

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insulin receptor (Homo sapiens (Human)) | BDBM50506238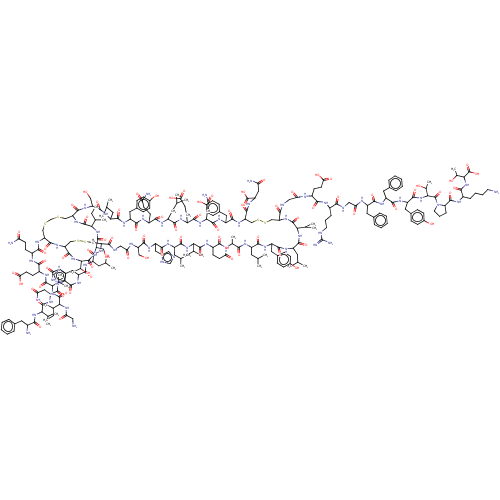 (CHEMBL4457859) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Agonist activity at human IR-beta signalling expressed in mouse 3T3 cells assessed as AKT phosphorylation at Ser473 residue after 30 mins by HTRF ass... | J Med Chem 62: 11437-11443 (2019) Article DOI: 10.1021/acs.jmedchem.9b01589 BindingDB Entry DOI: 10.7270/Q26Q21HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50548050 (CHEMBL4762540) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human IR-B expressed in mouse NIH3T3 cells assessed as increase in Akt phosphorylation at Ser473 residue incubated for 30 mins by HTRF ... | Citation and Details Article DOI: 10.1039/d0md00173b BindingDB Entry DOI: 10.7270/Q20005PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50548049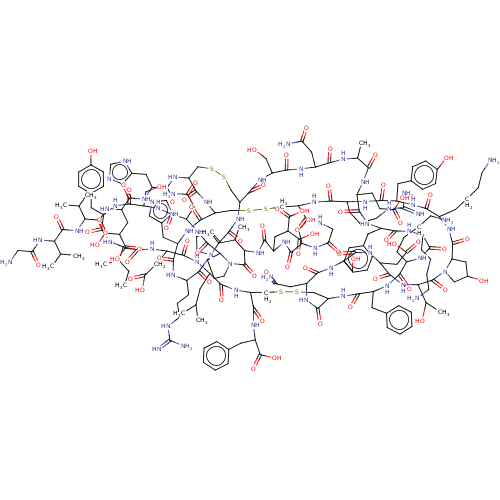 (CHEMBL4751178) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human IR-B expressed in mouse NIH3T3 cells assessed as increase in Akt phosphorylation at Ser473 residue incubated for 30 mins by HTRF ... | Citation and Details Article DOI: 10.1039/d0md00173b BindingDB Entry DOI: 10.7270/Q20005PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50548048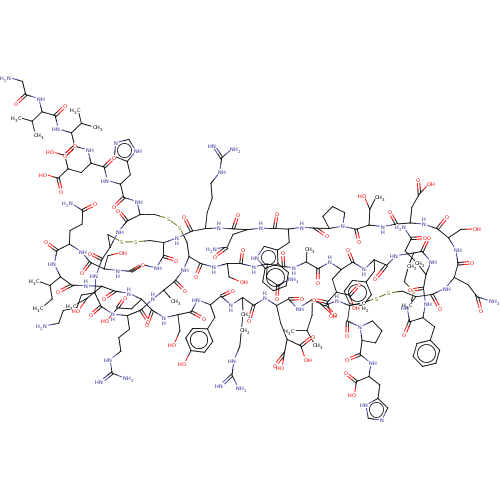 (CHEMBL4764550) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human IR-B expressed in mouse NIH3T3 cells assessed as increase in Akt phosphorylation at Ser473 residue incubated for 30 mins by HTRF ... | Citation and Details Article DOI: 10.1039/d0md00173b BindingDB Entry DOI: 10.7270/Q20005PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50548047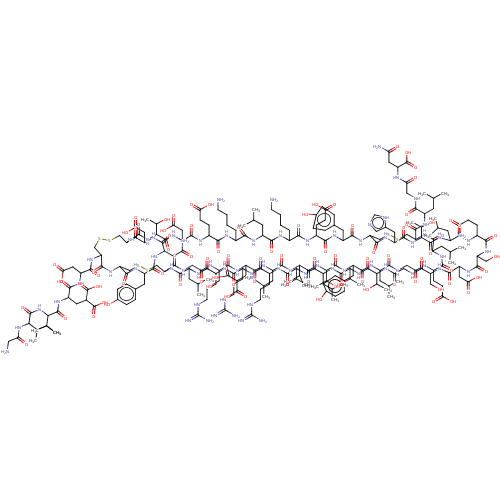 (CHEMBL4753825) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human IR-B expressed in mouse NIH3T3 cells assessed as increase in Akt