

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50571219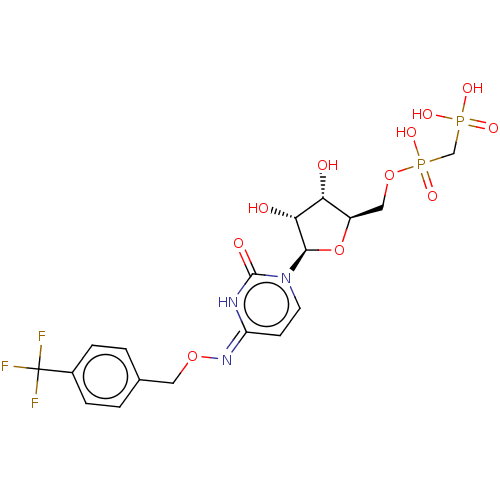 (CHEMBL4847547) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat CD73 using [3H]-AMP as substrate by radiometric assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128137 BindingDB Entry DOI: 10.7270/Q27P935K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50571222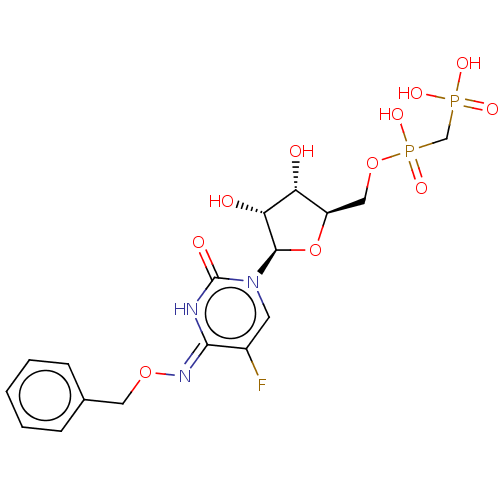 (CHEMBL4871928) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat CD73 using [3H]-AMP as substrate by radiometric assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128137 BindingDB Entry DOI: 10.7270/Q27P935K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437933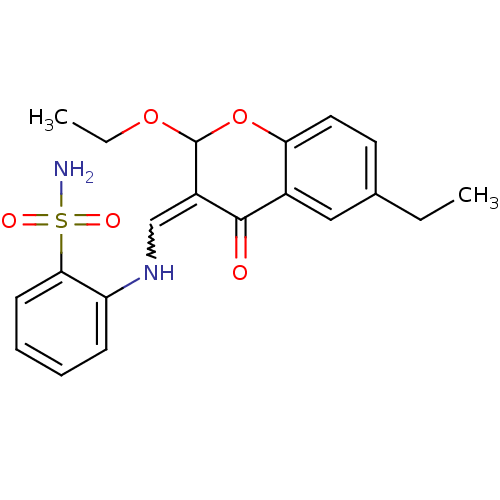 (CHEMBL2408703) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523521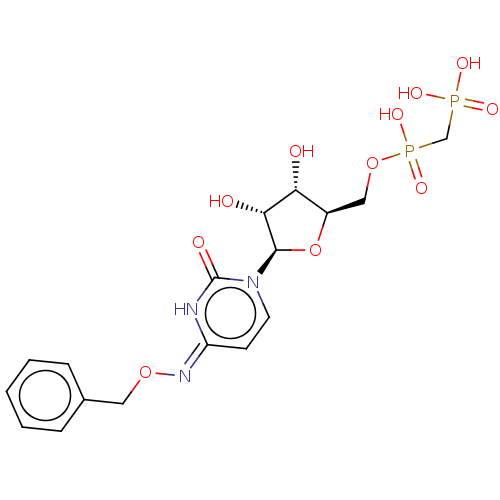 (CHEMBL4299847) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat CD73 using [3H]-AMP as substrate by radiometric assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128137 BindingDB Entry DOI: 10.7270/Q27P935K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM223077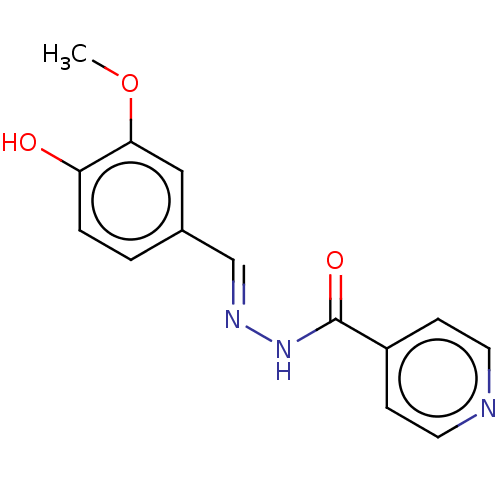 ((E)-N'-(4'-Hydroxy-3-methoxybenzylidene)is...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Quaid-I-Azam University | Assay Description The enzymatic assay of both human and rat ecto-5'-nucleotidase were performed with slight modifications in the previously described method. The stock... | Chem Biol Drug Des 89: 365-370 (2017) Article DOI: 10.1111/cbdd.12861 BindingDB Entry DOI: 10.7270/Q2F47N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50319142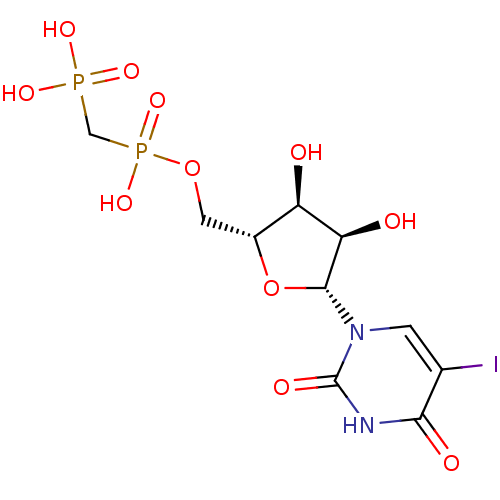 (5-Iodouridine5'-Methylenediphosphate Triethylammon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat