

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM217355 (4-benzyl-2-hydroxybenzenecarbohydroxamic acid (HX2...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Otago Christchurch | Assay Description The halogenation activities of LPO (EC 1.11.1.7) and TPO (EC 1.11.1.8) were determined using a modified method of that described previously [Ferrari ... | J Biol Chem 288: 36636-47 (2013) Article DOI: 10.1074/jbc.M113.507756 BindingDB Entry DOI: 10.7270/Q20K27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM92467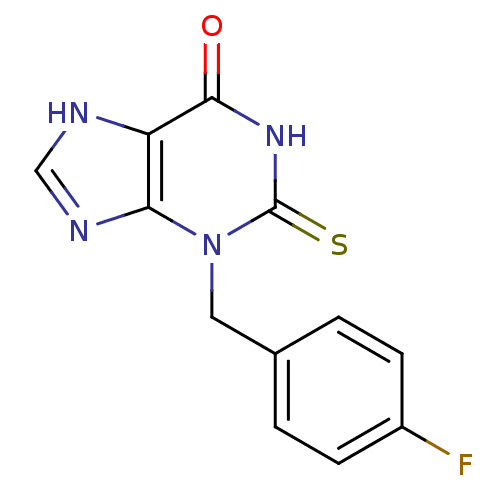 (2-Thioxanthine, TX2) | UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50567713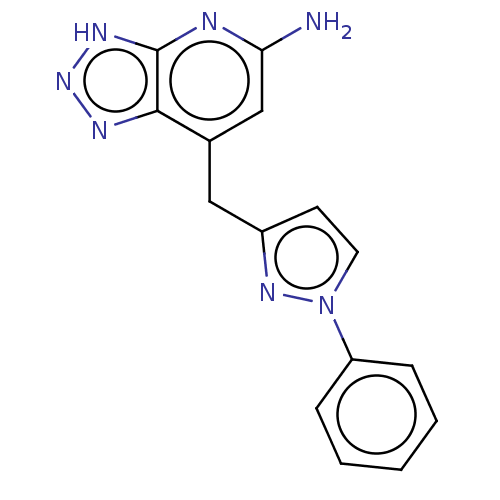 (CHEMBL4859908) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal hexaHis-tagged TPO (unknown origin) expressed in Trichoplusia ni insect cells using 3-iodo tyrosine as substrate preincubate... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50567712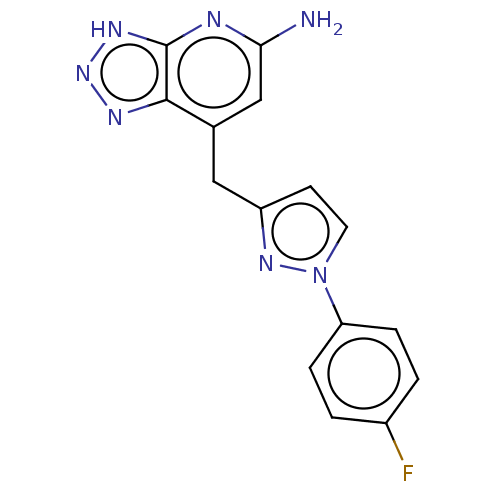 (CHEMBL4869433) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal hexaHis-tagged TPO (unknown origin) expressed in Trichoplusia ni insect cells using 3-iodo tyrosine as substrate preincubate... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50567715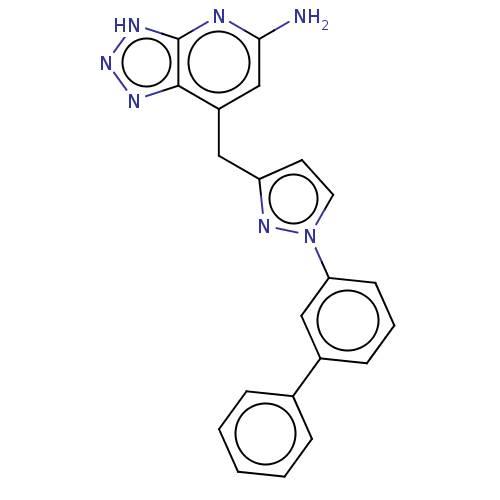 (CHEMBL4855931) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal hexaHis-tagged TPO (unknown origin) expressed in Trichoplusia ni insect cells using 3-iodo tyrosine as substrate preincubate... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50595667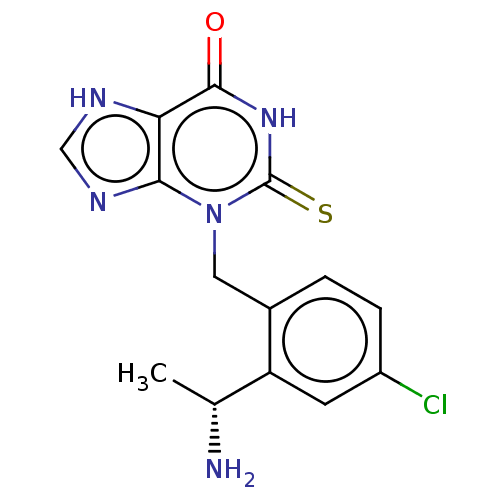 (CHEMBL5181350) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312172 (Alternative Preparation | US10016430, Example 3 | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50133595 (CHEMBL3633460) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50567722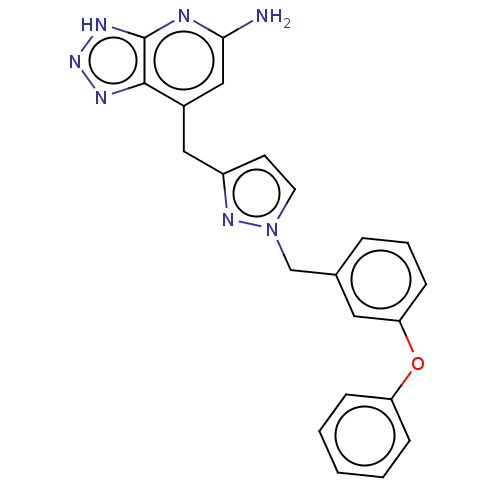 (CHEMBL4862053) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal hexaHis-tagged TPO (unknown origin) expressed in Trichoplusia ni insect cells using 3-iodo tyrosine as substrate preincubate... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50567718 (CHEMBL4863015) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal hexaHis-tagged TPO (unknown origin) expressed in Trichoplusia ni insect cells using 3-iodo tyrosine as substrate preincubate... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50567717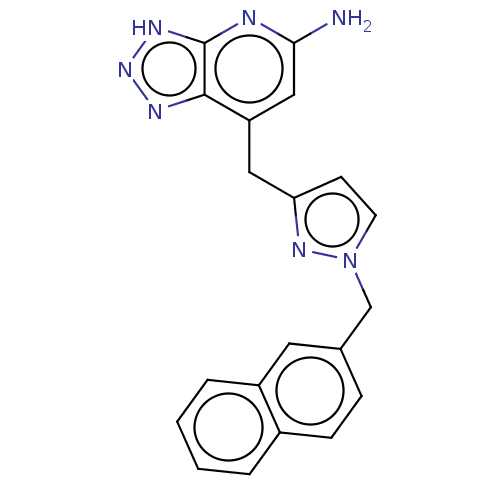 (CHEMBL4853722) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal hexaHis-tagged TPO (unknown origin) expressed in Trichoplusia ni insect cells using 3-iodo tyrosine as substrate preincubate... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312173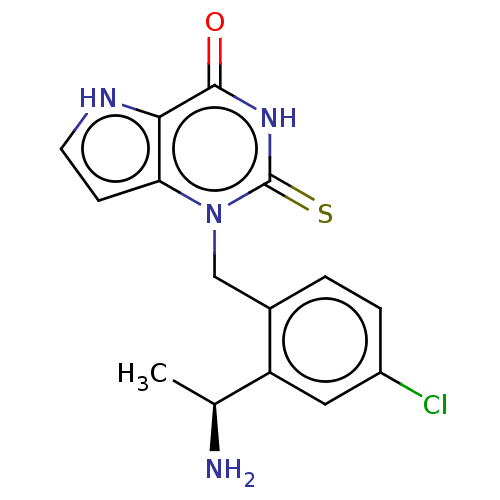 (1-{2-[(1S)-1-Aminoethyl]-4-chlorobenzyl}-2-thioxo-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50595666 (CHEMBL5200126) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312244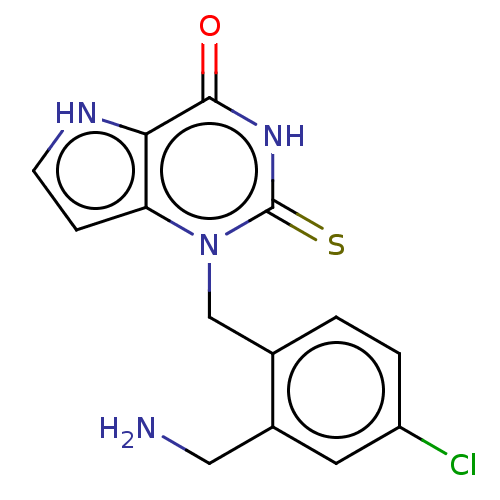 (1-[2-(Aminomethyl)-4-chlorobenzyl]-2-thioxo-1,2,3,...