

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insulin receptor (Homo sapiens (Human)) | BDBM50265869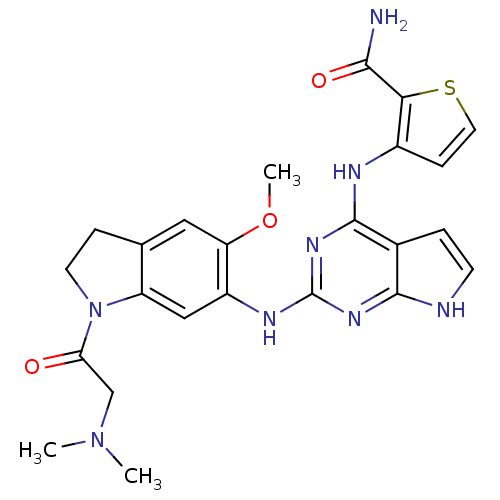 (3-(2-(1-(2-(dimethylamino)acetyl)-5-methoxyindolin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IR | Bioorg Med Chem Lett 19: 373-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.065 BindingDB Entry DOI: 10.7270/Q2F190PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50265888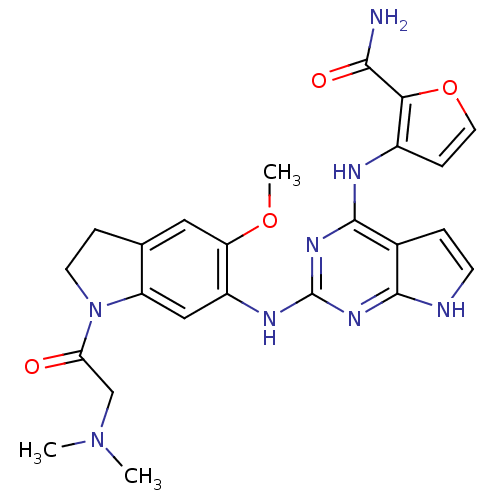 (3-(2-(1-(2-(dimethylamino)acetyl)-5-methoxyindolin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IR | Bioorg Med Chem Lett 19: 373-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.065 BindingDB Entry DOI: 10.7270/Q2F190PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50185288 (CHEMBL3824326) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50587680 (CHEMBL5182271) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00533 BindingDB Entry DOI: 10.7270/Q23X8BM3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50299148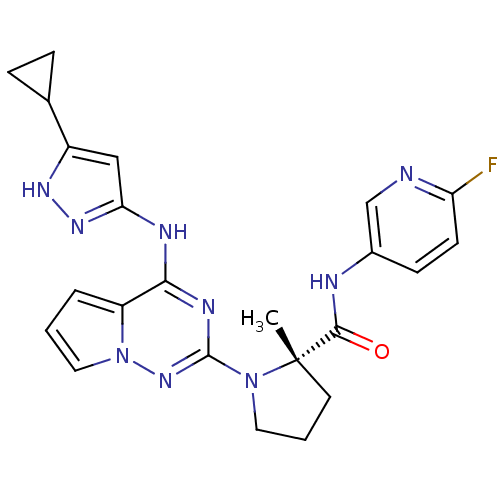 ((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of IR (unknown origin) | J Med Chem 62: 1731-1760 (2019) Article DOI: 10.1021/acs.jmedchem.8b01092 BindingDB Entry DOI: 10.7270/Q29Z986G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM291985 (US10100019, Example 55) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM482158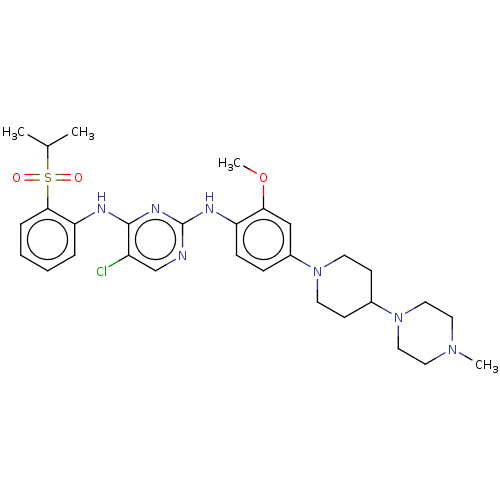 (BDBM50242742 | TAE684) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM291994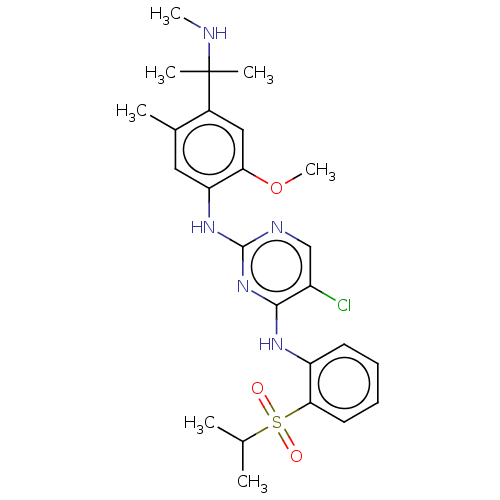 (US10100019, Example 64) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50116681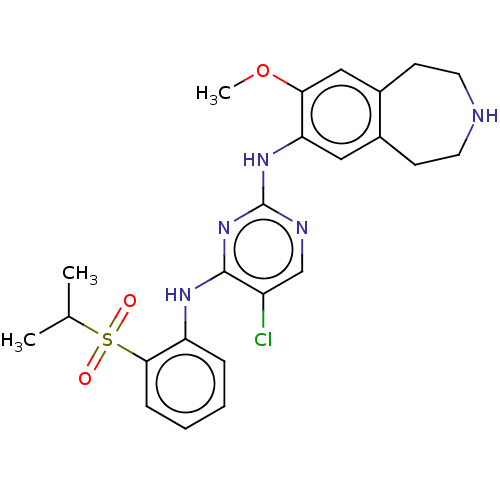 (CHEMBL3608524 | US10053458, 65) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor (unknown origin) by homogeneous time resolved fluorescence assay | Bioorg Med Chem Lett 25: 3992-8 (2015) Article DOI: 10.1016/j.bmcl.2015.07.004 BindingDB Entry DOI: 10.7270/Q2RF5WTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM291971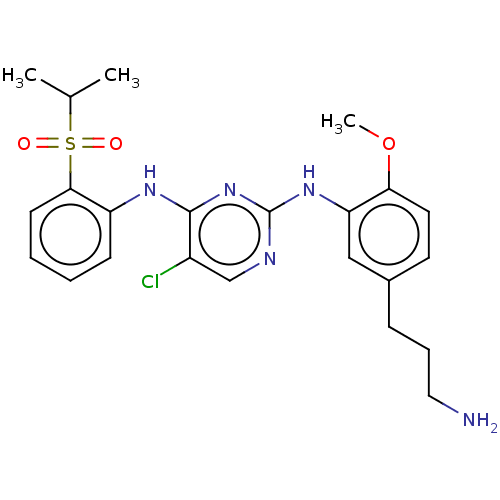 (US10100019, Example 41) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50240274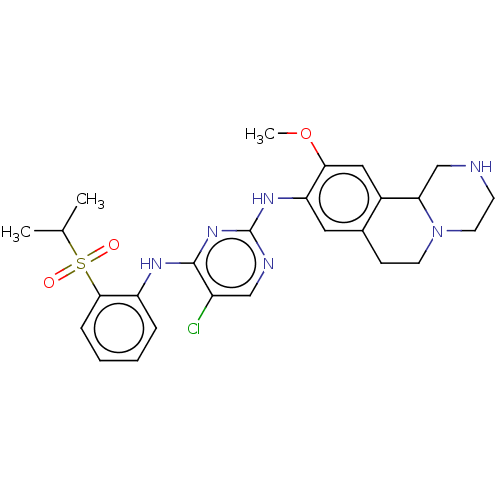 (CHEMBL4092174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes. | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50185160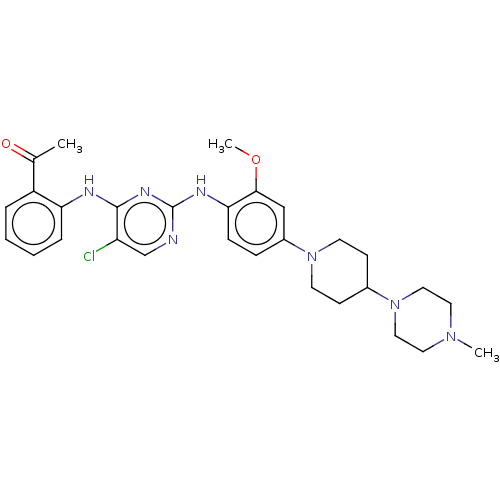 (CHEMBL3824301) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM244039 (10-((5-chloro-4-(2-(isopropylsulfonyl)phenyl)amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes. | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM291934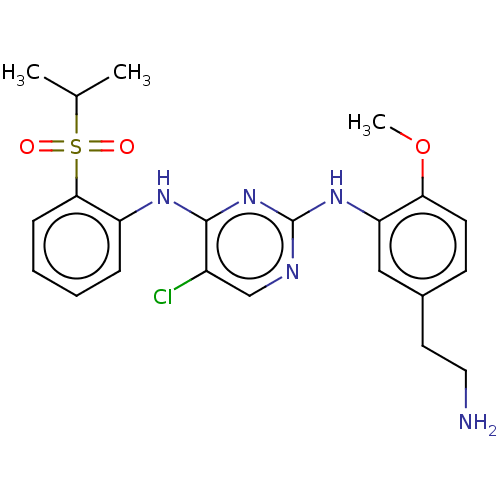 (US10100019, Example 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM291988 (US10100019, Example 58) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM291987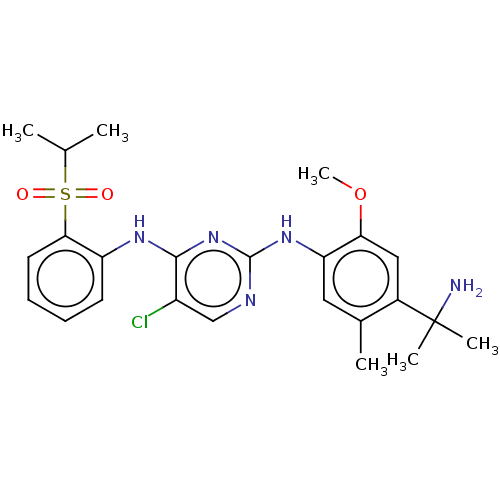 (US10100019, Example 57) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM291995 (US10100019, Example 65) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM244025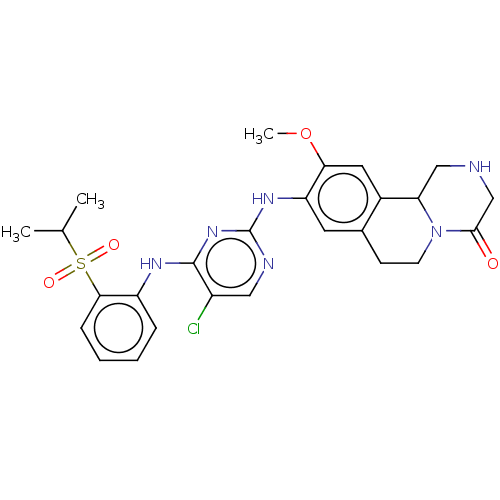 (9-((5-chloro-4-(2-(isopropylsulfonyl)phenyl)aminop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50340121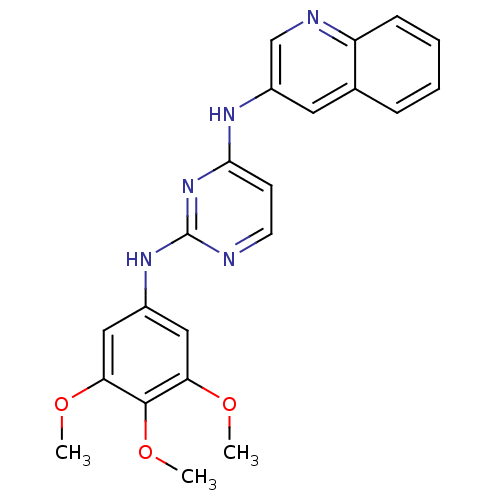 (CHEMBL1760035 | N4-(quinolin-3-yl)-N2-(3,4,5-trime...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of INSR | Bioorg Med Chem Lett 21: 2394-9 (2011) Article DOI: 10.1016/j.bmcl.2011.02.075 BindingDB Entry DOI: 10.7270/Q28G8M0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50363711 (CHEMBL370967) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University College of Medicine Curated by ChEMBL | Assay Description Displacement of thioflavin T from insulin receptor by thioflavin-T fluorescent dye assay | Bioorg Med Chem 20: 1434-41 (2012) Article DOI: 10.1016/j.bmc.2011.12.062 BindingDB Entry DOI: 10.7270/Q2M32W7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM291986 (US10100019, Example 56) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50185163 (CHEMBL3824246) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM291993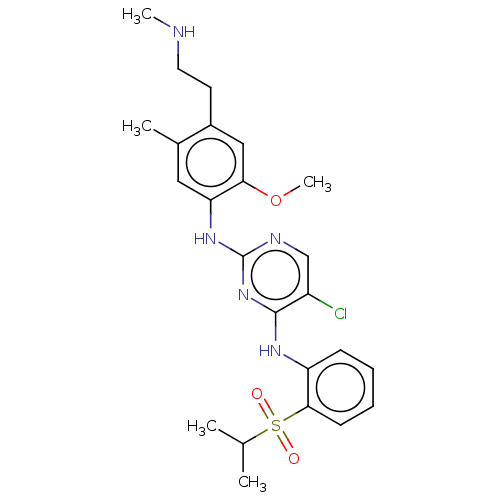 (US10100019, Example 63) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM291998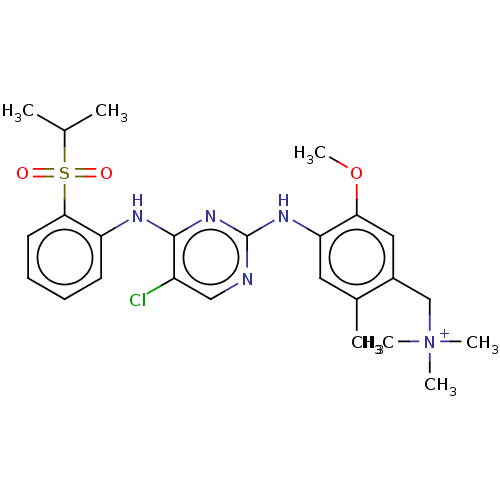 (US10100019, Example 69) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM292001 (US10100019, Example 72) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM291990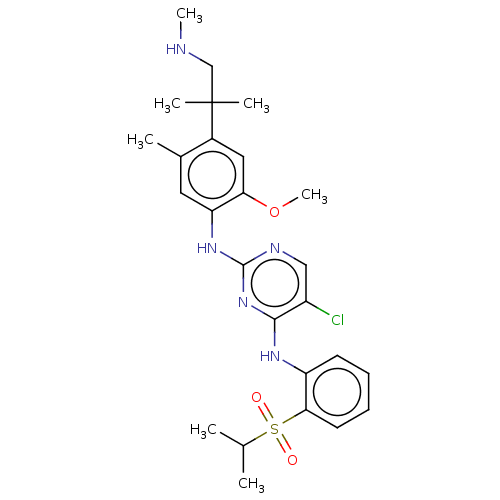 (US10100019, Example 60) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50334080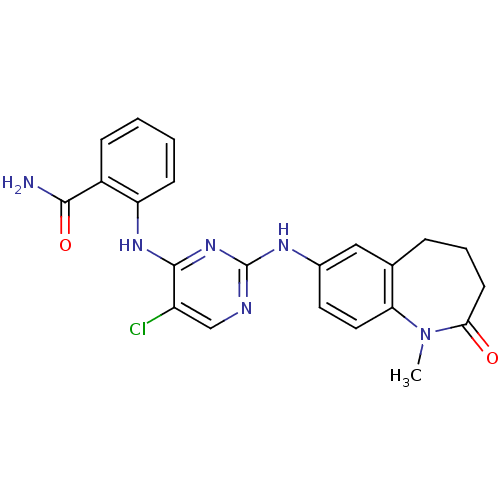 (2-[5-Chloro-2-(1-methyl-2-oxo-2,3,4,5-tetrahydro-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc Curated by ChEMBL | Assay Description Inhibition of IR kinase | Bioorg Med Chem Lett 21: 164-7 (2010) Checked by Author Article DOI: 10.1016/j.bmcl.2010.11.045 BindingDB Entry DOI: 10.7270/Q28W3F85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50311921 (CHEMBL1086731 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of Tel-fused InsR expressed in mouse BAF3 cells | Bioorg Med Chem Lett 19: 6691-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.123 BindingDB Entry DOI: 10.7270/Q2Q52PRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50185286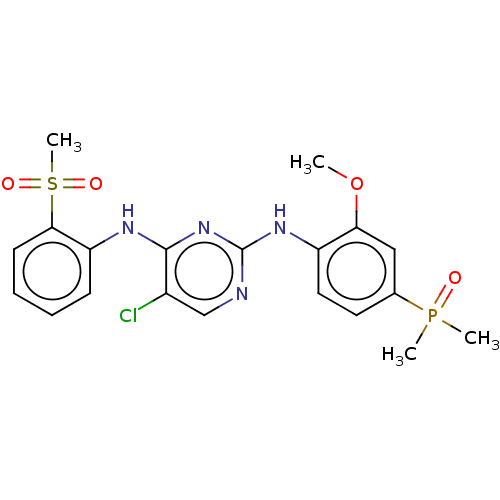 (CHEMBL3823256) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM291999 (US10100019, Example 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50256478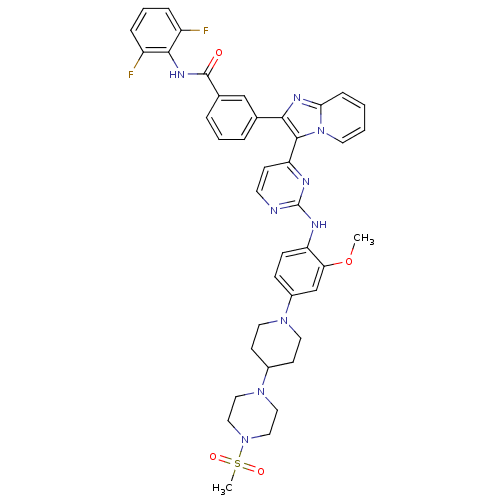 (CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged insulin receptor expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50116680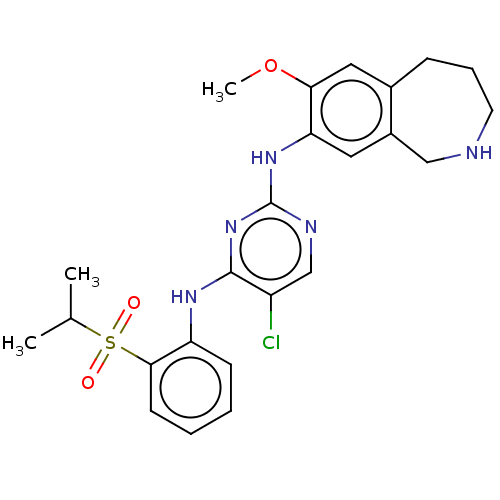 (CHEMBL3608523 | US10053458, 56) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor (unknown origin) by homogeneous time resolved fluorescence assay | Bioorg Med Chem Lett 25: 3992-8 (2015) Article DOI: 10.1016/j.bmcl.2015.07.004 BindingDB Entry DOI: 10.7270/Q2RF5WTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50185152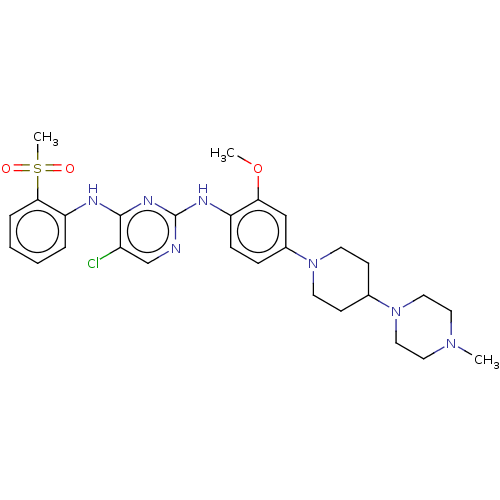 (CHEMBL3822475) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50363712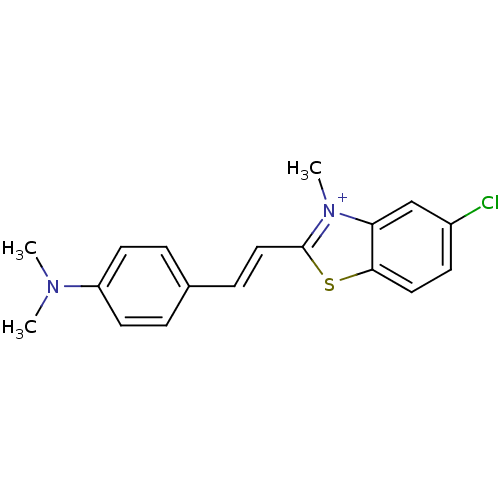 (CHEMBL1945931) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University College of Medicine Curated by ChEMBL | Assay Description Displacement of thioflavin T from insulin receptor by thioflavin-T fluorescent dye assay | Bioorg Med Chem 20: 1434-41 (2012) Article DOI: 10.1016/j.bmc.2011.12.062 BindingDB Entry DOI: 10.7270/Q2M32W7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50116679 (CHEMBL3608522 | US10053458, 47) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor (unknown origin) by homogeneous time resolved fluorescence assay | Bioorg Med Chem Lett 25: 3992-8 (2015) Article DOI: 10.1016/j.bmcl.2015.07.004 BindingDB Entry DOI: 10.7270/Q2RF5WTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM291997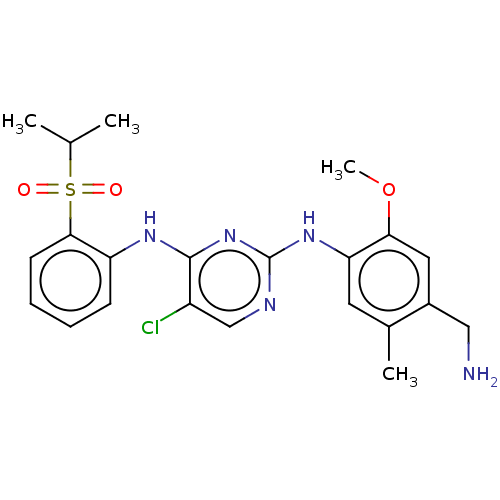 (US10100019, Example 68) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50240270 (CHEMBL4099922) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50363710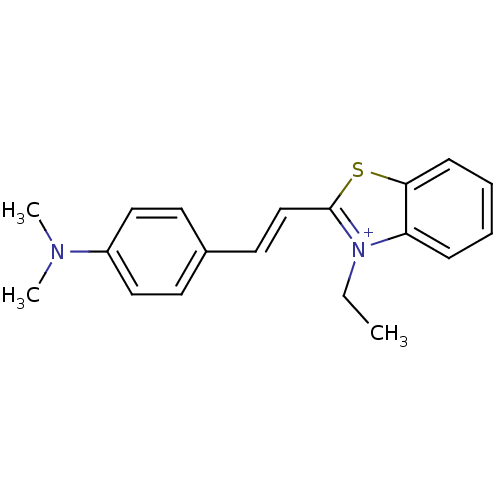 (CHEMBL370968) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University College of Medicine Curated by ChEMBL | Assay Description Displacement of thioflavin T from insulin receptor by thioflavin-T fluorescent dye assay | Bioorg Med Chem 20: 1434-41 (2012) Article DOI: 10.1016/j.bmc.2011.12.062 BindingDB Entry DOI: 10.7270/Q2M32W7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM291996 (US10100019, Example 66) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM292000 (US10100019, Example 71) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50363715 (CHEMBL1944955) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University College of Medicine Curated by ChEMBL | Assay Description Displacement of thioflavin T from insulin receptor by thioflavin-T fluorescent dye assay | Bioorg Med Chem 20: 1434-41 (2012) Article DOI: 10.1016/j.bmc.2011.12.062 BindingDB Entry DOI: 10.7270/Q2M32W7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50185162 (CHEMBL3823016) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hr | J Med Chem 59: 4948-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00306 BindingDB Entry DOI: 10.7270/Q2NK3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM291991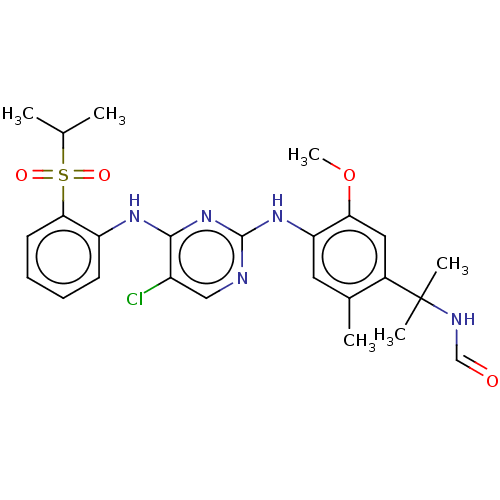 (US10100019, Example 61) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50436850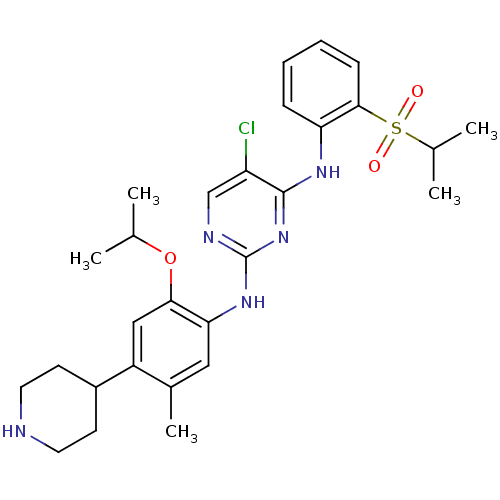 (CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of insulin receptor (unknown origin) after 60 mins | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50256479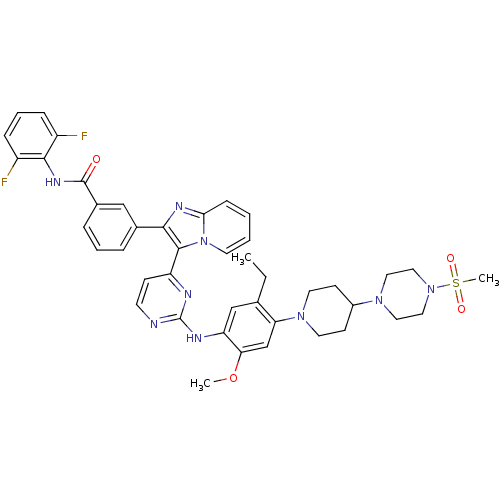 (CHEMBL448929 | N-(2,6-difluorophenyl)-3-(3-(2-(5-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged insulin receptor expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50363714 (CHEMBL1944954) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University College of Medicine Curated by ChEMBL | Assay Description