CD73 using [3H]-AMP as substrate by radiometric assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128137 BindingDB Entry DOI: 10.7270/Q27P935K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50561936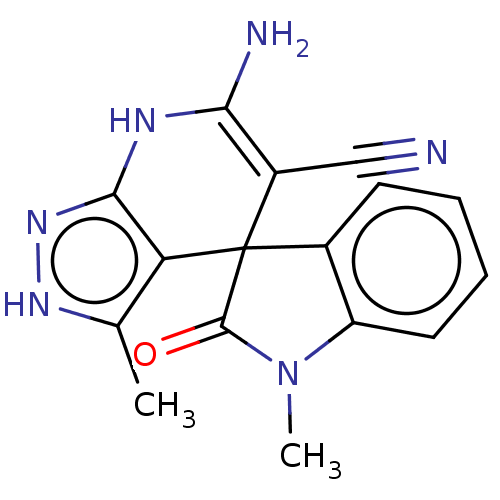 (CHEMBL4741833) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM223076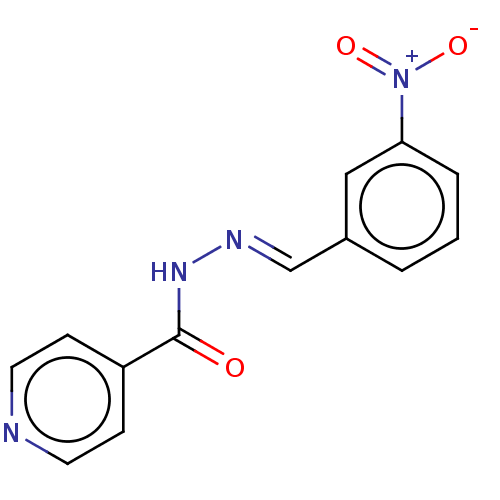 ((E)-N'-(3'-Nitrobenzylidene)isonicotinohyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Quaid-I-Azam University | Assay Description The enzymatic assay of both human and rat ecto-5'-nucleotidase were performed with slight modifications in the previously described method. The stock... | Chem Biol Drug Des 89: 365-370 (2017) Article DOI: 10.1111/cbdd.12861 BindingDB Entry DOI: 10.7270/Q2F47N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523547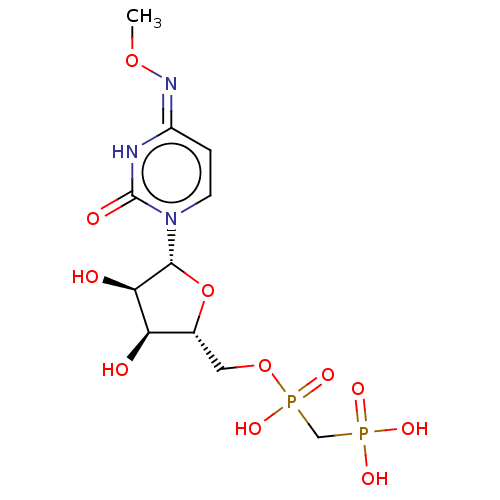 (CHEMBL1198873) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat CD73 using [3H]-AMP as substrate by radiometric assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128137 BindingDB Entry DOI: 10.7270/Q27P935K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM223074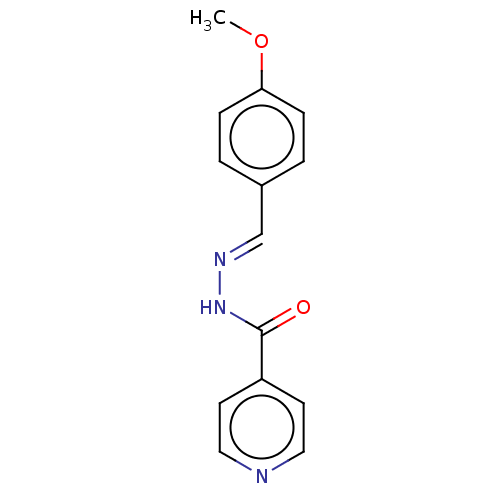 ((E)-N'-(4'-Methoxybenzylidene)isonicotinoh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Quaid-I-Azam University | Assay Description The enzymatic assay of both human and rat ecto-5'-nucleotidase were performed with slight modifications in the previously described method. The stock... | Chem Biol Drug Des 89: 365-370 (2017) Article DOI: 10.1111/cbdd.12861 BindingDB Entry DOI: 10.7270/Q2F47N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50571223 (CHEMBL4875403) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 321 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat CD73 using [3H]-AMP as substrate by radiometric assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128137 BindingDB Entry DOI: 10.7270/Q27P935K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM222261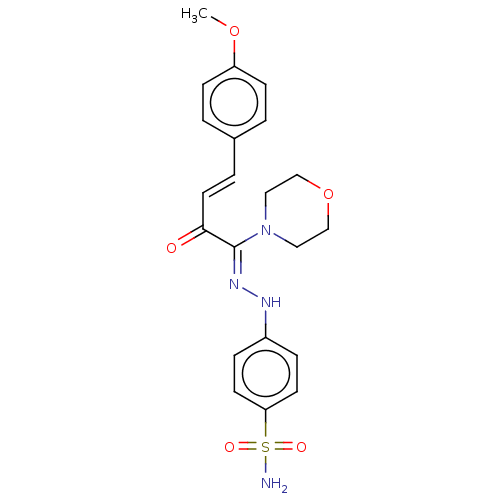 ((Z)-4-(2-((E)-4-(4-methoxyphenyl)-1-morpholino-2-o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | 9.8 | 37 |
COMSATS Institute of Information Technology | Assay Description Alkaline phosphatase assay was optimized and performed in the same way as previously reported method with slight modifications [Sergienko et al., Nat... | Bioorg Chem 70: 229-236 (2017) Article DOI: 10.1016/j.bioorg.2017.01.003 BindingDB Entry DOI: 10.7270/Q2K07334 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM223080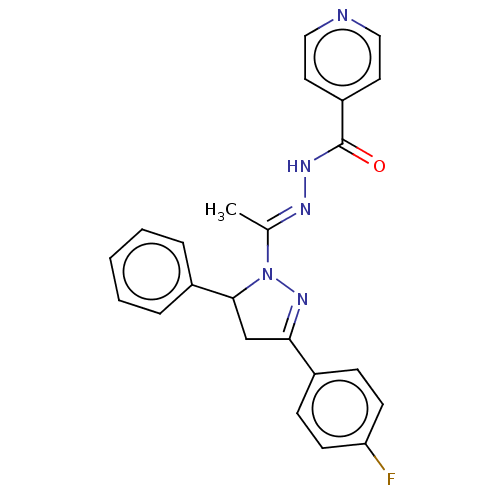 ((E)-N'-(1-(3-(4-fluorophenyl)-5-phenyl-4,5-dih...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Quaid-I-Azam University | Assay Description The enzymatic assay of both human and rat ecto-5'-nucleotidase were performed with slight modifications in the previously described method. The stock... | Chem Biol Drug Des 89: 365-370 (2017) Article DOI: 10.1111/cbdd.12861 BindingDB Entry DOI: 10.7270/Q2F47N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM223073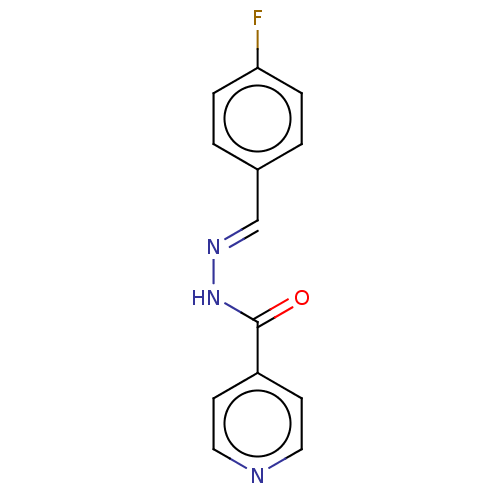 ((E)-N'-(4'-Fluorobenzylidene)isonicotinohy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Quaid-I-Azam University | Assay Description The enzymatic assay of both human and rat ecto-5'-nucleotidase were performed with slight modifications in the previously described method. The stock... | Chem Biol Drug Des 89: 365-370 (2017) Article DOI: 10.1111/cbdd.12861 BindingDB Entry DOI: 10.7270/Q2F47N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM223075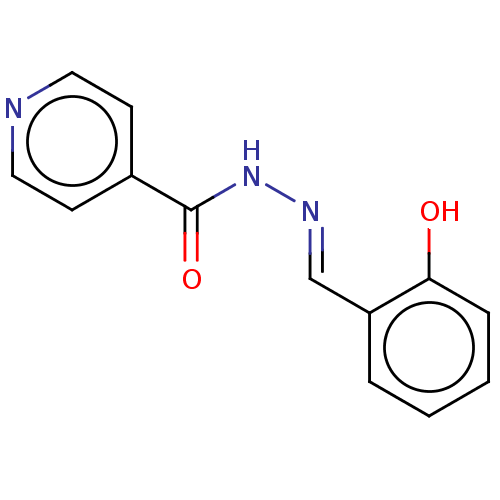 ((E)-N'-(2'-hydroxybenzylidene)isonicotinoh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Quaid-I-Azam University | Assay Description The enzymatic assay of both human and rat ecto-5'-nucleotidase were performed with slight modifications in the previously described method. The stock... | Chem Biol Drug Des 89: 365-370 (2017) Article DOI: 10.1111/cbdd.12861 BindingDB Entry DOI: 10.7270/Q2F47N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50561931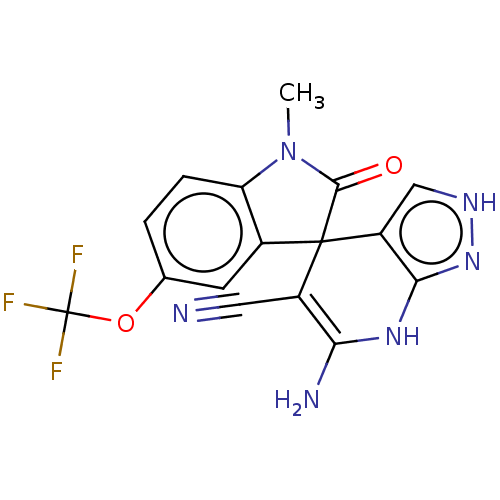 (CHEMBL4746318) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165143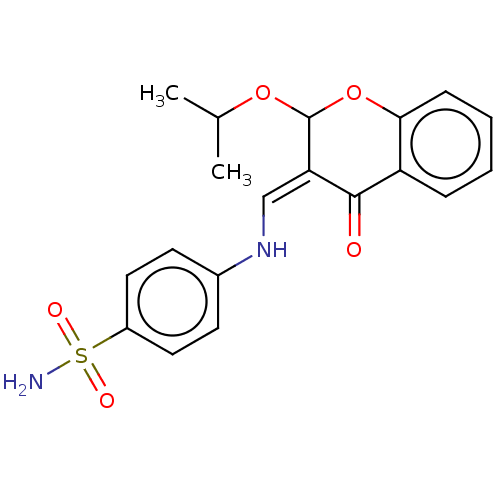 (CHEMBL3798555) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437939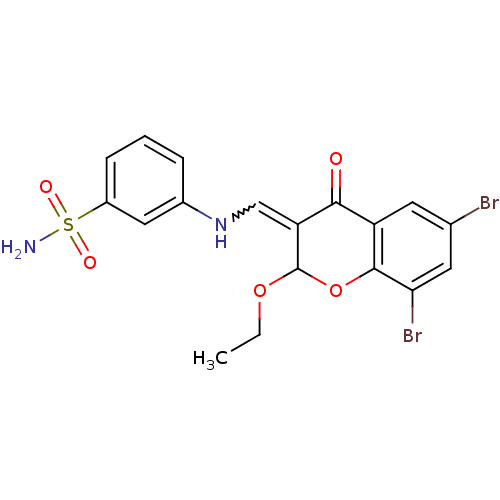 (CHEMBL1814399) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 583 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50561934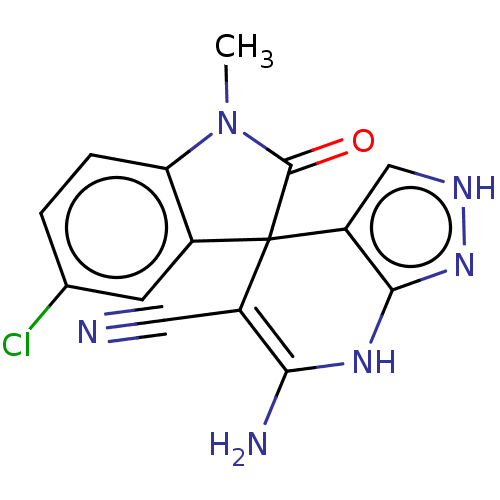 (CHEMBL4753669) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM222263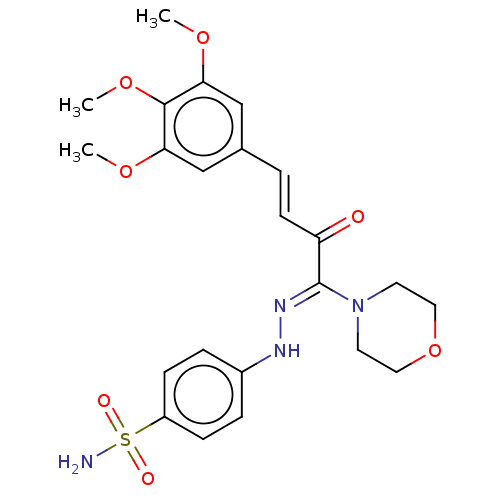 ((Z)-4-(2-((E)-1-morpholino-2-oxo-4-(3,4,5-Trimetho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | 7.4 | 37 |
COMSATS Institute of Information Technology | Assay Description The ecto-5'-nucleotidase inhibition assay was performed according to our previously reported protocol [Raza et al., Med. Chem., 8:1133-1139]. The com... | Bioorg Chem 70: 229-236 (2017) Article DOI: 10.1016/j.bioorg.2017.01.003 BindingDB Entry DOI: 10.7270/Q2K07334 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437941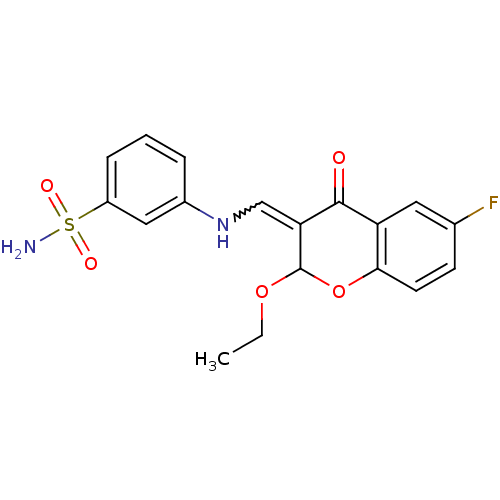 (CHEMBL1814397) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523526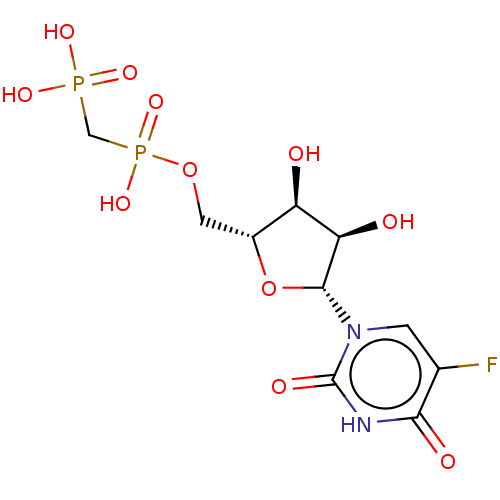 (CHEMBL3606064) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat CD73 using [3H]-AMP as substrate by radiometric assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128137 BindingDB Entry DOI: 10.7270/Q27P935K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM223071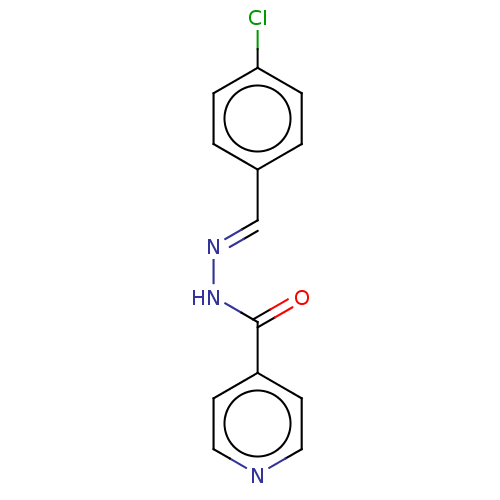 ((E)-N'-(4'-Chlorobenzylidene)isonicotinohy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Quaid-I-Azam University | Assay Description The enzymatic assay of both human and rat ecto-5'-nucleotidase were performed with slight modifications in the previously described method. The stock... | Chem Biol Drug Des 89: 365-370 (2017) Article DOI: 10.1111/cbdd.12861 BindingDB Entry DOI: 10.7270/Q2F47N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165126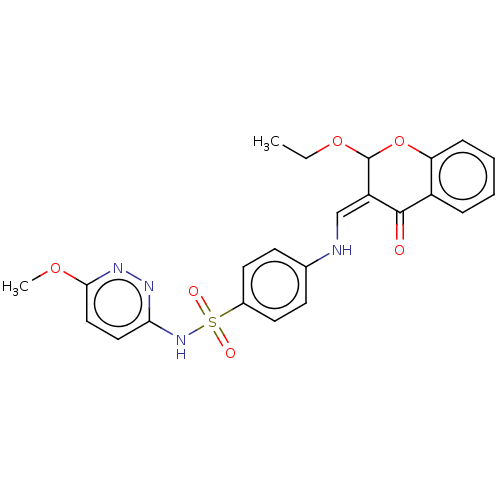 (CHEMBL3797319) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165150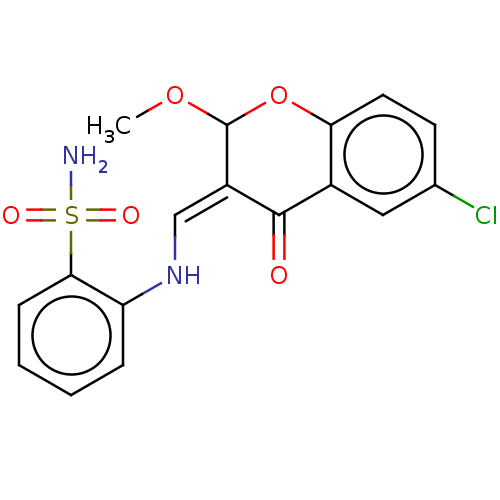 (CHEMBL3799027) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50561937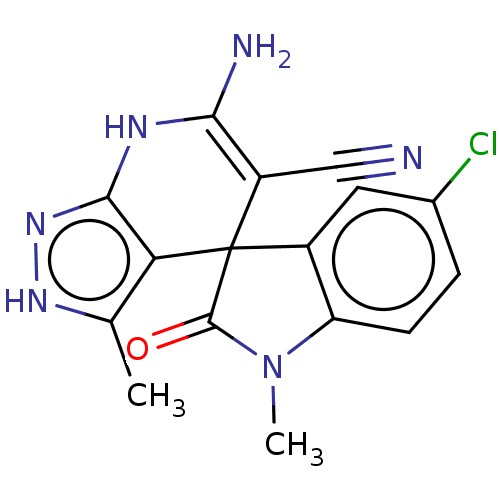 (CHEMBL4780721) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165148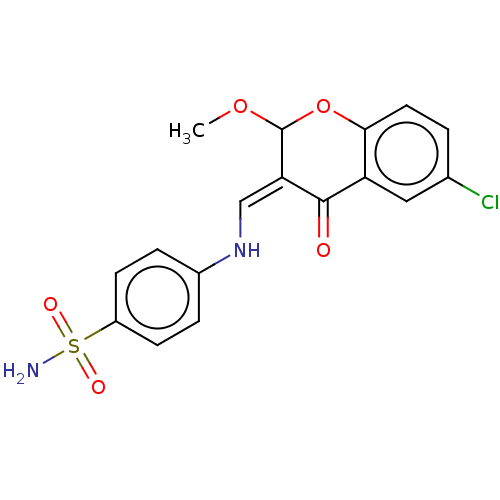 (CHEMBL3797640) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50271102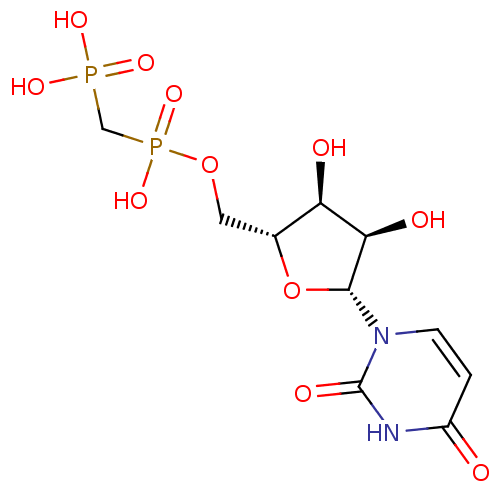 (((((2R,3S,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat CD73 using [3H]-AMP as substrate by radiometric assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128137 BindingDB Entry DOI: 10.7270/Q27P935K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165137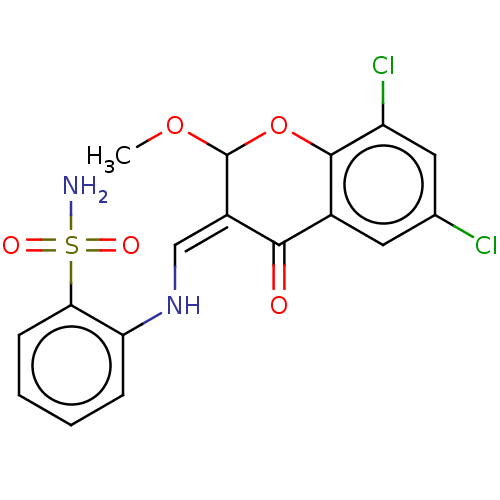 (CHEMBL3800324) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50395084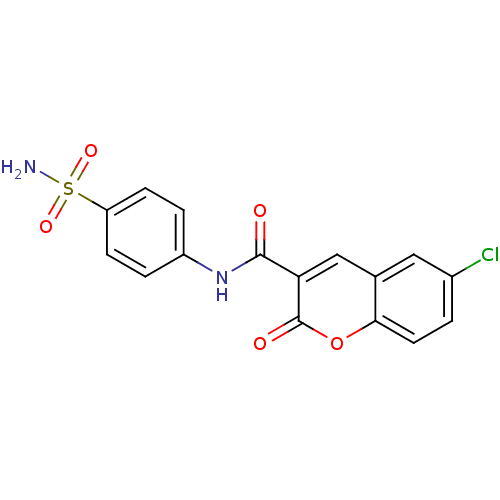 (CHEMBL2164286) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t Curated by ChEMBL | Assay Description