 Found 36 hits of ic50 data for polymerid = 9627
Found 36 hits of ic50 data for polymerid = 9627 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM200723

(US9233086, 10L)Show SMILES CCC(CC)(NC(=O)C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC1(CCCC1)C(=O)NC(c1ccc(F)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C35H49F2N7O4/c1-5-34(6-2,43-29(45)22(3)4)31(47)41-27(10-9-21-40-33(38)39)30(46)44-35(19-7-8-20-35)32(48)42-28(23-11-15-25(36)16-12-23)24-13-17-26(37)18-14-24/h11-18,22,27-28H,5-10,19-21H2,1-4H3,(H,41,47)(H,42,48)(H,43,45)(H,44,46)(H4,38,39,40)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of MLL1 binding to N-terminal His-tagged WRD5 23 deletion mutant (24 to 334 residues) (unknown origin) expressed in Escherichia coli Roset... |
Bioorg Med Chem 26: 356-365 (2018)
Article DOI: 10.1016/j.bmc.2017.11.045
BindingDB Entry DOI: 10.7270/Q2SQ92ZS |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM200712
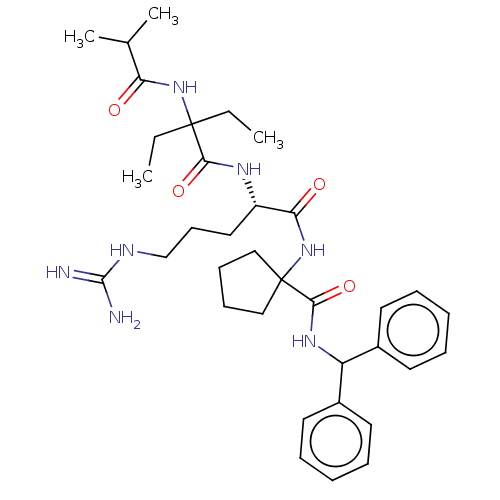
(US9233086, 10A)Show SMILES CCC(CC)(NC(=O)C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC1(CCCC1)C(=O)NC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C35H51N7O4/c1-5-34(6-2,41-29(43)24(3)4)31(45)39-27(20-15-23-38-33(36)37)30(44)42-35(21-13-14-22-35)32(46)40-28(25-16-9-7-10-17-25)26-18-11-8-12-19-26/h7-12,16-19,24,27-28H,5-6,13-15,20-23H2,1-4H3,(H,39,45)(H,40,46)(H,41,43)(H,42,44)(H4,36,37,38)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of MLL1 binding to N-terminal His-tagged WRD5 23 deletion mutant (24 to 334 residues) (unknown origin) expressed in Escherichia coli Roset... |
Bioorg Med Chem 26: 356-365 (2018)
Article DOI: 10.1016/j.bmc.2017.11.045
BindingDB Entry DOI: 10.7270/Q2SQ92ZS |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM200722

(US9233086, 10K)Show SMILES CCC(CC)(NC(=O)C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC1(CCCC1)C(=O)NC(c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C35H49Cl2N7O4/c1-5-34(6-2,43-29(45)22(3)4)31(47)41-27(10-9-21-40-33(38)39)30(46)44-35(19-7-8-20-35)32(48)42-28(23-11-15-25(36)16-12-23)24-13-17-26(37)18-14-24/h11-18,22,27-28H,5-10,19-21H2,1-4H3,(H,41,47)(H,42,48)(H,43,45)(H,44,46)(H4,38,39,40)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of MLL1 binding to N-terminal His-tagged WRD5 23 deletion mutant (24 to 334 residues) (unknown origin) expressed in Escherichia coli Roset... |
Bioorg Med Chem 26: 356-365 (2018)
Article DOI: 10.1016/j.bmc.2017.11.045
BindingDB Entry DOI: 10.7270/Q2SQ92ZS |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50601694
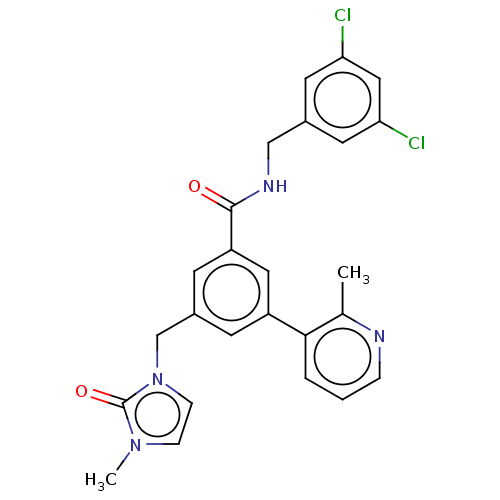
(CHEMBL5183439)Show SMILES Cc1ncccc1-c1cc(Cn2ccn(C)c2=O)cc(c1)C(=O)NCc1cc(Cl)cc(Cl)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00037
BindingDB Entry DOI: 10.7270/Q2B85D61 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50574672

