

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM588052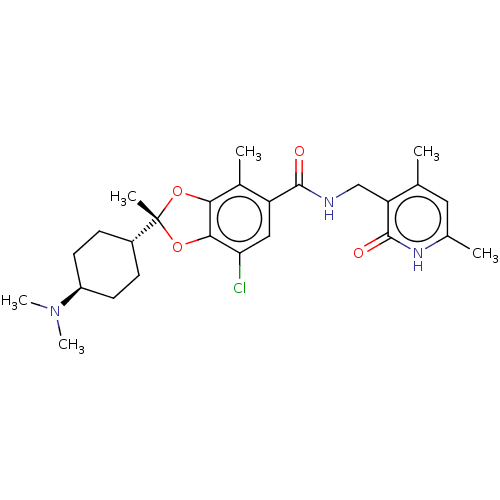 ((2R)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE UniChem | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00047 BindingDB Entry DOI: 10.7270/Q27D3059 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50594132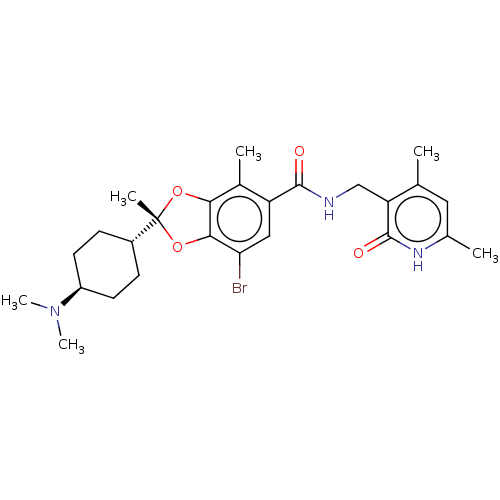 (CHEMBL5197386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00047 BindingDB Entry DOI: 10.7270/Q27D3059 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50594116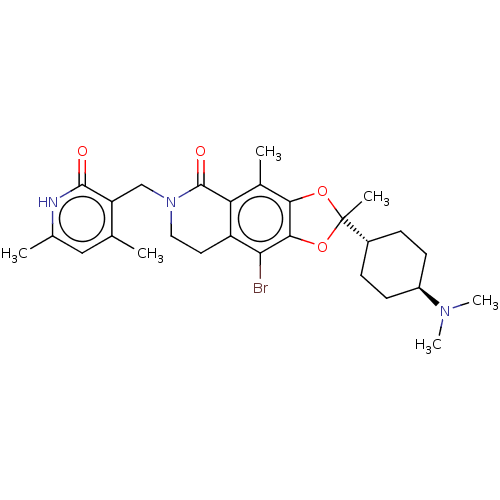 (CHEMBL5185991) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00047 BindingDB Entry DOI: 10.7270/Q27D3059 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM588043 ((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 8.31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM588044 ((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM588042 (6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-yl)me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50075054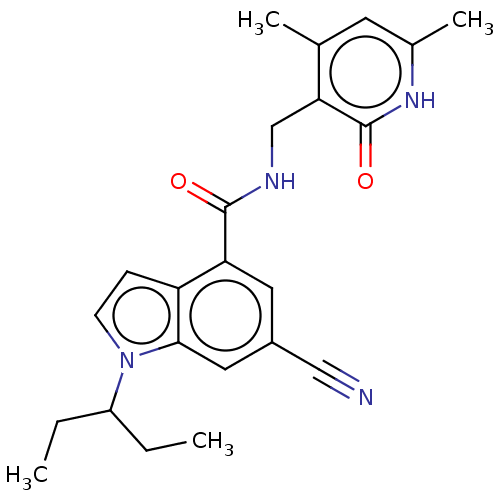 (CHEMBL3414574) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00047 