Inhibition of rat recombinant ecto-5'-nucleotidase using [3H]AMP substrate | J Med Chem 55: 6576-81 (2012) Article DOI: 10.1021/jm300658n BindingDB Entry DOI: 10.7270/Q2DR2WNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50561935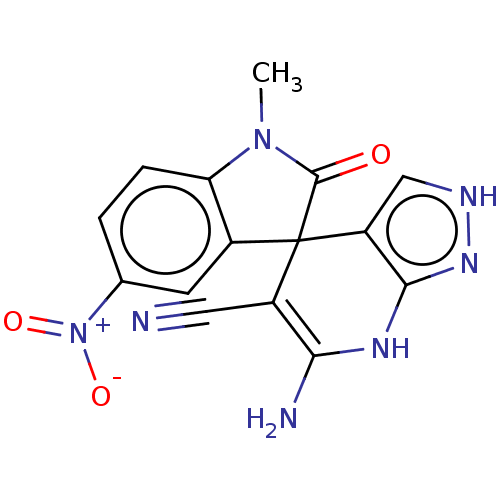 (CHEMBL4761355) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437946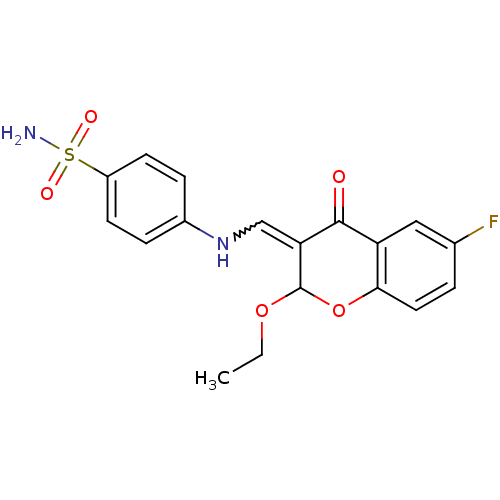 (CHEMBL1814392) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165149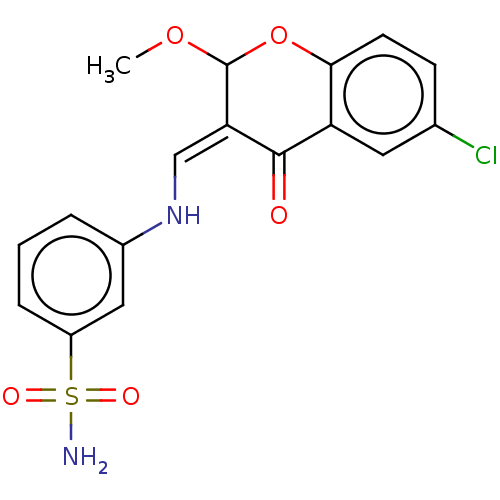 (CHEMBL3799691) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165129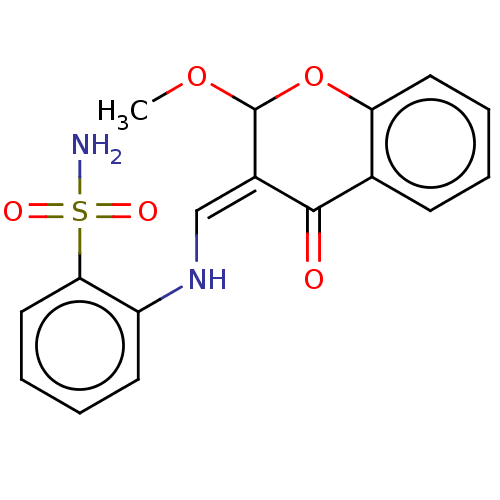 (CHEMBL3799469) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437936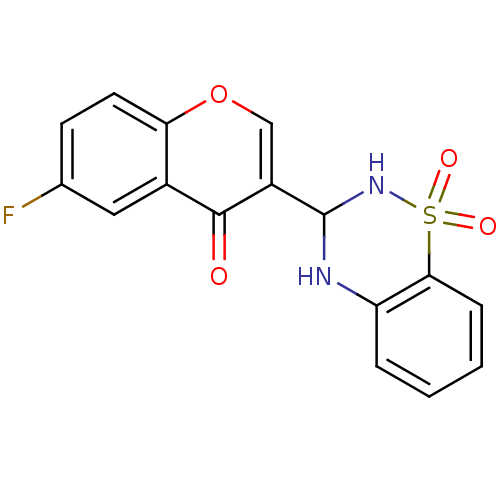 (CHEMBL2408700) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165147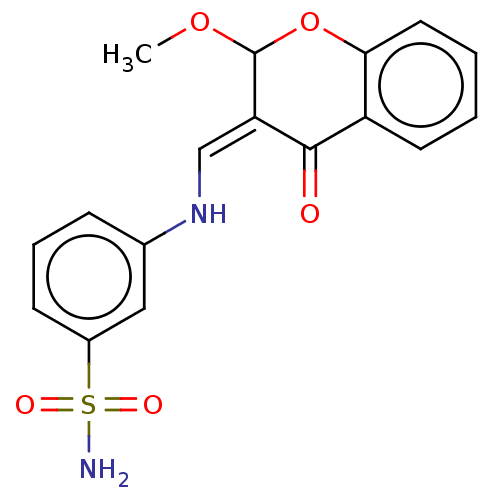 (CHEMBL3797302) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM222259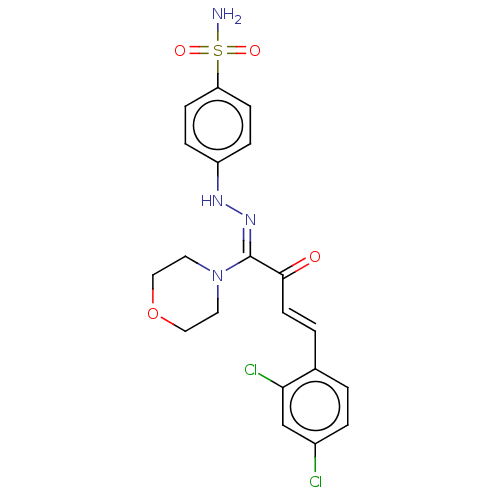 ((Z)-4-(2-((E)-4-(2,4-dichlorophenyl)-1-morpholino-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
COMSATS Institute of Information Technology | Assay Description The ecto-5'-nucleotidase inhibition assay was performed according to our previously reported protocol [Raza et al., Med. Chem., 8:1133-1139]. The com... | Bioorg Chem 70: 229-236 (2017) Article DOI: 10.1016/j.bioorg.2017.01.003 BindingDB Entry DOI: 10.7270/Q2K07334 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM223078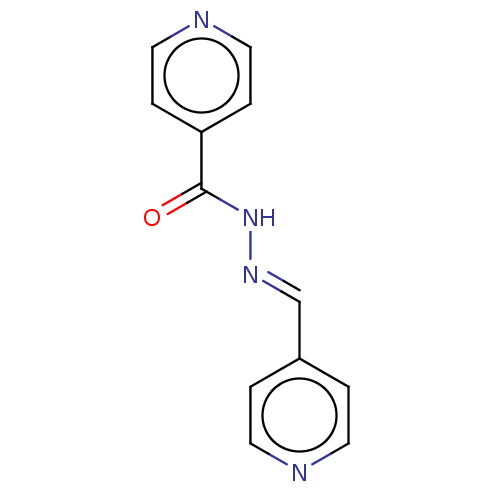 ((E)-N'-(Pyridin-4'-ylmethylene)isonicotino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.89E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Quaid-I-Azam University | Assay Description The enzymatic assay of both human and rat ecto-5'-nucleotidase were