(CHEMBL4861912)Show SMILES CN1CCN(CC1)c1ccc(cc1NC(=O)c1cc(N)c(F)c(C)c1Cl)-n1cc(nn1)C(=O)NCCCN1CCOCC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MLL1 (unknown origin)-mediated H3K4 methylation using histone H3 and SAM as substrate incubated for 30 mins by AlphaScreen assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113677
BindingDB Entry DOI: 10.7270/Q2J38XCR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50601692

(CHEMBL5172154)Show SMILES CN1CCN(CC1)c1ccc(cc1NC(=O)c1cc(N)c(F)c(C)c1Cl)-c1ccc(cc1)C(=O)NCCCN | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00037
BindingDB Entry DOI: 10.7270/Q2B85D61 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50164787
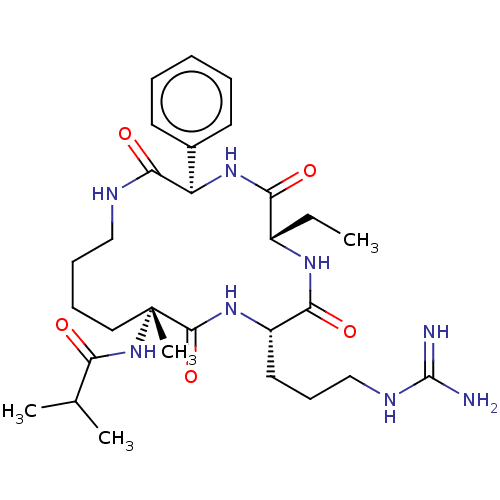
(CHEMBL3798088)Show SMILES CC[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@](C)(CCCCNC(=O)[C@H](NC1=O)c1ccccc1)NC(=O)C(C)C |r| Show InChI InChI=1S/C29H46N8O5/c1-5-20-24(39)36-22(19-12-7-6-8-13-19)26(41)32-16-10-9-15-29(4,37-23(38)18(2)3)27(42)35-21(25(40)34-20)14-11-17-33-28(30)31/h6-8,12-13,18,20-22H,5,9-11,14-17H2,1-4H3,(H,32,41)(H,34,40)(H,35,42)(H,36,39)(H,37,38)(H4,30,31,33)/t20-,21-,22+,29+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of SUMO-His-tagged MLL1 activity (unknown origin) expressed in Escherichia coli BL21(DE3) pLysS cells using histone H3 as substrate by sci... |
J Med Chem 59: 2478-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01630
BindingDB Entry DOI: 10.7270/Q2VT1V0C |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50164787

(CHEMBL3798088)Show SMILES CC[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@](C)(CCCCNC(=O)[C@H](NC1=O)c1ccccc1)NC(=O)C(C)C |r| Show InChI InChI=1S/C29H46N8O5/c1-5-20-24(39)36-22(19-12-7-6-8-13-19)26(41)32-16-10-9-15-29(4,37-23(38)18(2)3)27(42)35-21(25(40)34-20)14-11-17-33-28(30)31/h6-8,12-13,18,20-22H,5,9-11,14-17H2,1-4H3,(H,32,41)(H,34,40)(H,35,42)(H,36,39)(H,37,38)(H4,30,31,33)/t20-,21-,22+,29+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of recombinant SUMO-tagged MLL1 (unknown origin) by histone methyl transferase assay |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50581147

(CHEMBL5094242)Show SMILES CN(C)C(=O)c1ccc(cc1)-c1cc(Nc2ncnc(Cl)c2N)c(cc1F)N1CCN(C)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MLL1 (unknown origin) histamine methyltransferase activity assessed as inhibition of H3K4 methylation incubated for 30 mins by Alphascr... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00091
BindingDB Entry DOI: 10.7270/Q22B92X1 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50530867