phosphorylation at Ser473 residue incubated for 30 mins by HTRF ... | Citation and Details Article DOI: 10.1039/d0md00173b BindingDB Entry DOI: 10.7270/Q20005PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50548051 (CHEMBL4748140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human IR-B expressed in mouse NIH3T3 cells assessed as increase in Akt phosphorylation at Ser473 residue incubated for 30 mins by HTRF ... | Citation and Details Article DOI: 10.1039/d0md00173b BindingDB Entry DOI: 10.7270/Q20005PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM313069 (1-(6-chloroquinazolin-4-yl)-N3-(4-(2-(pyrrolidin-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, 1180 Veterans Blvd., South San Francisco, CA 94080, USA. Electronic address: dagoff@att.net. Curated by ChEMBL | Assay Description Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by... | Bioorg Med Chem Lett 27: 3766-3771 (2017) Article DOI: 10.1016/j.bmcl.2017.06.071 BindingDB Entry DOI: 10.7270/Q26D5WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM313084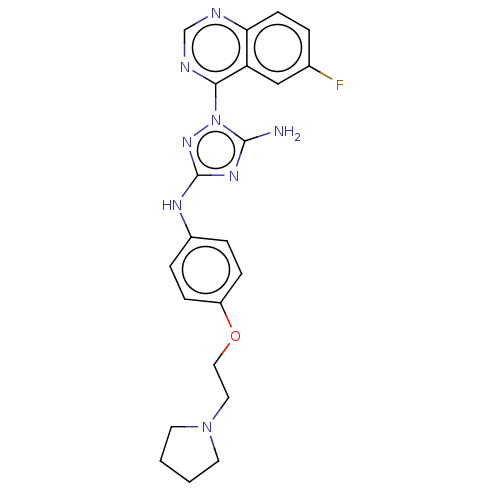 (1-(6-fluoroquinazolin-4-yl)-N3-(4-(2-(pyrrolidin-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 126 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, 1180 Veterans Blvd., South San Francisco, CA 94080, USA. Electronic address: dagoff@att.net. Curated by ChEMBL | Assay Description Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by... | Bioorg Med Chem Lett 27: 3766-3771 (2017) Article DOI: 10.1016/j.bmcl.2017.06.071 BindingDB Entry DOI: 10.7270/Q26D5WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM313027 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 241 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, 1180 Veterans Blvd., South San Francisco, CA 94080, USA. Electronic address: dagoff@att.net. Curated by ChEMBL | Assay