BindingDB Entry DOI: 10.7270/Q27D3059 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM588047 ((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM588031 (9-chloro-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM588041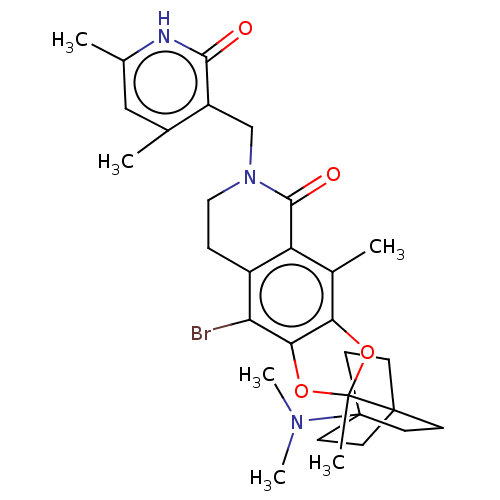 (9-bromo-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM588032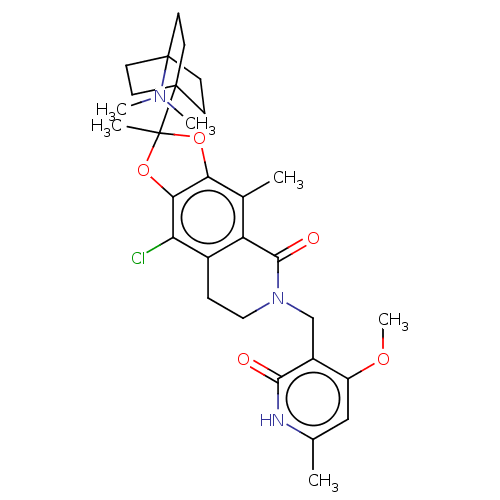 (9-chloro-2-(4- (dimethylamino)bicyclo[2.2.2]octan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM588050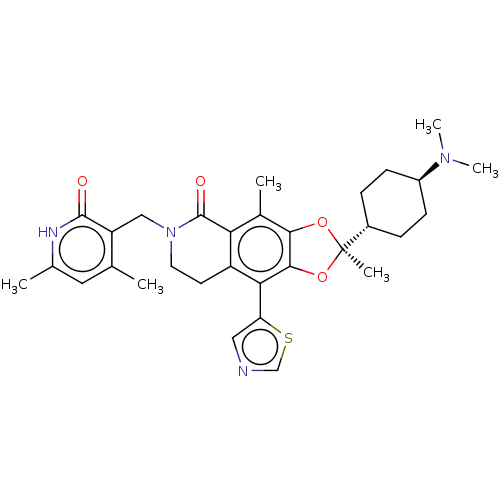 ((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM588048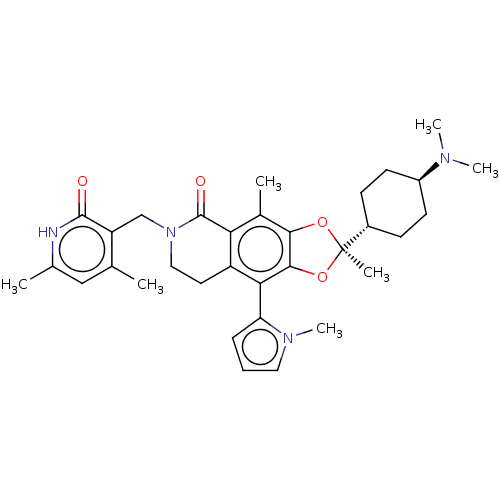 ((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50590375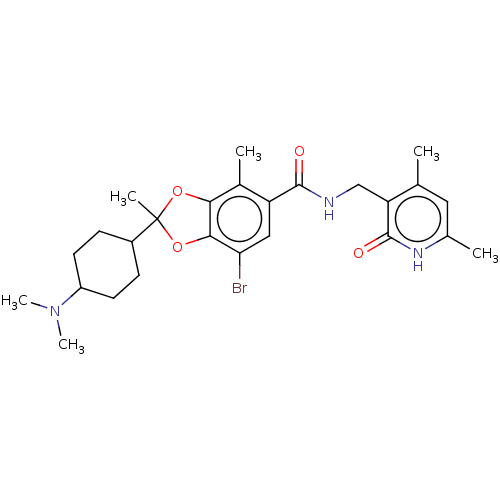 (CHEMBL5187396) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114419 BindingDB Entry DOI: 10.7270/Q2PR80Z0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM588049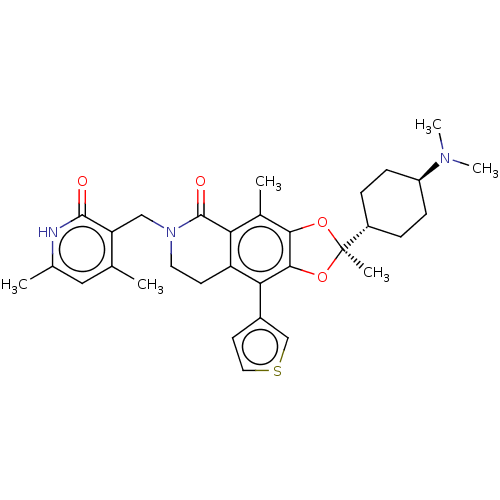 ((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM588046 ((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50590376 (CHEMBL5193486) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114419 BindingDB Entry DOI: 10.7270/Q2PR80Z0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM588052 ((2R)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE UniChem | US Patent | n/a | n/a | 31.