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50595661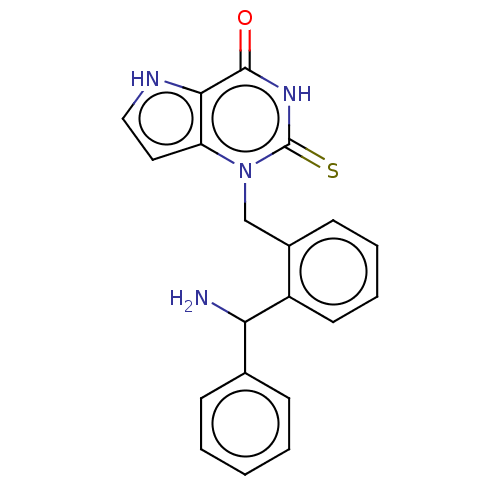 (CHEMBL5197968) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50595663 (CHEMBL5175091) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50595660 (CHEMBL5185576) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM217354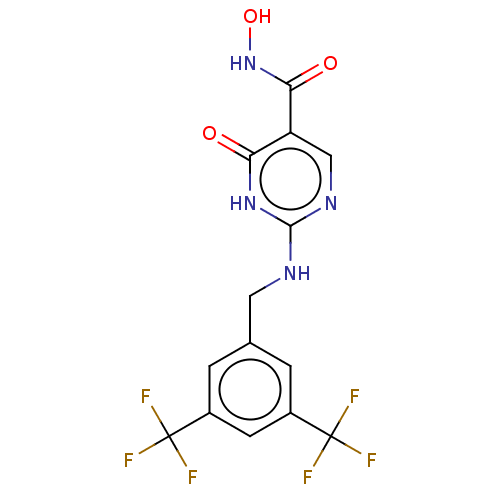 (2-(3,5-bistrifluoromethylbenzylamino)-6-oxo-1H-pyr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Otago Christchurch | Assay Description The halogenation activities of LPO (EC 1.11.1.7) and TPO (EC 1.11.1.8) were determined using a modified method of that described previously [Ferrari ... | J Biol Chem 288: 36636-47 (2013) Article DOI: 10.1074/jbc.M113.507756 BindingDB Entry DOI: 10.7270/Q20K27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM217356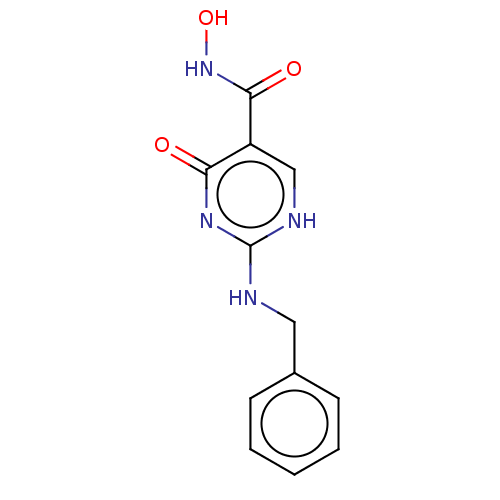 (2-(benzylamino)-6-oxo-3H-pyrimidine-5-carbohydroxa...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Otago Christchurch | Assay Description The halogenation activities of LPO (EC 1.11.1.7) and TPO (EC 1.11.1.8) were determined using a modified method of that described previously [Ferrari ... | J Biol Chem 288: 36636-47 (2013) Article DOI: 10.1074/jbc.M113.507756 BindingDB Entry DOI: 10.7270/Q20K27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50567723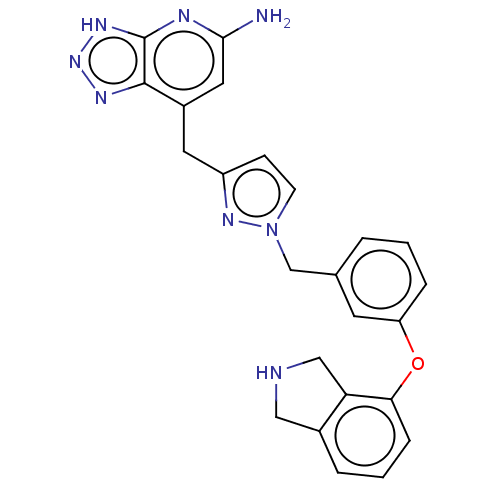 (CHEMBL4860429) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal hexaHis-tagged TPO (unknown origin) expressed in Trichoplusia ni insect cells using 3-iodo tyrosine as substrate preincubate... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50567719 (CHEMBL4871587) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal hexaHis-tagged TPO (unknown origin) expressed in Trichoplusia ni insect cells using 3-iodo tyrosine as substrate preincubate... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50595665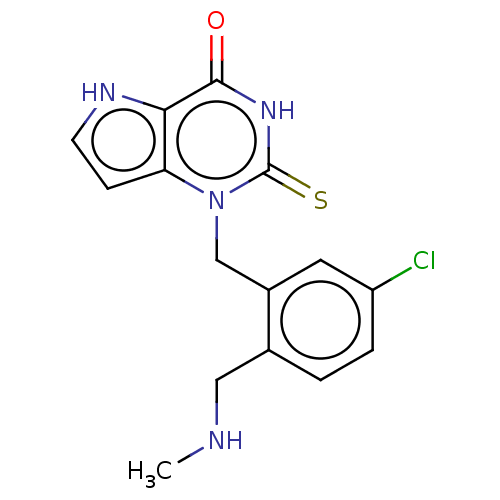 (CHEMBL5186129) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50567721 (CHEMBL4878518) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal hexaHis-tagged TPO (unknown origin) expressed in Trichoplusia ni insect cells using 3-iodo tyrosine as substrate preincubate... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50015089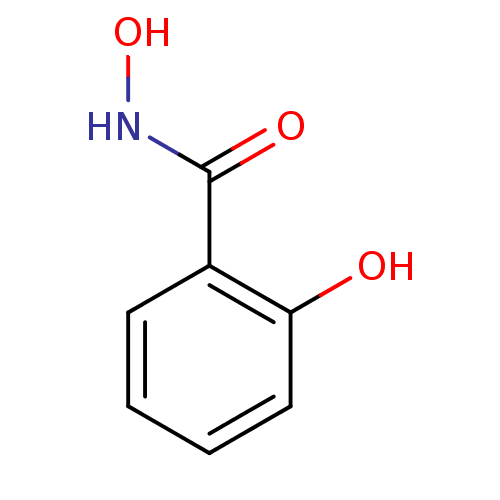 (2,N-Dihydroxy-benzamide | CHEMBL309339 | N,2-dihyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Otago Christchurch | Assay Description The halogenation activities of LPO (EC 1.11.1.7) and TPO (EC 1.11.1.8) were determined using a modified method of that described previously [Ferrari ... | J Biol Chem 288: 36636-47 (2013) Article DOI: 10.1074/jbc.M113.507756 BindingDB Entry DOI: 10.7270/Q20K27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50567714 (CHEMBL4855030) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal hexaHis-tagged TPO (unknown origin) expressed in Trichoplusia ni insect cells using 3-iodo tyrosine as substrate preincubate... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312245 (1-{4-Chloro-2-[(methylamino)methyl]benzyl}-2-thiox...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM357629 (7-benzyl-3H-[1,2,3]triazolo[4,5-b]pyridin-5-amine ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TPO (unknown origin) using 3-iodo tyrosine as substrate incubated for 10 mins | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50567716 (CHEMBL4846080) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal hexaHis-tagged TPO (unknown origin) expressed in Trichoplusia ni insect cells using 3-iodo tyrosine as substrate preincubate... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM92469 (2-Thioxanthine, TX4) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50133597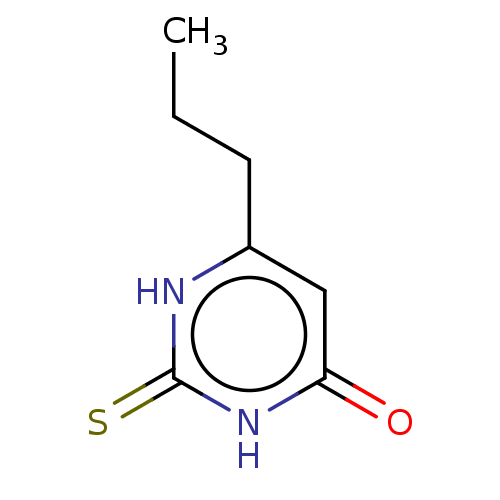 (CHEBI:8502 | Propacil | Propylthiouracil | Prothyr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of TPO (unknown origin) using Amplex Red as substrate assessed as formation of resorufin measured every 20 secs by spectrophotometric anal... | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50595662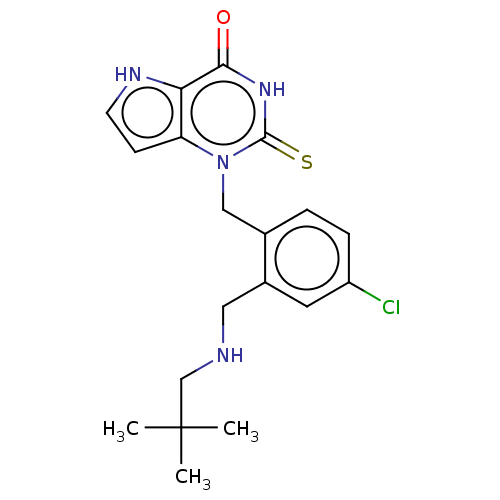 (CHEMBL5197383) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50507390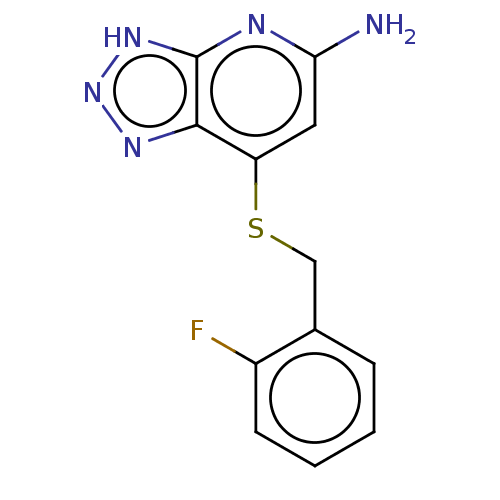 (CHEMBL4482878 | US10981879, Example 3) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human TPO assessed as reduction in H2O2 catalyzed 3,5-iodo tyrosine formation from 3-iodotyrosine and potassium iodide preincubated for... | ACS Med Chem Lett 9: 1175-1180 (2018) Article DOI: 10.1021/acsmedchemlett.8b00308 BindingDB Entry DOI: 10.7270/Q2W0997Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50595659 (CHEMBL5181827) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50595664 (CHEMBL5207346) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312172 (Alternative Preparation | US10016430, Example 3 | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description To detect thyroid peroxidase (TPO) inhibitory activity, the production of hypoiodous acid (HOI) was quantified. HOI was detected by reacting it with ... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312172 (Alternative Preparation | US10016430, Example 3 | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312172 (Alternative Preparation | US10016430, Example 3 | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312157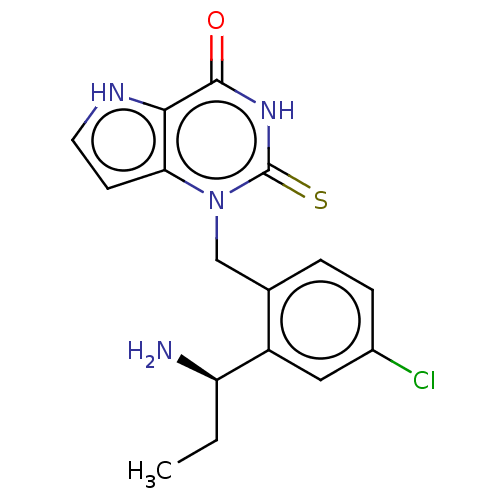 (1-{2-[(1R)-1-Aminopropyl]-4-chlorobenzyl}-2-thioxo...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312157 (1-{2-[(1R)-1-Aminopropyl]-4-chlorobenzyl}-2-thioxo...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312157 (1-{2-[(1R)-1-Aminopropyl]-4-chlorobenzyl}-2-thioxo...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description To detect thyroid peroxidase (TPO) inhibitory activity, the production of hypoiodous acid (HOI) was quantified. HOI was detected by reacting it with ... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50543850 (CHEMBL4645343) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of thyroid peroxidase (unknown origin) | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115548 BindingDB Entry DOI: 10.7270/Q2668HRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50543852 (CHEMBL4633000) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of thyroid peroxidase (unknown origin) | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115548 BindingDB Entry DOI: 10.7270/Q2668HRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50554044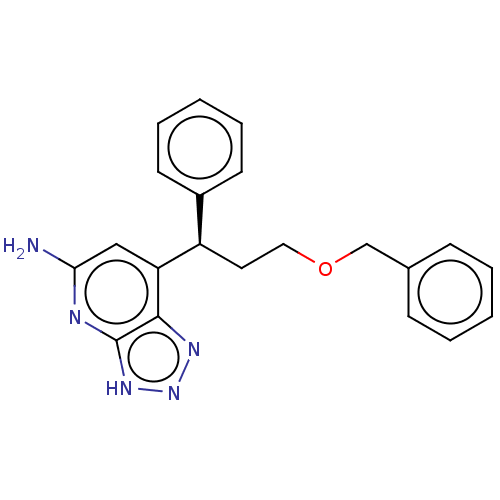 (CHEMBL4792720) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TPO (unknown origin) using 3-iodo tyrosine as substrate incubated for 10 mins | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM50567720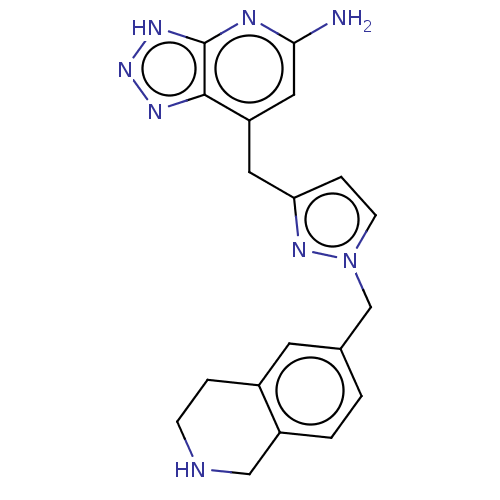 (CHEMBL4855956) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal hexaHis-tagged TPO (unknown origin) expressed in Trichoplusia ni insect cells using 3-iodo tyrosine as substrate preincubate... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312261 (1-{2-[(Propan-2-ylamino)methyl]benzyl}-2-thioxo-1,...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312171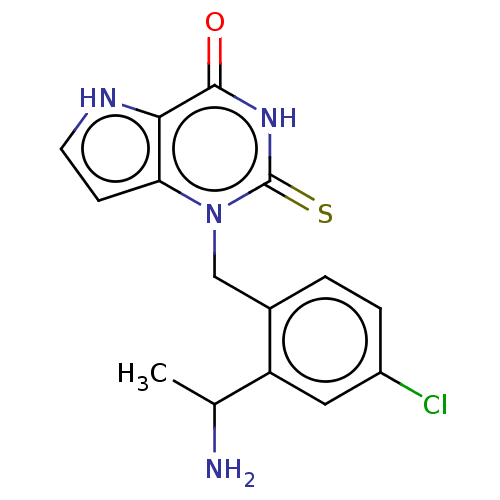 (1-[2-(1-Aminoethyl)-4-chlorobenzyl]-2-thioxo-1,2,3...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description To detect thyroid peroxidase (TPO) inhibitory activity, the production of hypoiodous acid (HOI) was quantified. HOI was detected by reacting it with ... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312171 (1-[2-(1-Aminoethyl)-4-chlorobenzyl]-2-thioxo-1,2,3...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312171 (1-[2-(1-Aminoethyl)-4-chlorobenzyl]-2-thioxo-1,2,3...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312176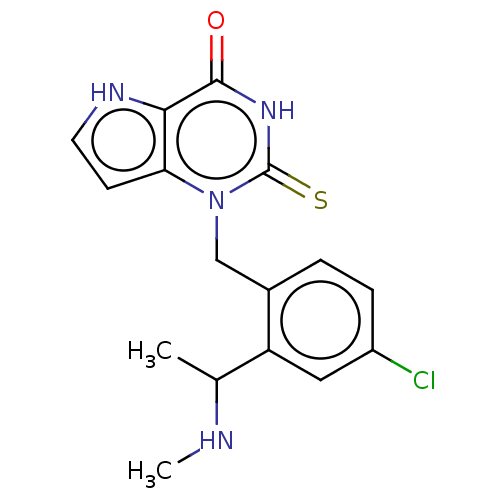 (1-{4-Chloro-2-[1-(methylamino)ethyl]benzyl}-2-thio...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description To detect thyroid peroxidase (TPO) inhibitory activity, the production of hypoiodous acid (HOI) was quantified. HOI was detected by reacting it with ... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid peroxidase (Homo sapiens (Human)) | BDBM312176 (1-{4-Chloro-2-[1-(methylamino)ethyl]benzyl}-2-thio...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 100 total ) | Next | Last >> |