 Found 19 hits of ki for UniProtKB: P08263
Found 19 hits of ki for UniProtKB: P08263 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50054198

(CHEMBL3310888)Show InChI InChI=1S/C13H9BrO3/c14-8-5-6-12(16)10(7-8)13(17)9-3-1-2-4-11(9)15/h1-7,15-16H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens
Curated by ChEMBL
| Assay Description
Competitive inhibition of human GSTA1 activity by double reciprocal Lineweaver-Burk graph |
Bioorg Med Chem 22: 3957-70 (2014)
Article DOI: 10.1016/j.bmc.2014.06.007
BindingDB Entry DOI: 10.7270/Q28P625B |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50054196
(CHEMBL3310887)Show SMILES [#8]-c1ccccc1\[#6](=[#7]\[#7]-[#6](=O)-c1ccccc1)-c1ccccc1-[#8] Show InChI InChI=1S/C20H16N2O3/c23-17-12-6-4-10-15(17)19(16-11-5-7-13-18(16)24)21-22-20(25)14-8-2-1-3-9-14/h1-13,23-24H,(H,22,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens
Curated by ChEMBL
| Assay Description
Competitive inhibition of human GSTA1 activity by double reciprocal Lineweaver-Burk graph |
Bioorg Med Chem 22: 3957-70 (2014)
Article DOI: 10.1016/j.bmc.2014.06.007
BindingDB Entry DOI: 10.7270/Q28P625B |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50043758

(2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-hexylsulf...)Show InChI InChI=1S/C16H29N3O6S/c1-2-3-4-5-8-26-10-12(15(23)18-9-14(21)22)19-13(20)7-6-11(17)16(24)25/h11-12H,2-10,17H2,1H3,(H,18,23)(H,19,20)(H,21,22)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50054194

(CHEMBL3310886)Show InChI InChI=1S/C19H14O3/c20-17-9-5-4-8-15(17)19(22)16-12-14(10-11-18(16)21)13-6-2-1-3-7-13/h1-12,20-21H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens
Curated by ChEMBL
| Assay Description
Competitive inhibition of human GSTA1 activity by double reciprocal Lineweaver-Burk graph |
Bioorg Med Chem 22: 3957-70 (2014)
Article DOI: 10.1016/j.bmc.2014.06.007
BindingDB Entry DOI: 10.7270/Q28P625B |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50295556

(C6H5-SO2-Glu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-G...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NS(=O)(=O)c1ccccc1)C(C)C)C(N)=O |r,wU:102.110,82.92,53.61,34.35,8.12,wD:93.101,74.81,62.69,39.52,30.31,16.23,4.4,(58.32,-8.81,;56.96,-8.06,;56.94,-6.52,;55.58,-5.78,;55.55,-4.23,;54.21,-3.5,;52.89,-4.29,;52.92,-5.83,;51.54,-3.55,;51.51,-2.01,;52.83,-1.21,;52.8,.33,;54.17,-1.95,;50.22,-4.34,;48.87,-3.6,;48.85,-2.06,;47.55,-4.39,;47.58,-5.94,;48.93,-6.68,;50.33,-6.03,;51.38,-7.15,;50.63,-8.5,;49.12,-8.2,;46.21,-3.65,;44.89,-4.45,;44.92,-5.98,;43.54,-3.7,;42.22,-4.49,;40.87,-3.75,;40.84,-2.21,;39.55,-4.55,;38.21,-3.81,;36.89,-4.6,;36.92,-6.13,;35.53,-3.85,;35.51,-2.32,;34.22,-4.65,;32.87,-3.91,;32.84,-2.36,;31.55,-4.7,;31.58,-6.24,;32.93,-6.99,;34.32,-6.33,;35.38,-7.46,;34.64,-8.8,;35.13,-10.26,;34.13,-11.42,;32.62,-11.13,;32.11,-9.68,;33.12,-8.52,;30.21,-3.96,;28.89,-4.75,;28.92,-6.29,;27.54,-4.01,;27.51,-2.47,;28