(CHEMBL4472654)Show SMILES [H][C@]12Nc3ccccc3[C@]1([C@H](O)[C@]13SS[C@](C)(N(C)C1=O)C(=O)N23)[C@]12[C@H](O)[C@]34SS[C@](C)(N(C)C3=O)C(=O)N4[C@@]1([H])Nc1ccccc21 |r| Show InChI InChI=1S/C30H28N6O6S4/c1-25-21(39)35-19-27(13-9-5-7-11-15(13)31-19,17(37)29(35,45-43-25)23(41)33(25)3)28-14-10-6-8-12-16(14)32-20(28)36-22(40)26(2)34(4)24(42)30(36,18(28)38)46-44-26/h5-12,17-20,31-32,37-38H,1-4H3/t17-,18-,19+,20+,25-,26-,27+,28+,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Greensboro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MLL1 using [3H]S-adenosyl-methionine as substrate |
J Nat Prod 82: 3104-3110 (2019)
Article DOI: 10.1021/acs.jnatprod.9b00711
BindingDB Entry DOI: 10.7270/Q2C53QB2 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50530867

(CHEMBL4472654)Show SMILES [H][C@]12Nc3ccccc3[C@]1([C@H](O)[C@]13SS[C@](C)(N(C)C1=O)C(=O)N23)[C@]12[C@H](O)[C@]34SS[C@](C)(N(C)C3=O)C(=O)N4[C@@]1([H])Nc1ccccc21 |r| Show InChI InChI=1S/C30H28N6O6S4/c1-25-21(39)35-19-27(13-9-5-7-11-15(13)31-19,17(37)29(35,45-43-25)23(41)33(25)3)28-14-10-6-8-12-16(14)32-20(28)36-22(40)26(2)34(4)24(42)30(36,18(28)38)46-44-26/h5-12,17-20,31-32,37-38H,1-4H3/t17-,18-,19+,20+,25-,26-,27+,28+,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Greensboro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MLL1 using [3H]S-adenosyl-methionine as substrate |
J Nat Prod 82: 3104-3110 (2019)
Article DOI: 10.1021/acs.jnatprod.9b00711
BindingDB Entry DOI: 10.7270/Q2C53QB2 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MLL (unknown origin) by HMT assay |
Bioorg Med Chem Lett 25: 1532-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.017
BindingDB Entry DOI: 10.7270/Q2TM7CSS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM23411

(5,6,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...)Show InChI InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)11-5-9(17)13-12(21-11)6-10(18)14(19)15(13)20/h1-6,16,18-20H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of MLL1 (unknown origin) preincubated for 15 mins followed by addition of substrate measured after 1 hr by AlphaLISA assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115372
BindingDB Entry DOI: 10.7270/Q2NS0ZG9 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594525
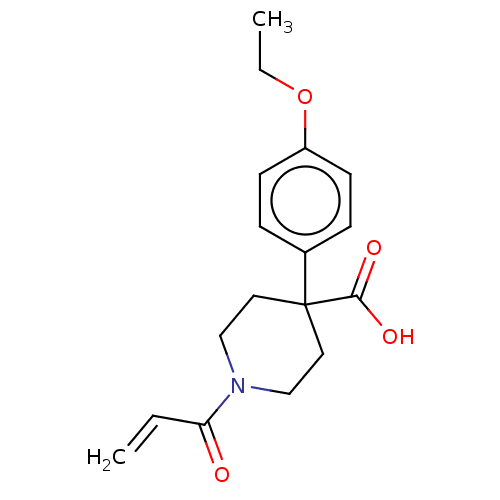
(CHEMBL5189434) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594527
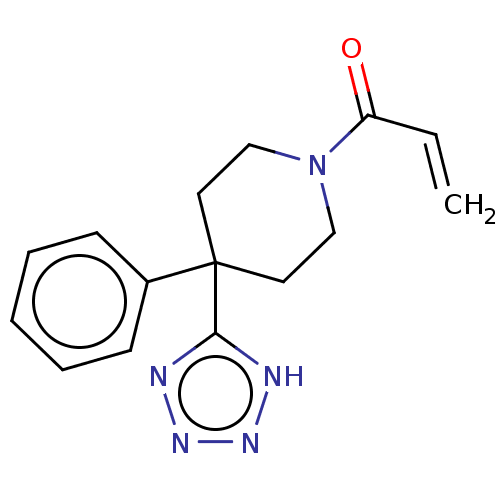
(CHEMBL5177921) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594520
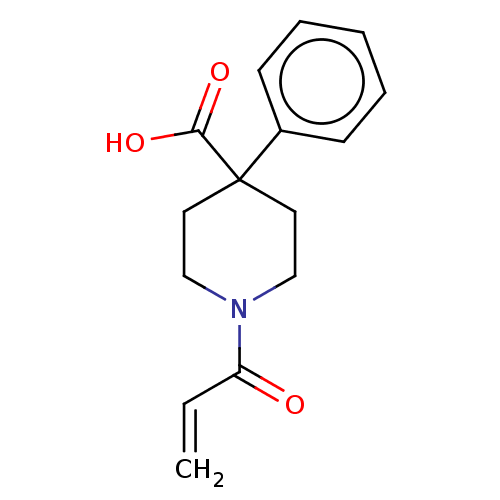
(CHEMBL5194845) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594530