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM588045 ((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50594130 (CHEMBL5196227) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00047 BindingDB Entry DOI: 10.7270/Q27D3059 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50075051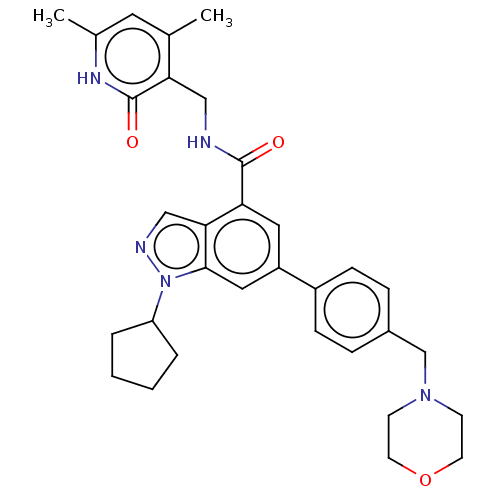 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH1 in PRC2 complex (unknown origin) using biotinylated peptide as substrate in presence of S-adenosylmethionine | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50541920 (Cpi-1205 | Lirametostat) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH1 (unknown origin) | Citation and Details BindingDB Entry DOI: 10.7270/Q2QZ2FQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536604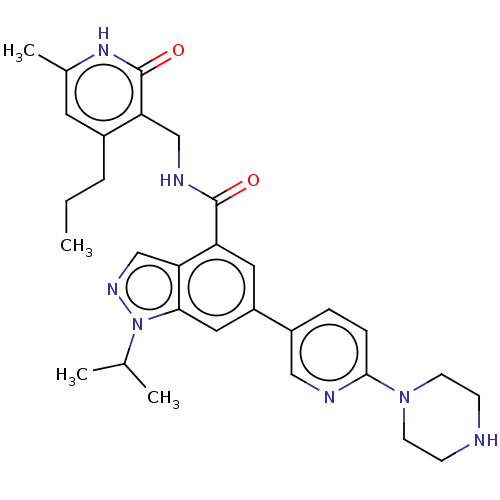 (CHEMBL4594174) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50075071 (CHEMBL3414619) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50594155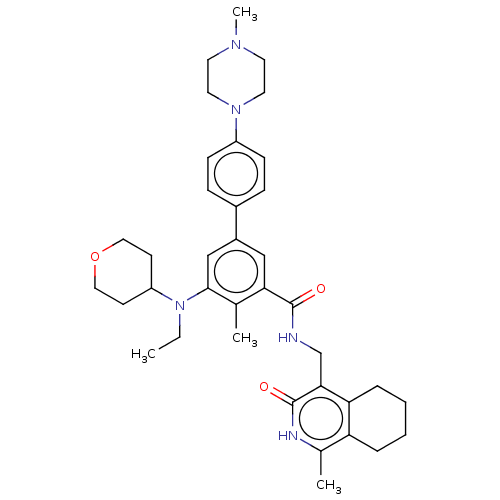 (CHEMBL5203667) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00047 BindingDB Entry DOI: 10.7270/Q27D3059 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50544756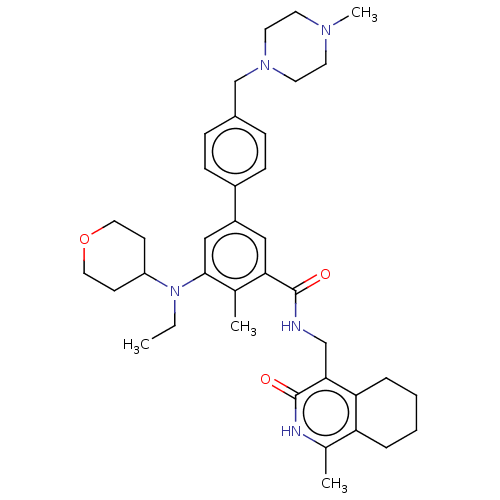 (CHEMBL4637350) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00047 BindingDB Entry DOI: 10.7270/Q27D3059 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536560 (CHEMBL4567859) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536579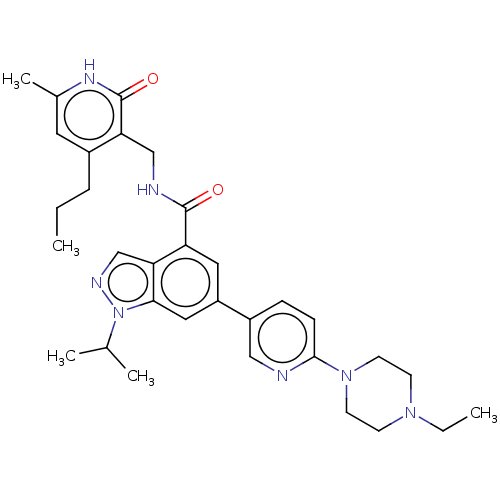 (CHEMBL4557033) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50590382 (CHEMBL5197902) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00047 BindingDB Entry DOI: 10.7270/Q27D3059 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536573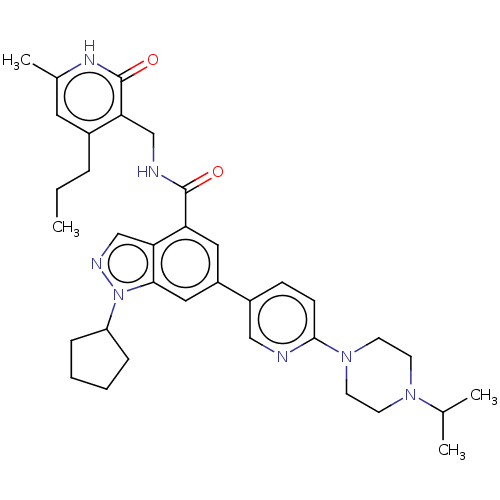 (CHEMBL4559875) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536589 (CHEMBL4545986) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536572 (CHEMBL4539055) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50594154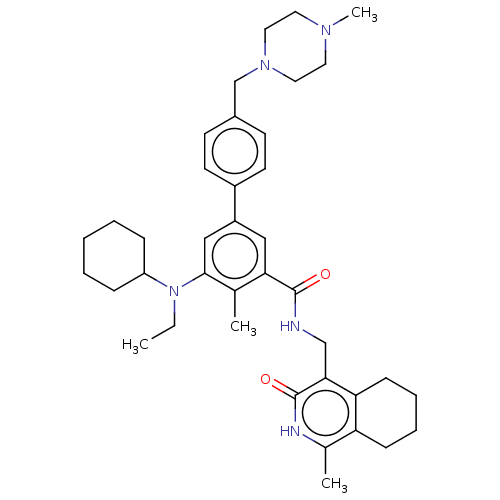 (CHEMBL5178720) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00047 BindingDB Entry DOI: 10.7270/Q27D3059 