.83,-1.68,;28.8,-.13,;27.45,.6,;30.11,.66,;26.22,-4.8,;24.87,-4.07,;24.84,-2.52,;23.55,-4.86,;23.59,-6.4,;24.93,-7.14,;26.25,-6.35,;24.96,-8.68,;22.21,-4.11,;20.89,-4.91,;20.91,-6.45,;19.53,-4.16,;18.22,-4.95,;16.87,-4.22,;16.84,-2.67,;15.56,-5.01,;15.59,-6.55,;16.93,-7.29,;16.96,-8.83,;18.25,-6.5,;14.21,-4.27,;12.9,-5.06,;12.93,-6.59,;11.55,-4.31,;11.52,-2.78,;12.84,-1.97,;12.8,-.44,;14.12,.36,;14.08,1.89,;12.74,2.64,;15.4,2.69,;10.22,-5.11,;8.88,-4.37,;8.85,-2.82,;7.55,-5.16,;7.59,-6.7,;8.94,-7.45,;8.97,-8.98,;7.64,-9.78,;10.32,-9.73,;6.21,-4.42,;4.89,-5.21,;4.92,-6.76,;3.54,-4.48,;3.5,-2.93,;4.83,-2.14,;4.81,-.59,;3.45,.15,;6.11,.2,;2.23,-5.26,;.88,-4.52,;1.96,-3.43,;.1,-3.19,;-.19,-5.62,;-1.68,-5.24,;-2.75,-6.34,;-2.34,-7.82,;-.84,-8.2,;.23,-7.1,;39.58,-6.09,;40.93,-6.83,;38.27,-6.88,;56.87,-3.44,;58.23,-4.19,;56.84,-1.9,)| Show InChI InChI=1S/C77H116N24O21S2/c1-39(2)29-53(97-68(112)49(19-14-27-85-77(82)83)94-69(113)50(20-23-58(78)102)95-71(115)52(22-25-63(107)108)101-124(121,122)45-15-10-9-11-16-45)67(111)87-36-61(105)92-57(33-60(80)104)75(119)96-51(21-24-59(79)103)70(114)99-55(31-43-34-86-47-18-13-12-17-46(43)47)72(116)90-42(7)66(110)100-64(41(5)6)76(120)88-37-62(106)91-56(32-44-35-84-38-89-44)74(118)98-54(30-40(3)4)73(117)93-48(65(81)109)26-28-123-8/h9-13,15-18,34-35,38-42,48-57,64,86,101H,14,19-33,36-37H2,1-8H3,(H2,78,102)(H2,79,103)(H2,80,104)(H2,81,109)(H,84,89)(H,87,111)(H,88,120)(H,90,116)(H,91,106)(H,92,105)(H,93,117)(H,94,113)(H,95,115)(H,96,119)(H,97,112)(H,98,118)(H,99,114)(H,100,110)(H,107,108)(H4,82,83,85)/t42-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,64-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by 1-chloro-2,4-dinitrobenzene competitive assay |
Eur J Med Chem 44: 2009-16 (2009)
Article DOI: 10.1016/j.ejmech.2008.10.009
BindingDB Entry DOI: 10.7270/Q2S182J4 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50295554
(C6H5-SO2-Glu-Gln-Ser-Leu-Gly-Asn-Gln-Trp-Ala-Arg-G...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NS(=O)(=O)c1ccccc1)C(N)=O |r,wU:107.116,92.98,63.72,44.46,8.16,wD:98.107,84.92,72.80,49.63,33.42,19.27,4.4,(53.04,-39.54,;51.71,-38.78,;51.72,-37.23,;50.38,-36.46,;50.38,-34.92,;49.04,-34.15,;47.72,-34.92,;47.71,-36.46,;46.38,-34.15,;46.38,-32.6,;47.72,-31.84,;49.05,-32.61,;50.38,-31.84,;50.39,-30.3,;49.05,-29.53,;47.72,-30.29,;45.04,-34.91,;43.71,-34.15,;43.71,-32.6,;42.38,-34.91,;42.38,-36.46,;43.71,-37.22,;45.12,-36.6,;46.15,-37.74,;45.37,-39.08,;43.87,-38.75,;41.04,-34.14,;39.71,-34.91,;39.71,-36.45,;38.37,-34.14,;37.04,-34.9,;35.71,-34.14,;35.71,-32.59,;34.37,-34.9,;34.37,-36.45,;35.71,-37.21,;35.71,-38.76,;37.04,-39.53,;37.04,-41.07,;35.7,-41.84,;38.37,-41.84,;33.04,-34.14,;31.71,-34.9,;31.71,-36.45,;30.37,-34.14,;30.37,-32.59,;29.04,-34.9,;27.71,-34.13,;27.71,-32.59,;26.37,-34.9,;26.37,-36.44,;27.71,-37.21,;29.11,-36.58,;30.14,-37.73,;29.37,-39.07,;29.84,-40.53,;28.81,-41.67,;27.31,-41.35,;