Description Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by... | Bioorg Med Chem Lett 27: 3766-3771 (2017) Article DOI: 10.1016/j.bmcl.2017.06.071 BindingDB Entry DOI: 10.7270/Q26D5WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50269393 (CHEMBL4078564) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, 1180 Veterans Blvd., South San Francisco, CA 94080, USA. Electronic address: dagoff@att.net. Curated by ChEMBL | Assay Description Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by... | Bioorg Med Chem Lett 27: 3766-3771 (2017) Article DOI: 10.1016/j.bmcl.2017.06.071 BindingDB Entry DOI: 10.7270/Q26D5WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50092150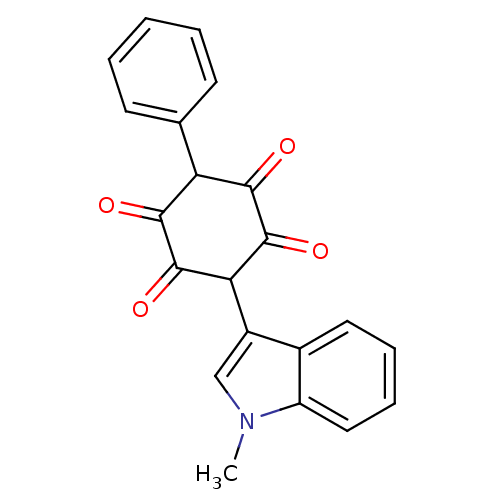 (2,5-Dihydroxy-3-(1-methyl-1H-indol-3-yl)-6-phenyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activation of human insulin receptor tyrosine kinase (IRTK) | J Med Chem 43: 3487-94 (2000) BindingDB Entry DOI: 10.7270/Q2NV9HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50092143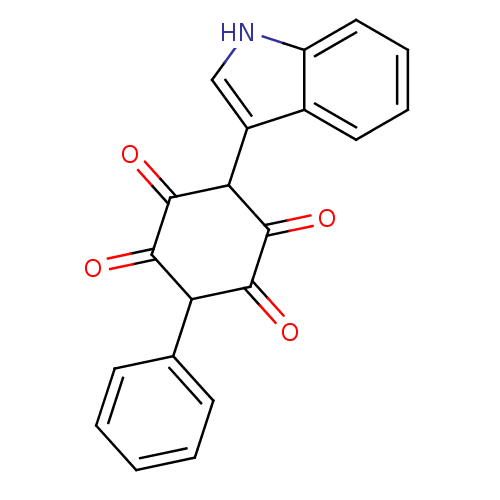 (2,5-Dihydroxy-3-(1H-indol-3-yl)-6-phenyl-[1,4]benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activation of human insulin receptor tyrosine kinase (IRTK) | J Med Chem 43: 3487-94 (2000) BindingDB Entry DOI: 10.7270/Q2NV9HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM313086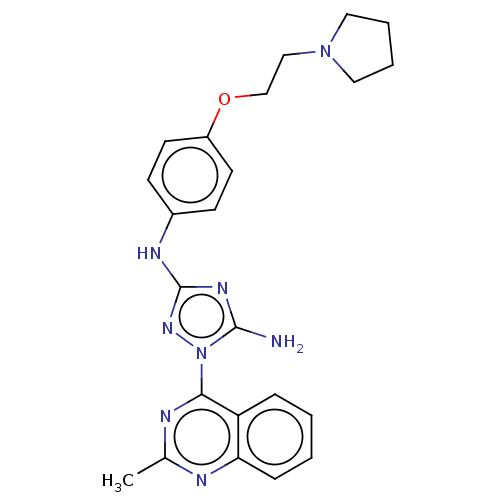 (1-(2-methylquinazolin-4-yl)-N3-(4-(2-(pyrrolidin-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, 1180 Veterans Blvd., South San Francisco, CA 94080, USA. Electronic address: dagoff@att.net. Curated by ChEMBL | Assay Description Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by... | Bioorg Med Chem Lett 27: 3766-3771 (2017) Article DOI: 10.1016/j.bmcl.2017.06.071 BindingDB Entry DOI: 10.7270/Q26D5WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM313081 (1-(2-chloro-7-methylthieno[3,2-d]pyrimidin-4-yl)-N...