performed with slight modifications in the previously described method. The stock... | Chem Biol Drug Des 89: 365-370 (2017) Article DOI: 10.1111/cbdd.12861 BindingDB Entry DOI: 10.7270/Q2F47N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM223079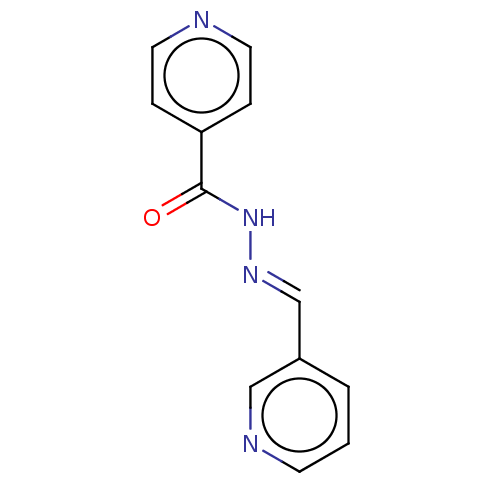 ((E)-N'-(Pyridin-3'-ylmethylene)isonicotino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Quaid-I-Azam University | Assay Description The enzymatic assay of both human and rat ecto-5'-nucleotidase were performed with slight modifications in the previously described method. The stock... | Chem Biol Drug Des 89: 365-370 (2017) Article DOI: 10.1111/cbdd.12861 BindingDB Entry DOI: 10.7270/Q2F47N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50561933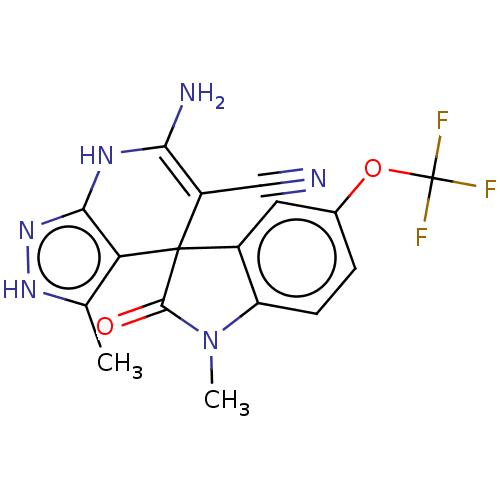 (CHEMBL4788305) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165146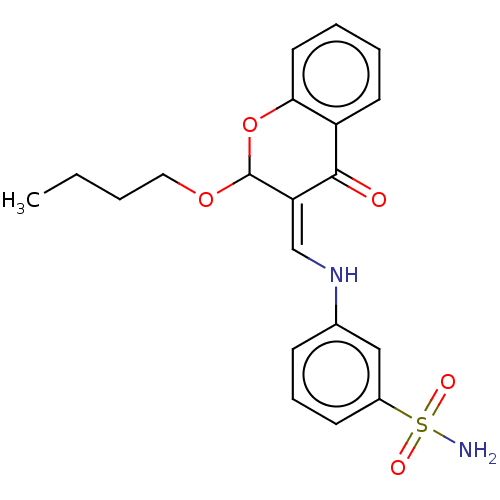 (CHEMBL3798795) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437943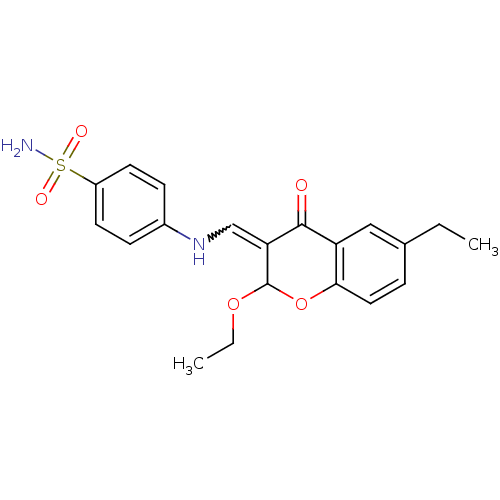 (CHEMBL1814395) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437937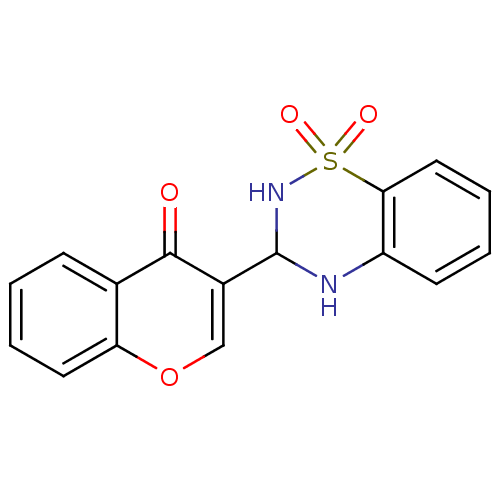 (CHEMBL2408699) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50395090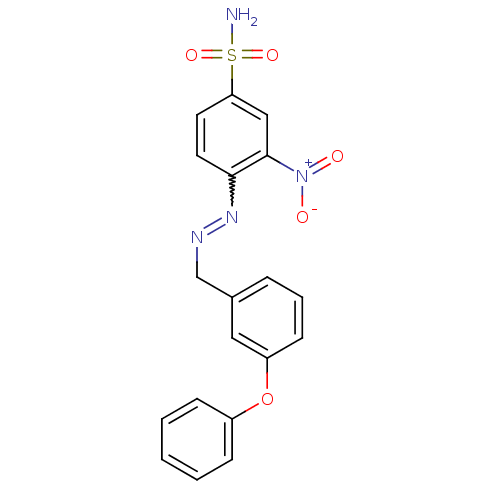 (CHEMBL2164288) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t Curated by ChEMBL | Assay Description Inhibition of rat recombinant ecto-5'-nucleotidase using [3H]AMP substrate | J Med Chem 55: 6576-81 (2012) Article DOI: 10.1021/jm300658n BindingDB Entry DOI: 10.7270/Q2DR2WNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165136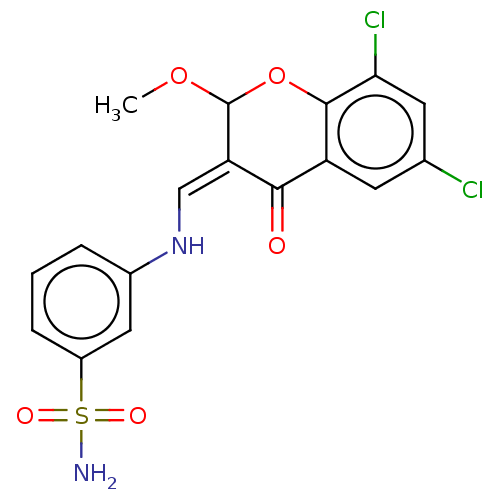 (CHEMBL3800429) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50561932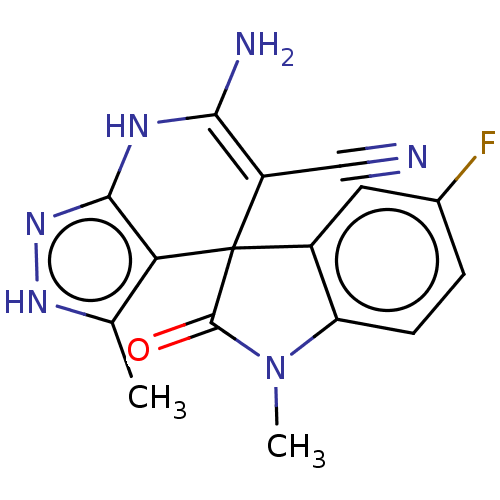 (CHEMBL4761451) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat ecto-5' nucleotidase expressed in COS7 cell membranes assessed as inorganic phosphate release using AMP as substrate uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00343 BindingDB Entry DOI: 10.7270/Q26T0RD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437940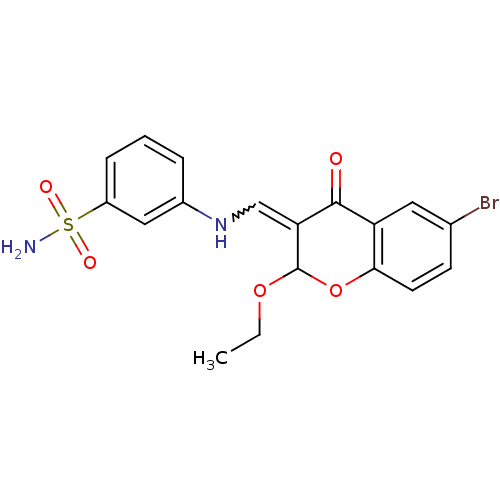 (CHEMBL1814398) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM222258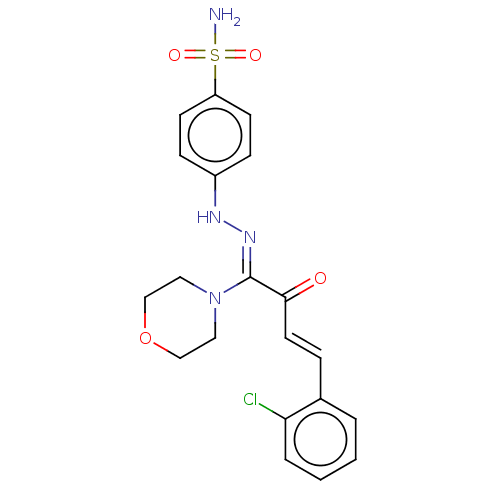 ((Z)-4-(2-((E)-4-(2-chlorophenyl)-1-morpholino-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.43E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
COMSATS Institute of Information Technology | Assay Description The ecto-5'-nucleotidase inhibition assay was performed according to our previously reported protocol [Raza et al., Med. Chem., 8:1133-1139]. The com... | Bioorg Chem 70: 229-236 (2017) Article DOI: 10.1016/j.bioorg.2017.01.003 BindingDB Entry DOI: 10.7270/Q2K07334 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50395091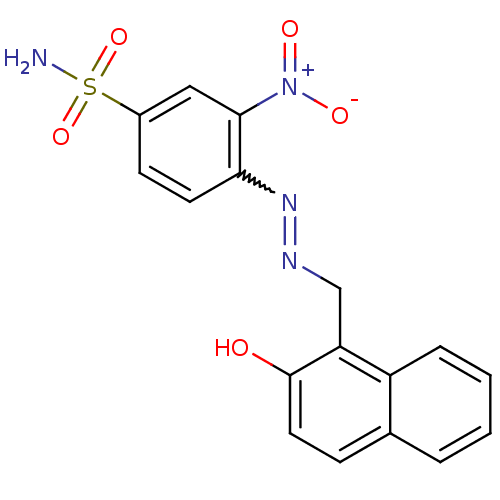 (CHEMBL2164289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t Curated by ChEMBL | Assay Description Inhibition of rat recombinant ecto-5'-nucleotidase using [3H]AMP substrate | J Med Chem 55: 6576-81 (2012) Article DOI: 10.1021/jm300658n BindingDB Entry DOI: 10.7270/Q2DR2WNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50395095 (CHEMBL2164294) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t Curated by ChEMBL | Assay Description Inhibition of rat recombinant ecto-5'-nucleotidase using [3H]AMP substrate | J Med Chem 55: 6576-81 (2012) Article DOI: 10.1021/jm300658n BindingDB Entry DOI: 10.7270/Q2DR2WNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 76 total ) | Next | Last >> |