26.83,-39.89,;27.87,-38.74,;25.04,-34.13,;23.7,-34.89,;23.7,-36.44,;22.37,-34.13,;22.37,-32.59,;23.71,-31.82,;23.71,-30.27,;22.37,-29.51,;25.04,-29.51,;21.03,-34.89,;19.7,-34.13,;19.71,-32.58,;18.37,-34.89,;18.37,-36.44,;19.7,-37.2,;21.03,-36.44,;19.7,-38.75,;17.04,-34.12,;15.7,-34.89,;15.7,-36.43,;14.36,-34.12,;13.03,-34.89,;11.7,-34.12,;11.7,-32.58,;10.36,-34.88,;10.36,-36.43,;11.7,-37.19,;11.7,-38.74,;13.03,-36.43,;9.04,-34.12,;7.7,-34.88,;7.7,-36.43,;6.36,-34.12,;6.36,-32.58,;7.7,-31.81,;5.03,-34.89,;3.7,-34.11,;3.7,-32.58,;2.36,-34.88,;2.36,-36.42,;3.7,-37.19,;3.69,-38.74,;2.36,-39.51,;5.03,-39.5,;1.03,-34.11,;-.31,-34.88,;-.31,-36.42,;-1.64,-34.11,;-1.64,-32.57,;-.3,-31.8,;-.3,-30.26,;-1.64,-29.49,;1.03,-29.5,;-2.97,-34.87,;-4.29,-34.11,;-3.21,-33.01,;-5.07,-32.77,;-5.37,-35.2,;-6.85,-34.82,;-7.93,-35.91,;-7.52,-37.39,;-6.02,-37.77,;-4.95,-36.68,;51.71,-34.15,;53.05,-34.92,;51.71,-32.61,)| Show InChI InChI=1S/C78H110N24O22S2/c1-41(2)30-54(98-77(122)59(39-103)101-71(116)52(22-25-61(80)105)96-72(117)53(23-26-65(109)110)102-126(123,124)46-16-9-6-10-17-46)69(114)89-38-64(108)93-58(34-62(81)106)76(121)97-51(21-24-60(79)104)70(115)100-56(32-44-35-87-48-19-12-11-18-47(44)48)73(118)91-42(3)67(112)95-50(20-13-28-86-78(83)84)68(113)88-37-63(107)92-57(33-45-36-85-40-90-45)75(120)99-55(31-43-14-7-5-8-15-43)74(119)94-49(66(82)111)27-29-125-4/h5-12,14-19,35-36,40-42,49-59,87,102-103H,13,20-34,37-39H2,1-4H3,(H2,79,104)(H2,80,105)(H2,81,106)(H2,82,111)(H,85,90)(H,88,113)(H,89,114)(H,91,118)(H,92,107)(H,93,108)(H,94,119)(H,95,112)(H,96,117)(H,97,121)(H,98,122)(H,99,120)(H,100,115)(H,101,116)(H,109,110)(H4,83,84,86)/t42-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by 1-chloro-2,4-dinitrobenzene competitive assay |
Eur J Med Chem 44: 2009-16 (2009)
Article DOI: 10.1016/j.ejmech.2008.10.009
BindingDB Entry DOI: 10.7270/Q2S182J4 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50295555
(C6H5-SO2-Glu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-G...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NS(=O)(=O)c1ccccc1)C(C)C)C(N)=O |r,wU:105.114,85.96,56.65,37.39,8.16,wD:96.105,77.85,65.73,42.56,33.35,19.27,4.4,(57.23,-24.89,;55.88,-24.13,;55.87,-22.59,;54.53,-21.83,;54.52,-20.28,;53.18,-19.53,;51.85,-20.31,;51.86,-21.85,;50.51,-19.56,;50.5,-18.01,;51.83,-17.23,;53.16,-17.99,;54.49,-17.21,;54.48,-15.67,;53.14,-14.91,;51.81,-15.69,;49.18,-20.33,;47.84,-19.58,;47.83,-18.04,;46.52,-20.36,;46.53,-21.9,;47.87,-22.65,;49.27,-22.02,;50.31,-23.15,;49.55,-24.5,;48.04,-24.18,;45.18,-19.6,;43.85,-20.38,;43.86,-21.92,;42.51,-19.62,;41.18,-20.4,;39.84,-19.65,;39.83,-18.1,;38.51,-20.42,;37.18,-19.67,;35.85,-20.45,;35.86,-21.98,;34.5,-19.69,;34.49,-18.15,;33.18,-20.47,;31.84,-19.71,;31.83,-18.17,;30.51,-20.49,;30.52,-22.03,;31.87,-22.79,;33.27,-22.15,;34.31,-23.29,;33.55,-24.62,;34.03,-26.09,;33.02,-27.24,;31.51,-26.93,;31.02,-25.48,;32.04,-24.32,;29.18,-19.73,;27.85