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 582 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, 1180 Veterans Blvd., South San Francisco, CA 94080, USA. Electronic address: dagoff@att.net. Curated by ChEMBL | Assay Description Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by... | Bioorg Med Chem Lett 27: 3766-3771 (2017) Article DOI: 10.1016/j.bmcl.2017.06.071 BindingDB Entry DOI: 10.7270/Q26D5WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM482158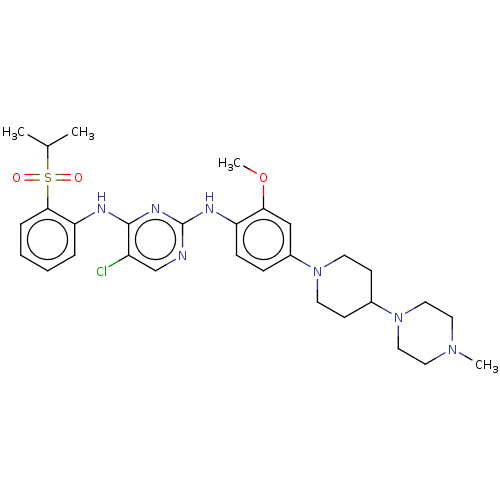 (BDBM50242742 | TAE684) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of InsR by cellular assay | Proc Natl Acad Sci USA 104: 19936-41 (2007) Article DOI: 10.1073/pnas.0707498104 BindingDB Entry DOI: 10.7270/Q24X58QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50092148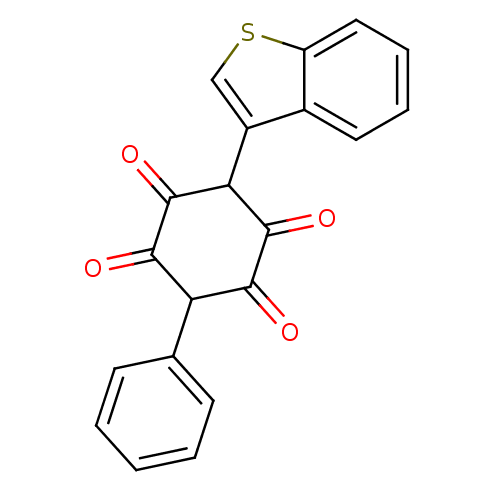 (2-Benzo[b]thiophen-3-yl-3,6-dihydroxy-5-phenyl-[1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activation of human insulin receptor tyrosine kinase (IRTK) | J Med Chem 43: 3487-94 (2000) BindingDB Entry DOI: 10.7270/Q2NV9HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50269375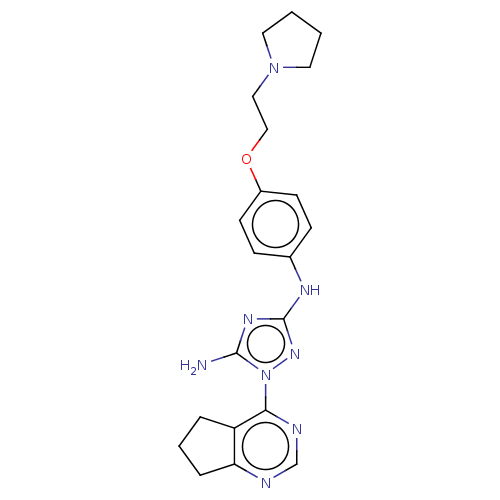 (CHEMBL4086420) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, 1180 Veterans Blvd., South San Francisco, CA 94080, USA. Electronic address: dagoff@att.net. Curated by ChEMBL | Assay Description Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by... | Bioorg Med Chem Lett 27: 3766-3771 (2017) Article DOI: 10.1016/j.bmcl.2017.06.071 BindingDB Entry DOI: 10.7270/Q26D5WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM313137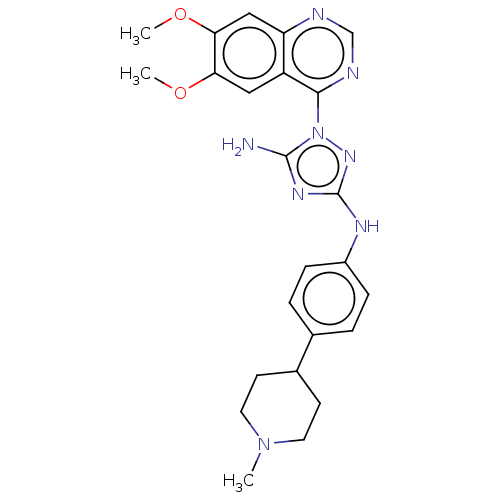 (1-(6,7-dimethoxyquinazolin-4-yl)-N3-(4-(1-methylpi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, 1180 Veterans Blvd., South San Francisco, CA 94080, USA. Electronic address: dagoff@att.net. Curated by ChEMBL | Assay