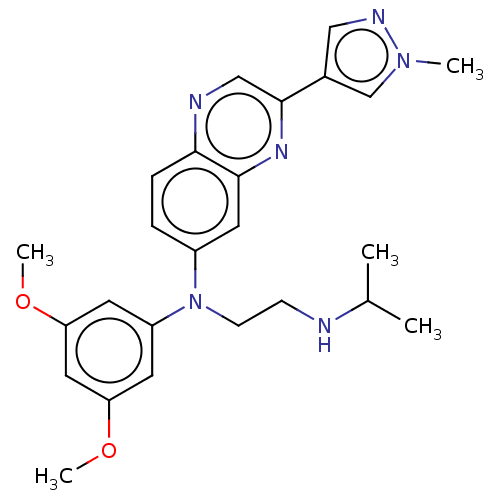
(Balversa | Erdafitinib | Jnj-42756493)Show SMILES COc1cc(OC)cc(c1)N(CCNC(C)C)c1ccc2ncc(nc2c1)-c1cnn(C)c1 Show InChI InChI=1S/C25H30N6O2/c1-17(2)26-8-9-31(20-10-21(32-4)13-22(11-20)33-5)19-6-7-23-24(12-19)29-25(15-27-23)18-14-28-30(3)16-18/h6-7,10-17,26H,8-9H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01776
BindingDB Entry DOI: 10.7270/Q2765KCQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM301310

(3-(2,6-difluoro-3,5-dimethoxyphenyl)-1-ethyl-8-(mo...)Show SMILES CCN1C(=O)N(Cc2cnc3[nH]c(CN4CCOCC4)cc3c12)c1c(F)c(OC)cc(OC)c1F Show InChI InChI=1S/C24H27F2N5O4/c1-4-30-21-14(11-27-23-16(21)9-15(28-23)13-29-5-7-35-8-6-29)12-31(24(30)32)22-19(25)17(33-2)10-18(34-3)20(22)26/h9-11H,4-8,12-13H2,1-3H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01776
BindingDB Entry DOI: 10.7270/Q2765KCQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50596831
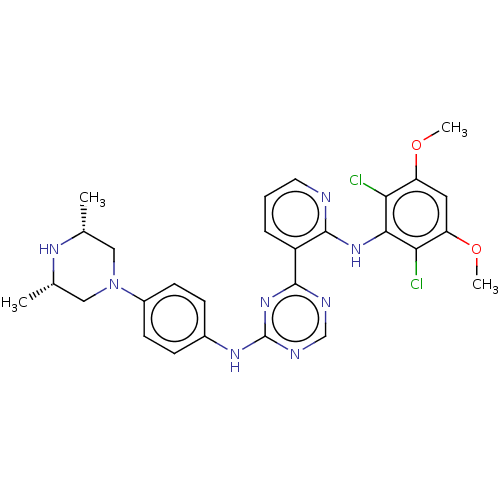
(CHEMBL5177100)Show SMILES COc1cc(OC)c(Cl)c(Nc2ncccc2-c2ncnc(Nc3ccc(cc3)N3C[C@H](C)N[C@H](C)C3)n2)c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01776
BindingDB Entry DOI: 10.7270/Q2765KCQ |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50596832
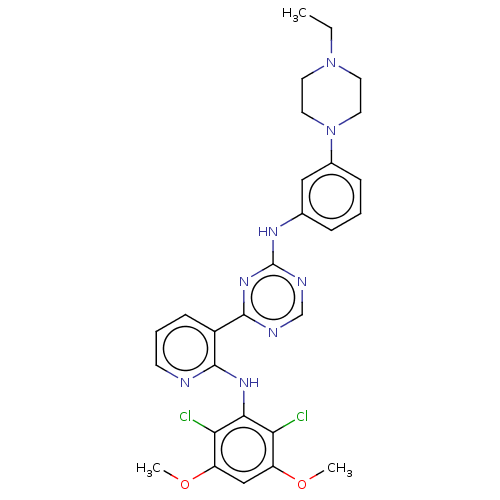
(CHEMBL5198569)Show SMILES CCN1CCN(CC1)c1cccc(Nc2ncnc(n2)-c2cccnc2Nc2c(Cl)c(OC)cc(OC)c2Cl)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01776
BindingDB Entry DOI: 10.7270/Q2765KCQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data