,-20.51,;27.86,-22.06,;26.5,-19.76,;26.49,-18.22,;27.82,-17.44,;27.81,-15.89,;26.47,-15.14,;29.13,-15.12,;25.18,-20.54,;23.84,-19.78,;23.83,-18.24,;22.51,-20.56,;22.53,-22.1,;23.86,-22.86,;25.19,-22.08,;23.88,-24.4,;21.17,-19.8,;19.85,-20.58,;19.86,-22.12,;18.5,-19.83,;17.18,-20.6,;15.84,-19.85,;15.83,-18.3,;14.51,-20.63,;14.52,-22.17,;15.87,-22.92,;15.88,-24.46,;17.19,-22.14,;13.18,-19.87,;11.86,-20.65,;11.87,-22.18,;10.51,-19.89,;10.5,-18.35,;11.84,-17.58,;11.81,-16.03,;13.13,-15.26,;13.13,-13.71,;11.78,-12.96,;14.45,-12.94,;9.18,-20.67,;7.85,-19.93,;7.83,-18.38,;6.51,-20.69,;6.53,-22.23,;7.87,-22.99,;7.88,-24.53,;6.55,-25.31,;9.23,-25.29,;5.18,-19.94,;3.85,-20.73,;3.86,-22.26,;2.5,-19.96,;2.49,-18.43,;3.82,-17.63,;3.81,-16.09,;2.46,-15.34,;5.13,-15.32,;1.18,-20.74,;-.16,-19.99,;.6,-18.66,;-.94,-18.66,;-1.02,-21.26,;-2.55,-21.14,;-3.41,-22.41,;-2.75,-23.79,;-1.21,-23.9,;-.35,-22.64,;38.52,-21.97,;39.87,-22.72,;37.2,-22.74,;55.84,-19.51,;57.19,-20.27,;55.83,-17.96,)| Show InChI InChI=1S/C80H114N24O21S2/c1-42(2)32-56(100-71(115)52(22-15-30-88-80(85)86)97-72(116)53(23-26-61(81)105)98-74(118)55(25-28-66(110)111)104-127(124,125)48-18-11-8-12-19-48)70(114)90-39-64(108)95-60(36-63(83)107)78(122)99-54(24-27-62(82)106)73(117)102-58(34-46-37-89-50-21-14-13-20-49(46)50)75(119)93-44(5)69(113)103-67(43(3)4)79(123)91-40-65(109)94-59(35-47-38-87-41-92-47)77(121)101-57(33-45-16-9-7-10-17-45)76(120)96-51(68(84)112)29-31-126-6/h7-14,16-21,37-38,41-44,51-60,67,89,104H,15,22-36,39-40H2,1-6H3,(H2,81,105)(H2,82,106)(H2,83,107)(H2,84,112)(H,87,92)(H,90,114)(H,91,123)(H,93,119)(H,94,109)(H,95,108)(H,96,120)(H,97,116)(H,98,118)(H,99,122)(H,100,115)(H,101,121)(H,102,117)(H,103,113)(H,110,111)(H4,85,86,88)/t44-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,67-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by 1-chloro-2,4-dinitrobenzene competitive assay |
Eur J Med Chem 44: 2009-16 (2009)
Article DOI: 10.1016/j.ejmech.2008.10.009
BindingDB Entry DOI: 10.7270/Q2S182J4 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50054205
(CHEMBL3310889)Show InChI InChI=1S/C15H13BrN2O3/c1-9(19)17-18-15(11-4-2-3-5-13(11)20)12-8-10(16)6-7-14(12)21/h2-8,20-21H,1H3,(H,17,19)/b18-15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens
Curated by ChEMBL
| Assay Description
Competitive inhibition of human GSTA1 activity by double reciprocal Lineweaver-Burk graph |
Bioorg Med Chem 22: 3957-70 (2014)
Article DOI: 10.1016/j.bmc.2014.06.007
BindingDB Entry DOI: 10.7270/Q28P625B |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50043764
(2-Amino-4-{1-[(carboxy-phenyl-methyl)-carbamoyl]-2...)Show SMILES CCCCCCSCC(NC(=O)CCC(N)C(O)=O)C(=O)NC(C(O)=O)c1ccccc1 Show InChI InChI=1S/C22H33N3O6S/c1-2-3-4-8-13-32-14-17(24-18(26)12-11-16(23)21(28)29)20(27)25-19(22(30)31)15-9-6-5-7-10-15/h5-7,9-10,16-17,19H,2-4,8,11-14,23H2,1H3,(H,24,26)(H,25,27)(H,28,29)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50295556