Description Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by... | Bioorg Med Chem Lett 27: 3766-3771 (2017) Article DOI: 10.1016/j.bmcl.2017.06.071 BindingDB Entry DOI: 10.7270/Q26D5WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50092146 (2-Benzofuran-3-yl-3,6-dihydroxy-5-phenyl-[1,4]benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activation of human insulin receptor tyrosine kinase (IRTK) | J Med Chem 43: 3487-94 (2000) BindingDB Entry DOI: 10.7270/Q2NV9HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM313124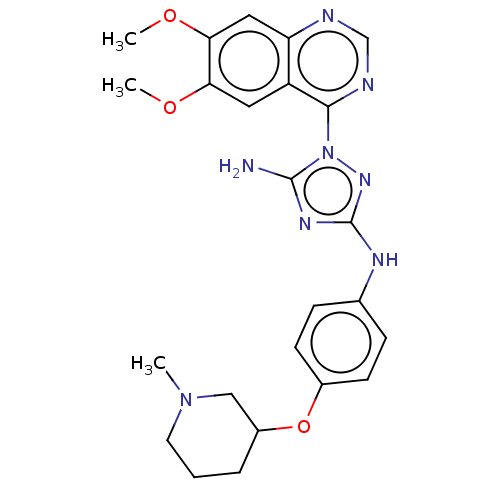 (1-(6,7-dimethoxyquinazolin-4-yl)-N3-(4-(1-methylpi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, 1180 Veterans Blvd., South San Francisco, CA 94080, USA. Electronic address: dagoff@att.net. Curated by ChEMBL | Assay Description Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by... | Bioorg Med Chem Lett 27: 3766-3771 (2017) Article DOI: 10.1016/j.bmcl.2017.06.071 BindingDB Entry DOI: 10.7270/Q26D5WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50242740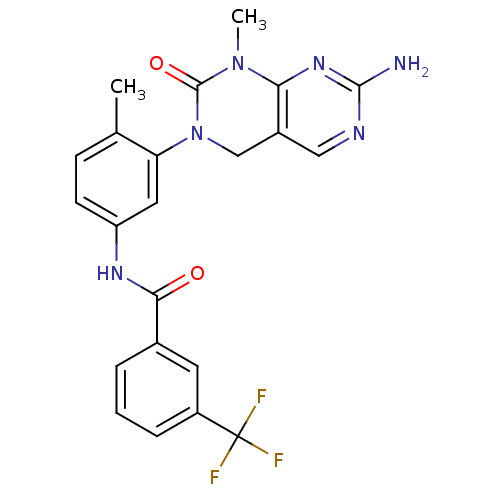 (CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of InsR by cellular assay | Proc Natl Acad Sci USA 104: 19936-41 (2007) Article DOI: 10.1073/pnas.0707498104 BindingDB Entry DOI: 10.7270/Q24X58QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50269384 (CHEMBL4095081) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, 1180 Veterans Blvd., South San Francisco, CA 94080, USA. Electronic address: dagoff@att.net. Curated by ChEMBL | Assay Description Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by... | Bioorg Med Chem Lett 27: 3766-3771 (2017) Article DOI: 10.1016/j.bmcl.2017.06.071 BindingDB Entry DOI: 10.7270/Q26D5WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM313080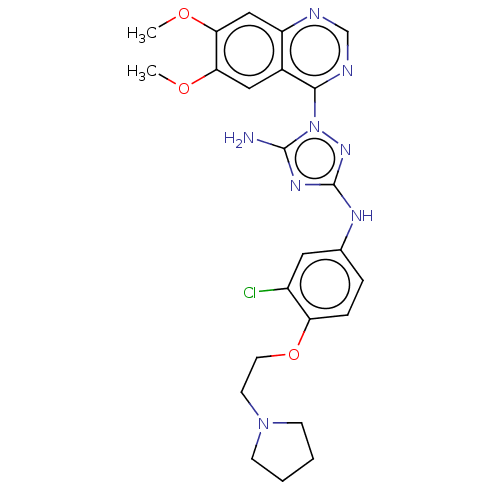 (N3-(3-chloro-4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.17E+3 