(CHEMBL5181110)Show SMILES CCOc1cccc(c1)C1(CCN(CC1)C(=O)C=C)c1nnn[nH]1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594528
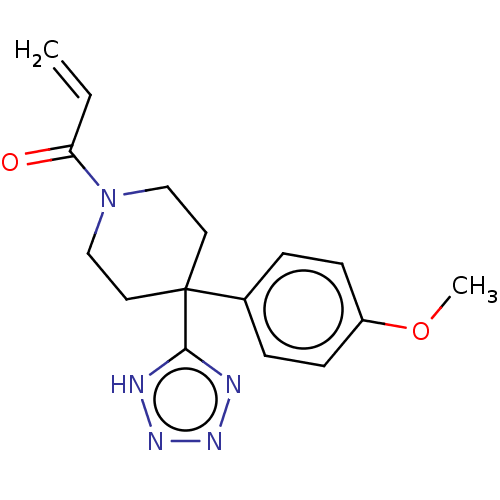
(CHEMBL5178563) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50063670
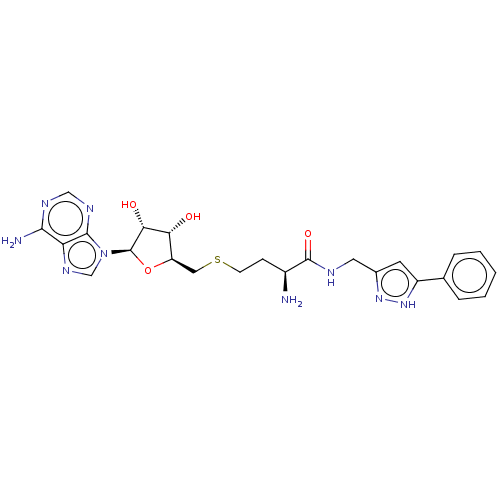
(CHEMBL3397332)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)NCc1cc([nH]n1)-c1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MLL (unknown origin) by HMT assay |
Bioorg Med Chem Lett 25: 1532-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.017
BindingDB Entry DOI: 10.7270/Q2TM7CSS |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50606100

(CHEMBL5173004)Show SMILES C[C@H](Oc1ccc(cc1)-c1cc2c(ncnc2[nH]1)C1=CCNCC1)c1ccccc1 |r,t:21| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00398
BindingDB Entry DOI: 10.7270/Q20K2DNQ |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50572749
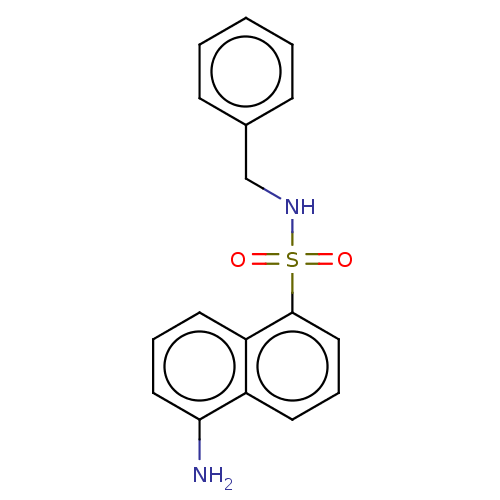
(CHEMBL4854710) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MLLI (unknown origin) using SAM as substrate preincubated for 10 mins followed by substrate addition measured after 4 hrs by HTRF metho... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113592
BindingDB Entry DOI: 10.7270/Q2HQ43QP |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594529