(C6H5-SO2-Glu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-G...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NS(=O)(=O)c1ccccc1)C(C)C)C(N)=O |r,wU:102.110,82.92,53.61,34.35,8.12,wD:93.101,74.81,62.69,39.52,30.31,16.23,4.4,(58.32,-8.81,;56.96,-8.06,;56.94,-6.52,;55.58,-5.78,;55.55,-4.23,;54.21,-3.5,;52.89,-4.29,;52.92,-5.83,;51.54,-3.55,;51.51,-2.01,;52.83,-1.21,;52.8,.33,;54.17,-1.95,;50.22,-4.34,;48.87,-3.6,;48.85,-2.06,;47.55,-4.39,;47.58,-5.94,;48.93,-6.68,;50.33,-6.03,;51.38,-7.15,;50.63,-8.5,;49.12,-8.2,;46.21,-3.65,;44.89,-4.45,;44.92,-5.98,;43.54,-3.7,;42.22,-4.49,;40.87,-3.75,;40.84,-2.21,;39.55,-4.55,;38.21,-3.81,;36.89,-4.6,;36.92,-6.13,;35.53,-3.85,;35.51,-2.32,;34.22,-4.65,;32.87,-3.91,;32.84,-2.36,;31.55,-4.7,;31.58,-6.24,;32.93,-6.99,;34.32,-6.33,;35.38,-7.46,;34.64,-8.8,;35.13,-10.26,;34.13,-11.42,;32.62,-11.13,;32.11,-9.68,;33.12,-8.52,;30.21,-3.96,;28.89,-4.75,;28.92,-6.29,;27.54,-4.01,;27.51,-2.47,;28.83,-1.68,;28.8,-.13,;27.45,.6,;30.11,.66,;26.22,-4.8,;24.87,-4.07,;24.84,-2.52,;23.55,-4.86,;23.59,-6.4,;24.93,-7.14,;26.25,-6.35,;24.96,-8.68,;22.21,-4.11,;20.89,-4.91,;20.91,-6.45,;19.53,-4.16,;18.22,-4.95,;16.87,-4.22,;16.84,-2.67,;15.56,-5.01,;15.59,-6.55,;16.93,-7.29,;16.96,-8.83,;18.25,-6.5,;14.21,-4.27,;12.9,-5.06,;12.93,-6.59,;11.55,-4.31,;11.52,-2.78,;12.84,-1.97,;12.8,-.44,;14.12,.36,;14.08,1.89,;12.74,2.64,;15.4,2.69,;10.22,-5.11,;8.88,-4.37,;8.85,-2.82,;7.55,-5.16,;7.59,-6.7,;8.94,-7.45,;8.97,-8.98,;7.64,-9.78,;10.32,-9.73,;6.21,-4.42,;4.89,-5.21,;4.92,-6.76,;3.54,-4.48,;3.5,-2.93,;4.83,-2.14,;4.81,-.59,;3.45,.15,;6.11,.2,;2.23,-5.26,;.88,-4.52,;1.96,-3.43,;.1,-3.19,;-.19,-5.62,;-1.68,-5.24,;-2.75,-6.34,;-2.34,-7.82,;-.84,-8.2,;.23,-7.1,;39.58,-6.09,;40.93,-6.83,;38.27,-6.88,;56.87,-3.44,;58.23,-4.19,;56.84,-1.9,)| Show InChI InChI=1S/C77H116N24O21S2/c1-39(2)29-53(97-68(112)49(19-14-27-85-77(82)83)94-69(113)50(20-23-58(78)102)95-71(115)52(22-25-63(107)108)101-124(121,122)45-15-10-9-11-16-45)67(111)87-36-61(105)92-57(33-60(80)104)75(119)96-51(21-24-59(79)103)70(114)99-55(31-43-34-86-47-18-13-12-17-46(43)47)72(116)90-42(7)66(110)100-64(41(5)6)76(120)88-37-62(106)91-56(32-44-35-84-38-89-44)74(118)98-54(30-40(3)4)73(117)93-48(65(81)109)26-28-123-8/h9-13,15-18,34-35,38-42,48-57,64,86,101H,14,19-33,36-37H2,1-8H3,(H2,78,102)(H2,79,103)(H2,80,104)(H2,81,109)(H,84,89)(H,87,111)(H,88,120)(H,90,116)(H,91,106)(H,92,105)(H,93,117)(H,94,113)(H,95,115)(H,96,119)(H,97,112)(H,98,118)(H,99,114)(H,100,110)(H,107,108)(H4,82,83,85)/t42-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,64-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by glutathione competitive assay |
Eur J Med Chem 44: 2009-16 (2009)
Article DOI: 10.1016/j.ejmech.2008.10.009
BindingDB Entry DOI: 10.7270/Q2S182J4 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50295554