Displacement of thioflavin T from insulin receptor by thioflavin-T fluorescent dye assay | Bioorg Med Chem 20: 1434-41 (2012) Article DOI: 10.1016/j.bmc.2011.12.062 BindingDB Entry DOI: 10.7270/Q2M32W7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50240272 (CHEMBL4063965) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50334082 (2-[5-Chloro-2-(1-methyl-2-oxo-2,3,4,5-tetrahydro-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc Curated by ChEMBL | Assay Description Inhibition of IR kinase | Bioorg Med Chem Lett 21: 164-7 (2010) Checked by Author Article DOI: 10.1016/j.bmcl.2010.11.045 BindingDB Entry DOI: 10.7270/Q28W3F85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50520589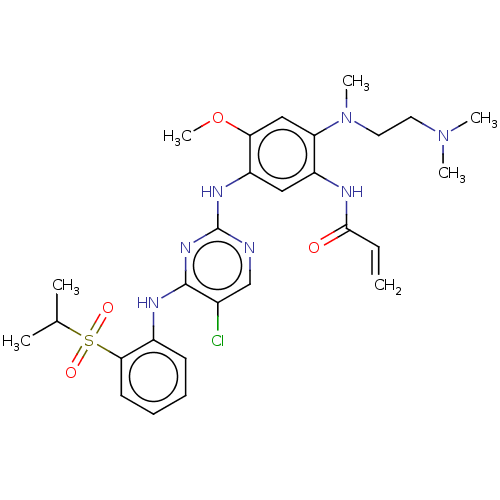 (CHEMBL4473365) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human InsR (1011 to 1382 residues) expressed in baculovirus expression system by Z-LYTE assay | Eur J Med Chem 136: 497-510 (2017) Article DOI: 10.1016/j.ejmech.2017.04.079 BindingDB Entry DOI: 10.7270/Q2WH2TCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50363716 (CHEMBL1944956) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University College of Medicine Curated by ChEMBL | Assay Description Displacement of thioflavin T from insulin receptor by thioflavin-T fluorescent dye assay | Bioorg Med Chem 20: 1434-41 (2012) Article DOI: 10.1016/j.bmc.2011.12.062 BindingDB Entry DOI: 10.7270/Q2M32W7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1188 total ) | Next | Last >> |