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, 1180 Veterans Blvd., South San Francisco, CA 94080, USA. Electronic address: dagoff@att.net. Curated by ChEMBL | Assay Description Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by... | Bioorg Med Chem Lett 27: 3766-3771 (2017) Article DOI: 10.1016/j.bmcl.2017.06.071 BindingDB Entry DOI: 10.7270/Q26D5WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50269389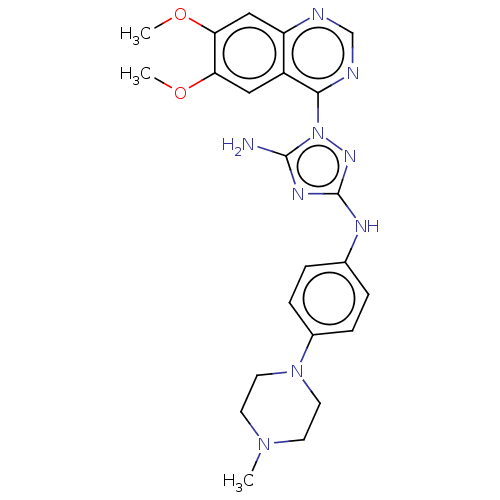 (CHEMBL4069483) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.58E+3 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, 1180 Veterans Blvd., South San Francisco, CA 94080, USA. Electronic address: dagoff@att.net. Curated by ChEMBL | Assay Description Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by... | Bioorg Med Chem Lett 27: 3766-3771 (2017) Article DOI: 10.1016/j.bmcl.2017.06.071 BindingDB Entry DOI: 10.7270/Q26D5WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM313140 (1-(6,7-dimethoxyquinazolin-4-yl)-N3-(4-((S)-3-(dim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.76E+3 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, 1180 Veterans Blvd., South San Francisco, CA 94080, USA. Electronic address: dagoff@att.net. Curated by ChEMBL | Assay Description Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by... | Bioorg Med Chem Lett 27: 3766-3771 (2017) Article DOI: 10.1016/j.bmcl.2017.06.071 BindingDB Entry DOI: 10.7270/Q26D5WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50092149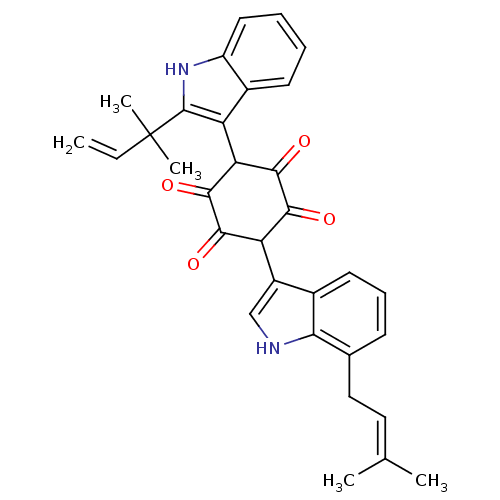 (2-[2-(1,1-Dimethyl-allyl)-1H-indol-3-yl]-3,6-dihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activation of human insulin receptor tyrosine kinase (IRTK) | J Med Chem 43: 3487-94 (2000) BindingDB Entry DOI: 10.7270/Q2NV9HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50269390 (CHEMBL4080190) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.03E+3 