(C6H5-SO2-Glu-Gln-Ser-Leu-Gly-Asn-Gln-Trp-Ala-Arg-G...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NS(=O)(=O)c1ccccc1)C(N)=O |r,wU:107.116,92.98,63.72,44.46,8.16,wD:98.107,84.92,72.80,49.63,33.42,19.27,4.4,(53.04,-39.54,;51.71,-38.78,;51.72,-37.23,;50.38,-36.46,;50.38,-34.92,;49.04,-34.15,;47.72,-34.92,;47.71,-36.46,;46.38,-34.15,;46.38,-32.6,;47.72,-31.84,;49.05,-32.61,;50.38,-31.84,;50.39,-30.3,;49.05,-29.53,;47.72,-30.29,;45.04,-34.91,;43.71,-34.15,;43.71,-32.6,;42.38,-34.91,;42.38,-36.46,;43.71,-37.22,;45.12,-36.6,;46.15,-37.74,;45.37,-39.08,;43.87,-38.75,;41.04,-34.14,;39.71,-34.91,;39.71,-36.45,;38.37,-34.14,;37.04,-34.9,;35.71,-34.14,;35.71,-32.59,;34.37,-34.9,;34.37,-36.45,;35.71,-37.21,;35.71,-38.76,;37.04,-39.53,;37.04,-41.07,;35.7,-41.84,;38.37,-41.84,;33.04,-34.14,;31.71,-34.9,;31.71,-36.45,;30.37,-34.14,;30.37,-32.59,;29.04,-34.9,;27.71,-34.13,;27.71,-32.59,;26.37,-34.9,;26.37,-36.44,;27.71,-37.21,;29.11,-36.58,;30.14,-37.73,;29.37,-39.07,;29.84,-40.53,;28.81,-41.67,;27.31,-41.35,;26.83,-39.89,;27.87,-38.74,;25.04,-34.13,;23.7,-34.89,;23.7,-36.44,;22.37,-34.13,;22.37,-32.59,;23.71,-31.82,;23.71,-30.27,;22.37,-29.51,;25.04,-29.51,;21.03,-34.89,;19.7,-34.13,;19.71,-32.58,;18.37,-34.89,;18.37,-36.44,;19.7,-37.2,;21.03,-36.44,;19.7,-38.75,;17.04,-34.12,;15.7,-34.89,;15.7,-36.43,;14.36,-34.12,;13.03,-34.89,;11.7,-34.12,;11.7,-32.58,;10.36,-34.88,;10.36,-36.43,;11.7,-37.19,;11.7,-38.74,;13.03,-36.43,;9.04,-34.12,;7.7,-34.88,;7.7,-36.43,;6.36,-34.12,;6.36,-32.58,;7.7,-31.81,;5.03,-34.89,;3.7,-34.11,;3.7,-32.58,;2.36,-34.88,;2.36,-36.42,;3.7,-37.19,;3.69,-38.74,;2.36,-39.51,;5.03,-39.5,;1.03,-34.11,;-.31,-34.88,;-.31,-36.42,;-1.64,-34.11,;-1.64,-32.57,;-.3,-31.8,;-.3,-30.26,;-1.64,-29.49,;1.03,-29.5,;-2.97,-34.87,;-4.29,-34.11,;-3.21,-33.01,;-5.07,-32.77,;-5.37,-35.2,;-6.85,-34.82,;-7.93,-35.91,;-7.52,-37.39,;-6.02,-37.77,;-4.95,-36.68,;51.71,-34.15,;53.05,-34.92,;51.71,-32.61,)| Show InChI InChI=1S/C78H110N24O22S2/c1-41(2)30-54(98-77(122)59(39-103)101-71(116)52(22-25-61(80)105)96-72(117)53(23-26-65(109)110)102-126(123,124)46-16-9-6-10-17-46)69(114)89-38-64(108)93-58(34-62(81)106)76(121)97-51(21-24-60(79)104)70(115)100-56(32-44-35-87-48-19-12-11-18-47(44)48)73(118)91-42(3)67(112)95-50(20-13-28-86-78(83)84)68(113)88-37-63(107)92-57(33-45-36-85-40-90-45)75(120)99-55(31-43-14-7-5-8-15-43)74(119)94-49(66(82)111)27-29-125-4/h5-12,14-19,35-36,40-42,49-59,87,102-103H,13,20-34,37-39H2,1-4H3,(H2,79,104)(H2,80,105)(H2,81,106)(H2,82,111)(H,85,90)(H,88,113)(H,89,114)(H,91,118)(H,92,107)(H,93,108)(H,94,119)(H,95,112)(H,96,117)(H,97,121)(H,98,122)(H,99,120)(H,100,115)(H,101,116)(H,109,110)(H4,83,84,86)/t42-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by glutathione competitive assay |
Eur J Med Chem 44: 2009-16 (2009)
Article DOI: 10.1016/j.ejmech.2008.10.009
BindingDB Entry DOI: 10.7270/Q2S182J4 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50295555