(CHEMBL5201158)Show SMILES CCOc1ccc(cc1)C1(CCN(CC1)C(=O)C=C)c1nnn[nH]1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594521

(CHEMBL5193964) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594523
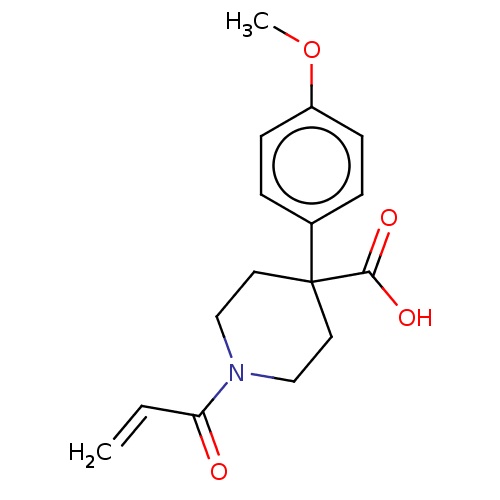
(CHEMBL5182454) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594531

(CHEMBL5198555)Show SMILES C=CC(=O)N1CCC(CC1)(C(=O)NS(=O)(=O)c1ccccc1)c1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594526

(CHEMBL5185919) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594533
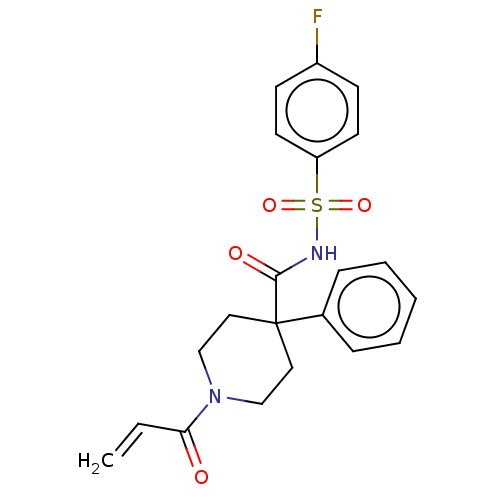
(CHEMBL5175295)Show SMILES Fc1ccc(cc1)S(=O)(=O)NC(=O)C1(CCN(CC1)C(=O)C=C)c1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594535
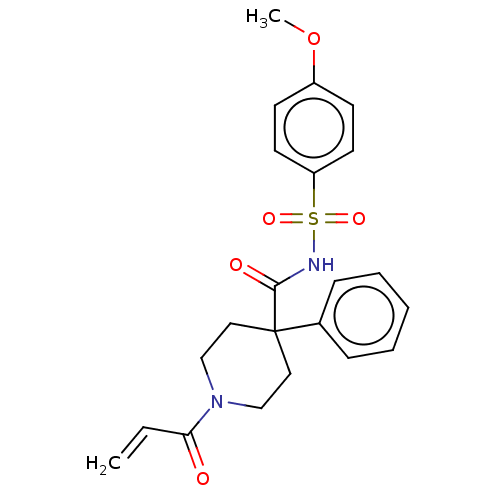
(CHEMBL5191621)Show SMILES COc1ccc(cc1)S(=O)(=O)NC(=O)C1(CCN(CC1)C(=O)C=C)c1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50051117
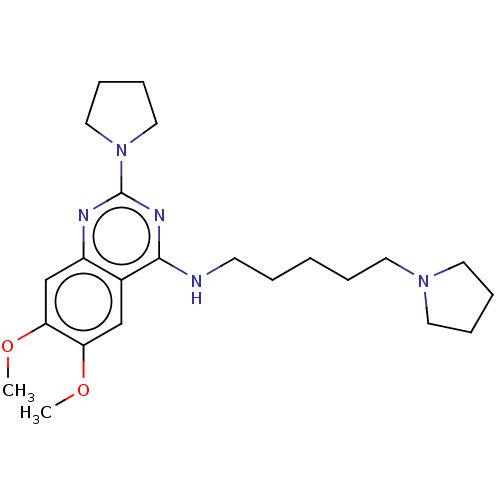
(CHEMBL3318285)Show InChI InChI=1S/C23H35N5O2/c1-29-20-16-18-19(17-21(20)30-2)25-23(28-14-8-9-15-28)26-22(18)24-10-4-3-5-11-27-12-6-7-13-27/h16-17H,3-15H2,1-2H3,(H,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of MLL1 (unknown origin) assessed as incorporation of tritium-labeled methyl group to lysine or arginine residues of peptide substrate by ... |
J Med Chem 57: 6822-33 (2014)
Article DOI: 10.1021/jm500871s
BindingDB Entry DOI: 10.7270/Q2J9681Q |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50400778
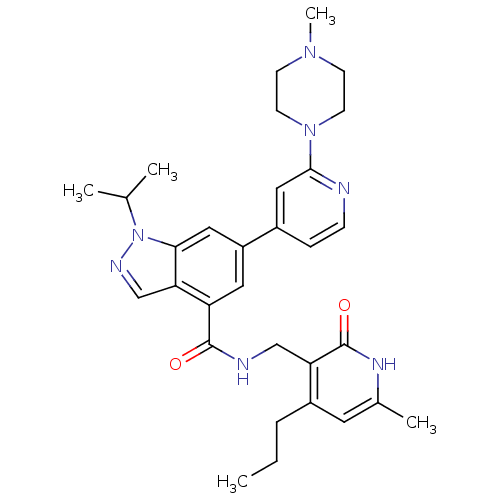
(CHEMBL2204995)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccnc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C31H39N7O2/c1-6-7-23-14-21(4)35-31(40)26(23)18-33-30(39)25-15-24(16-28-27(25)19-34-38(28)20(2)3)22-8-9-32-29(17-22)37-12-10-36(5)11-13-37/h8-9,14-17,19-20H,6-7,10-13,18H2,1-5H3,(H,33,39)(H,35,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MLL1 using core histone as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594536