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, 1180 Veterans Blvd., South San Francisco, CA 94080, USA. Electronic address: dagoff@att.net. Curated by ChEMBL | Assay Description Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by... | Bioorg Med Chem Lett 27: 3766-3771 (2017) Article DOI: 10.1016/j.bmcl.2017.06.071 BindingDB Entry DOI: 10.7270/Q26D5WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50092147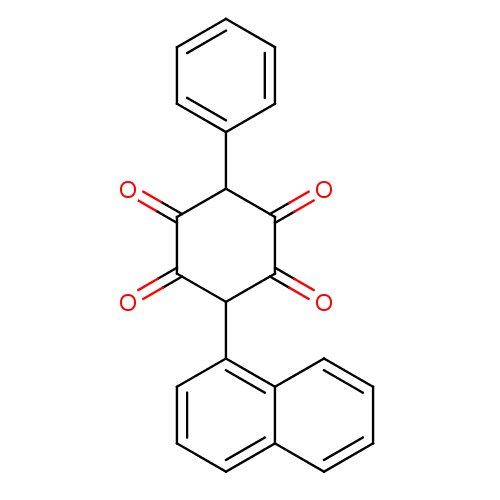 (2,5-Dihydroxy-3-naphthalen-1-yl-6-phenyl-[1,4]benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activation of human insulin receptor tyrosine kinase (IRTK) | J Med Chem 43: 3487-94 (2000) BindingDB Entry DOI: 10.7270/Q2NV9HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50092144 (2,5-Dihydroxy-3-(1H-indol-3-yl)-6-(7-methyl-1H-ind...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activation of human insulin receptor tyrosine kinase (IRTK) | J Med Chem 43: 3487-94 (2000) BindingDB Entry DOI: 10.7270/Q2NV9HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50269391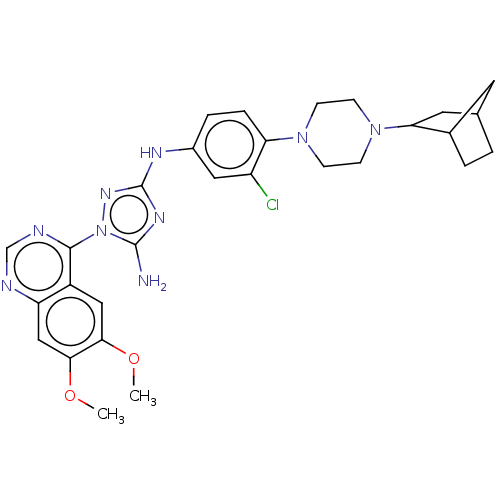 (CHEMBL4059790) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, 1180 Veterans Blvd., South San Francisco, CA 94080, USA. Electronic address: dagoff@att.net. Curated by ChEMBL | Assay Description Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by... | Bioorg Med Chem Lett 27: 3766-3771 (2017) Article DOI: 10.1016/j.bmcl.2017.06.071 BindingDB Entry DOI: 10.7270/Q26D5WH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50092145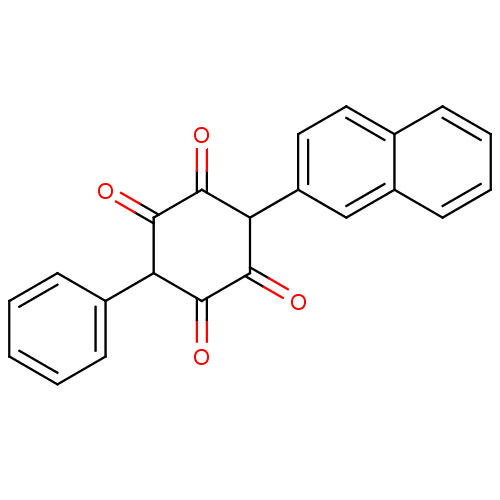 (2,5-Dihydroxy-3-naphthalen-2-yl-6-phenyl-[1,4]benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activation of human insulin receptor tyrosine kinase (IRTK) | J Med Chem 43: 3487-94 (2000) BindingDB Entry DOI: 10.7270/Q2NV9HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||