(C6H5-SO2-Glu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-G...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NS(=O)(=O)c1ccccc1)C(C)C)C(N)=O |r,wU:105.114,85.96,56.65,37.39,8.16,wD:96.105,77.85,65.73,42.56,33.35,19.27,4.4,(57.23,-24.89,;55.88,-24.13,;55.87,-22.59,;54.53,-21.83,;54.52,-20.28,;53.18,-19.53,;51.85,-20.31,;51.86,-21.85,;50.51,-19.56,;50.5,-18.01,;51.83,-17.23,;53.16,-17.99,;54.49,-17.21,;54.48,-15.67,;53.14,-14.91,;51.81,-15.69,;49.18,-20.33,;47.84,-19.58,;47.83,-18.04,;46.52,-20.36,;46.53,-21.9,;47.87,-22.65,;49.27,-22.02,;50.31,-23.15,;49.55,-24.5,;48.04,-24.18,;45.18,-19.6,;43.85,-20.38,;43.86,-21.92,;42.51,-19.62,;41.18,-20.4,;39.84,-19.65,;39.83,-18.1,;38.51,-20.42,;37.18,-19.67,;35.85,-20.45,;35.86,-21.98,;34.5,-19.69,;34.49,-18.15,;33.18,-20.47,;31.84,-19.71,;31.83,-18.17,;30.51,-20.49,;30.52,-22.03,;31.87,-22.79,;33.27,-22.15,;34.31,-23.29,;33.55,-24.62,;34.03,-26.09,;33.02,-27.24,;31.51,-26.93,;31.02,-25.48,;32.04,-24.32,;29.18,-19.73,;27.85,-20.51,;27.86,-22.06,;26.5,-19.76,;26.49,-18.22,;27.82,-17.44,;27.81,-15.89,;26.47,-15.14,;29.13,-15.12,;25.18,-20.54,;23.84,-19.78,;23.83,-18.24,;22.51,-20.56,;22.53,-22.1,;23.86,-22.86,;25.19,-22.08,;23.88,-24.4,;21.17,-19.8,;19.85,-20.58,;19.86,-22.12,;18.5,-19.83,;17.18,-20.6,;15.84,-19.85,;15.83,-18.3,;14.51,-20.63,;14.52,-22.17,;15.87,-22.92,;15.88,-24.46,;17.19,-22.14,;13.18,-19.87,;11.86,-20.65,;11.87,-22.18,;10.51,-19.89,;10.5,-18.35,;11.84,-17.58,;11.81,-16.03,;13.13,-15.26,;13.13,-13.71,;11.78,-12.96,;14.45,-12.94,;9.18,-20.67,;7.85,-19.93,;7.83,-18.38,;6.51,-20.69,;6.53,-22.23,;7.87,-22.99,;7.88,-24.53,;6.55,-25.31,;9.23,-25.29,;5.18,-19.94,;3.85,-20.73,;3.86,-22.26,;2.5,-19.96,;2.49,-18.43,;3.82,-17.63,;3.81,-16.09,;2.46,-15.34,;5.13,-15.32,;1.18,-20.74,;-.16,-19.99,;.6,-18.66,;-.94,-18.66,;-1.02,-21.26,;-2.55,-21.14,;-3.41,-22.41,;-2.75,-23.79,;-1.21,-23.9,;-.35,-22.64,;38.52,-21.97,;39.87,-22.72,;37.2,-22.74,;55.84,-19.51,;57.19,-20.27,;55.83,-17.96,)| Show InChI InChI=1S/C80H114N24O21S2/c1-42(2)32-56(100-71(115)52(22-15-30-88-80(85)86)97-72(116)53(23-26-61(81)105)98-74(118)55(25-28-66(110)111)104-127(124,125)48-18-11-8-12-19-48)70(114)90-39-64(108)95-60(36-63(83)107)78(122)99-54(24-27-62(82)106)73(117)102-58(34-46-37-89-50-21-14-13-20-49(46)50)75(119)93-44(5)69(113)103-67(43(3)4)79(123)91-40-65(109)94-59(35-47-38-87-41-92-47)77(121)101-57(33-45-16-9-7-10-17-45)76(120)96-51(68(84)112)29-31-126-6/h7-14,16-21,37-38,41-44,51-60,67,89,104H,15,22-36,39-40H2,1-6H3,(H2,81,105)(H2,82,106)(H2,83,107)(H2,84,112)(H,87,92)(H,90,114)(H,91,123)(H,93,119)(H,94,109)(H,95,108)(H,96,120)(H,97,116)(H,98,118)(H,99,122)(H,100,115)(H,101,121)(H,102,117)(H,103,113)(H,110,111)(H4,85,86,88)/t44-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,67-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by glutathione competitive assay |
Eur J Med Chem 44: 2009-16 (2009)
Article DOI: 10.1016/j.ejmech.2008.10.009