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM172038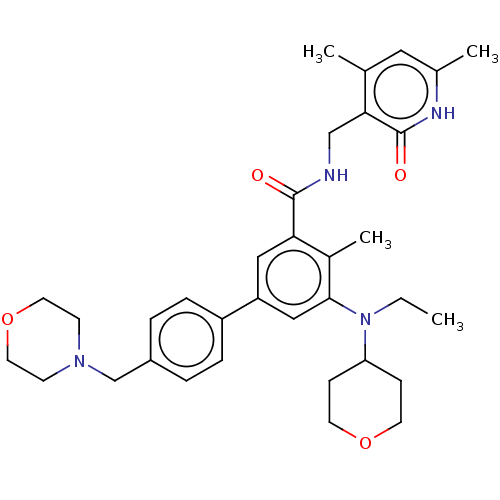 (US10155002, Compound 44 | US10647700, Compound EPZ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536585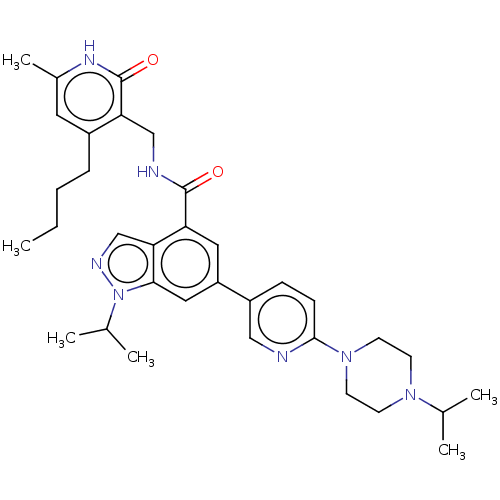 (CHEMBL4554286) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM588033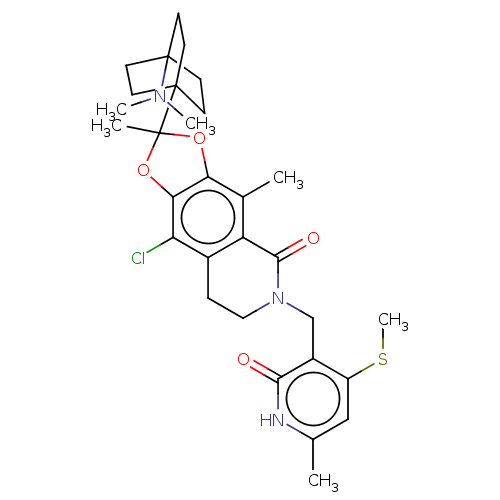 (9-chloro-2-(4- (dimethylamino)bicyclo[2.2.2]octan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50582389 (CHEMBL5089835) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length human N-terminal FLAG-tagged EZH1 in PRC2 complex expressed in baculovirus system using SAM as substrate preinc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02234 BindingDB Entry DOI: 10.7270/Q22V2M02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536598 (CHEMBL4551319) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536574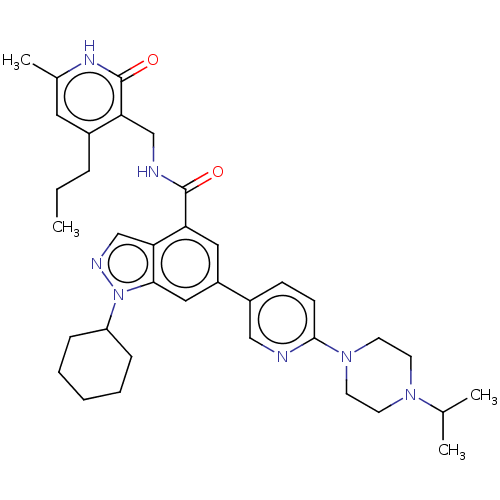 (CHEMBL4521710) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 315 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536563 (CHEMBL4559801) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536578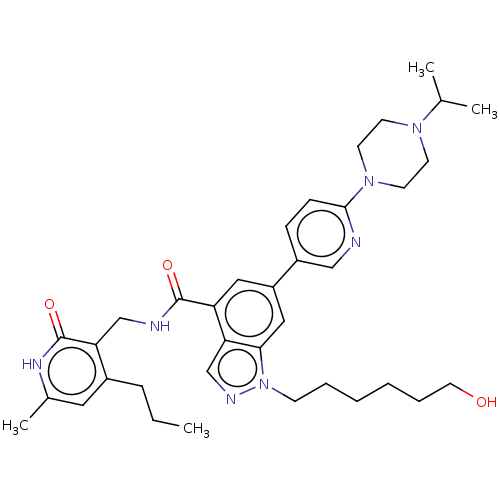 (CHEMBL4529085) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 394 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536593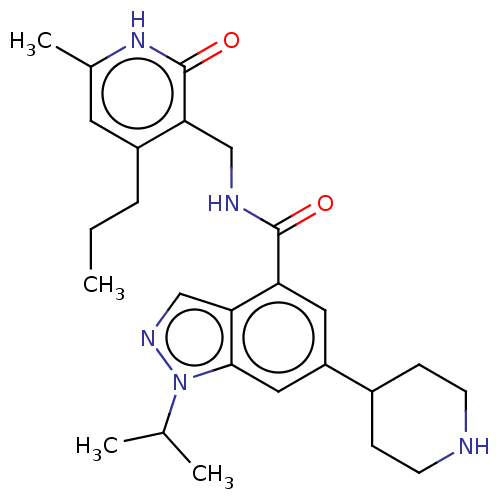 (CHEMBL4569399) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 613 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536562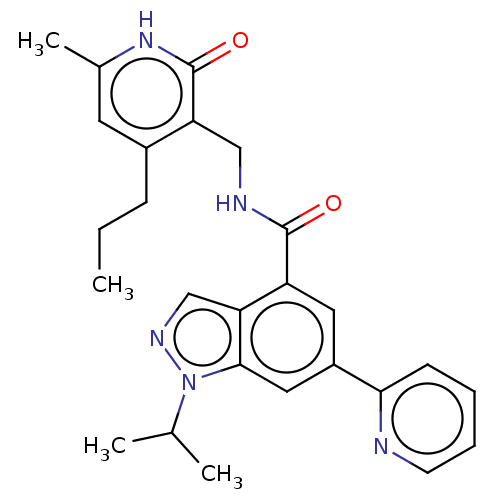 (CHEMBL4542135) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 681 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536581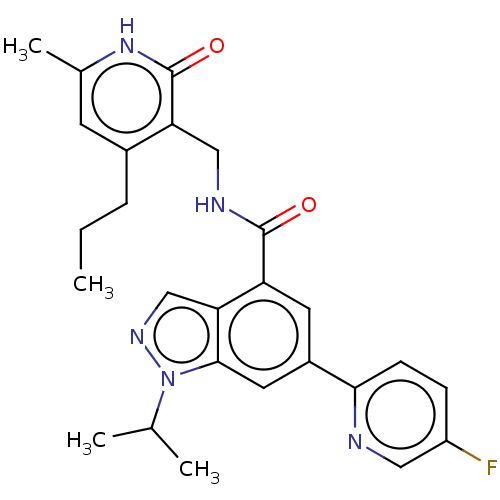 (CHEMBL4593352) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 773 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536584 (CHEMBL4544978) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536582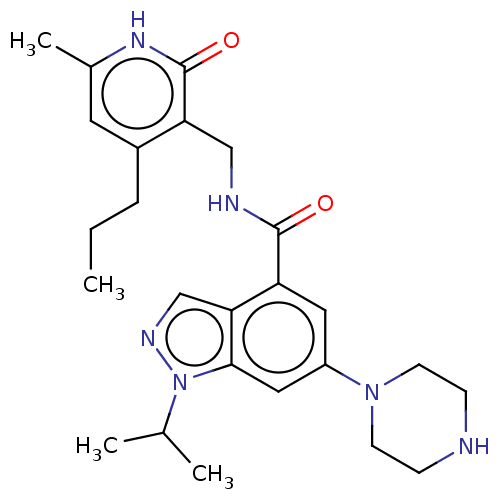 (CHEMBL4575460) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536554 (CHEMBL4535507) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536580 (CHEMBL4566302) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536595 (CHEMBL4535178) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536596 (CHEMBL4534613) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 96 total ) | Next | Last >> |