(CHEMBL5182357)Show SMILES CCS(=O)(=O)NC(=O)C1(CCN(CC1)C(=O)C=C)c1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594534

(CHEMBL5206543)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)C1(CCN(CC1)C(=O)C=C)c1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50031317
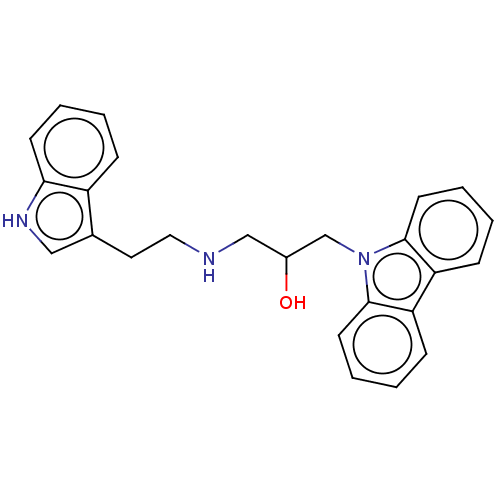
(CHEMBL3358015)Show SMILES OC(CNCCc1c[nH]c2ccccc12)Cn1c2ccccc2c2ccccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of MLL1 (unknown origin) incubated for 15 mins before addition of biotinylated peptide H3 (1 to 21) and SAM by methylation assay |
J Med Chem 57: 9028-41 (2014)
Article DOI: 10.1021/jm501134e
BindingDB Entry DOI: 10.7270/Q2JD4ZD6 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594532

(CHEMBL5169812)Show SMILES Clc1ccc(cc1)S(=O)(=O)NC(=O)C1(CCN(CC1)C(=O)C=C)c1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.53E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594522

(CHEMBL5172213) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50594524
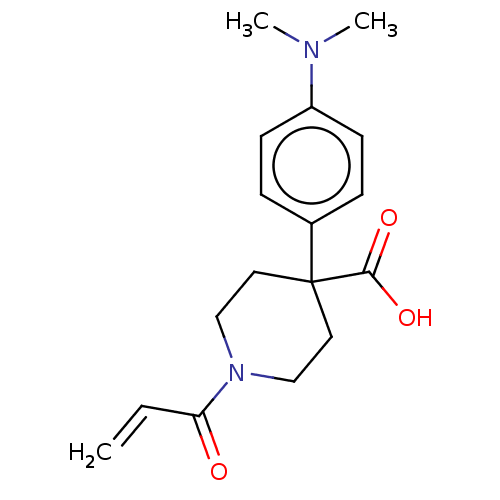
(CHEMBL5173950) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00198
BindingDB Entry DOI: 10.7270/Q2930Z58 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data