BindingDB Entry DOI: 10.7270/Q2S182J4 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50043760
(2-Amino-4-[1-[(carboxy-phenyl-methyl)-carbamoyl]-2...)Show SMILES NC(CCC(=O)NC(CSCc1ccc(Cl)cc1)C(=O)NC(C(O)=O)c1ccccc1)C(O)=O Show InChI InChI=1S/C23H26ClN3O6S/c24-16-8-6-14(7-9-16)12-34-13-18(26-19(28)11-10-17(25)22(30)31)21(29)27-20(23(32)33)15-4-2-1-3-5-15/h1-9,17-18,20H,10-13,25H2,(H,26,28)(H,27,29)(H,30,31)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50043762
(2-Amino-4-{2-benzylsulfanyl-1-[(carboxy-phenyl-met...)Show SMILES NC(CCC(=O)NC(CSCc1ccccc1)C(=O)NC(C(O)=O)c1ccccc1)C(O)=O Show InChI InChI=1S/C23H27N3O6S/c24-17(22(29)30)11-12-19(27)25-18(14-33-13-15-7-3-1-4-8-15)21(28)26-20(23(31)32)16-9-5-2-6-10-16/h1-10,17-18,20H,11-14,24H2,(H,25,27)(H,26,28)(H,29,30)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50043761

(2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-hexylsul...)Show SMILES CCCCCCSCC(NC(=O)CCC(N)C(O)=O)C(=O)NCCC(O)=O Show InChI InChI=1S/C17H31N3O6S/c1-2-3-4-5-10-27-11-13(16(24)19-9-8-15(22)23)20-14(21)7-6-12(18)17(25)26/h12-13H,2-11,18H2,1H3,(H,19,24)(H,20,21)(H,22,23)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50043763
(2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-(4-methy...)Show SMILES Cc1ccc(CSCC(NC(=O)CCC(N)C(O)=O)C(=O)NCCC(O)=O)cc1 Show InChI InChI=1S/C19H27N3O6S/c1-12-2-4-13(5-3-12)10-29-11-15(18(26)21-9-8-17(24)25)22-16(23)7-6-14(20)19(27)28/h2-5,14-15H,6-11,20H2,1H3,(H,21,26)(H,22,23)(H,24,25)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50395590
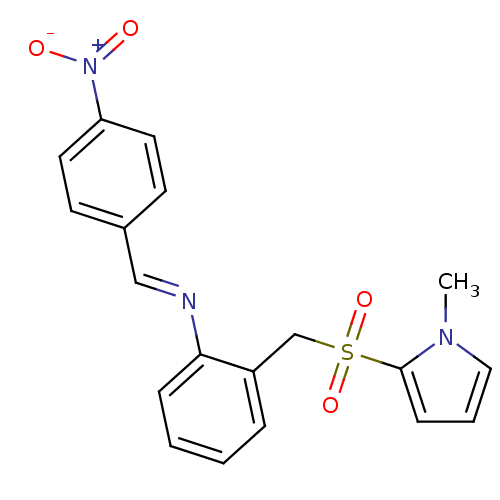
(CHEMBL2165141)Show SMILES Cn1cccc1S(=O)(=O)Cc1ccccc1\N=C\c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C19H17N3O4S/c1-21-12-4-7-19(21)27(25,26)14-16-5-2-3-6-18(16)20-13-15-8-10-17(11-9-15)22(23)24/h2-13H,14H2,1H3/b20-13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human GSTA1-1 expressed in Escherichia coli BL21 (DE3) using CDNB as substrate |
J Med Chem 55: 6802-13 (2012)
Article DOI: 10.1021/jm300385f
BindingDB Entry DOI: 10.7270/Q2DN4654 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50395591
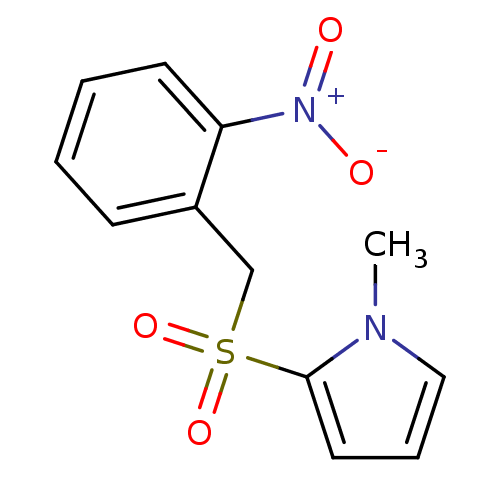
(CHEMBL2165146)Show InChI InChI=1S/C12H12N2O4S/c1-13-8-4-7-12(13)19(17,18)9-10-5-2-3-6-11(10)14(15)16/h2-8H,9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human GSTA1-1 expressed in Escherichia coli BL21 (DE3) using CDNB as substrate |
J Med Chem 55: 6802-13 (2012)
Article DOI: 10.1021/jm300385f
BindingDB Entry DOI: 10.7270/Q2DN4654 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50043759
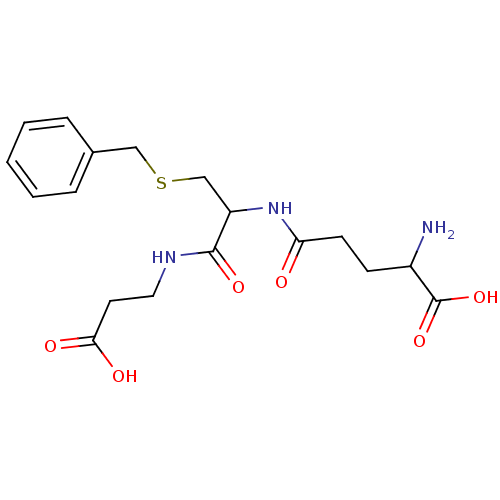
(2-Amino-4-[2-benzylsulfanyl-1-(2-carboxy-ethylcarb...)Show SMILES NC(CCC(=O)NC(CSCc1ccccc1)C(=O)NCCC(O)=O)C(O)=O Show InChI InChI=1S/C18H25N3O6S/c19-13(18(26)27)6-7-15(22)21-14(17(25)20-9-8-16(23)24)11-28-10-12-4-2-1-3-5-12/h1-5,13-14H,6-11,19H2,(H,20,25)(H,21,22)(H,23,24)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


















