

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50450426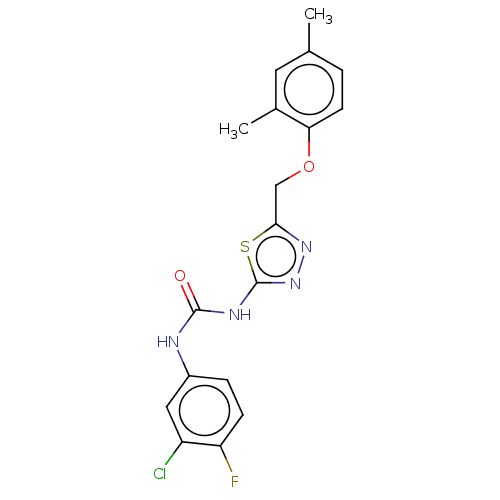 (CHEMBL4174865) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic PTPepsilon expressed in Escherichia coli BL21 (DE3) using E527-P-Q-pY530-Q-P-G-E-N-L536 as substrate after ... | Bioorg Med Chem 26: 5204-5211 (2018) Article DOI: 10.1016/j.bmc.2018.09.022 BindingDB Entry DOI: 10.7270/Q21C20F9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50450424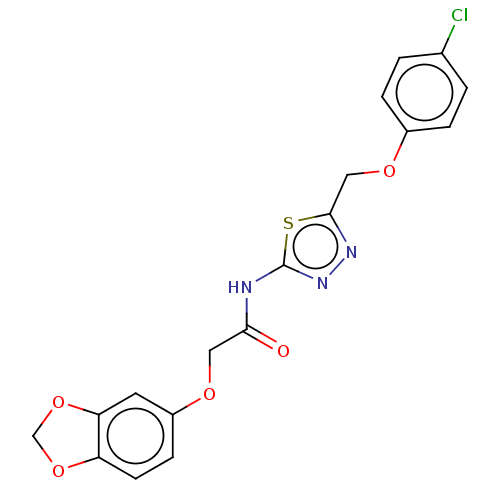 (CHEMBL4160163) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic PTPepsilon expressed in Escherichia coli BL21 (DE3) using E527-P-Q-pY530-Q-P-G-E-N-L536 as substrate after ... | Bioorg Med Chem 26: 5204-5211 (2018) Article DOI: 10.1016/j.bmc.2018.09.022 BindingDB Entry DOI: 10.7270/Q21C20F9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50450422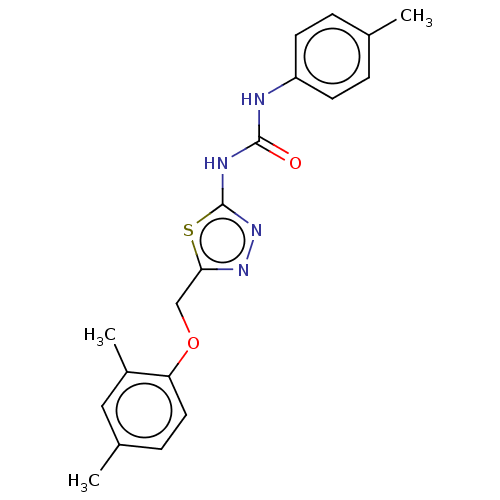 (CHEMBL4170383) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic PTPepsilon expressed in Escherichia coli BL21 (DE3) using E527-P-Q-pY530-Q-P-G-E-N-L536 as substrate after ... | Bioorg Med Chem 26: 5204-5211 (2018) Article DOI: 10.1016/j.bmc.2018.09.022 BindingDB Entry DOI: 10.7270/Q21C20F9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50450423 (CHEMBL4168082) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic PTPepsilon expressed in Escherichia coli BL21 (DE3) using E527-P-Q-pY530-Q-P-G-E-N-L536 as substrate after ... | Bioorg Med Chem 26: 5204-5211 (2018) Article DOI: 10.1016/j.bmc.2018.09.022 BindingDB Entry DOI: 10.7270/Q21C20F9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50450425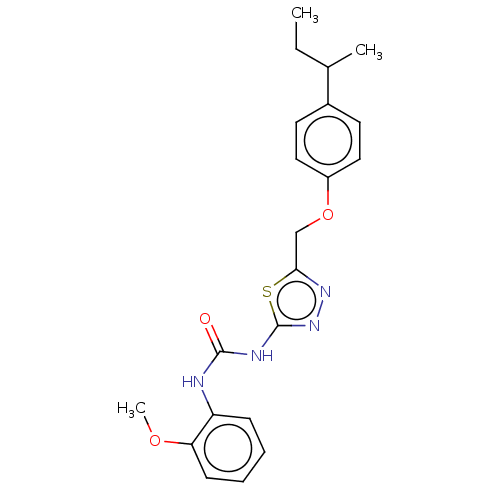 (CHEMBL4159748) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic PTPepsilon expressed in Escherichia coli BL21 (DE3) using E527-P-Q-pY530-Q-P-G-E-N-L536 as substrate after ... | Bioorg Med Chem 26: 5204-5211 (2018) Article DOI: 10.1016/j.bmc.2018.09.022 BindingDB Entry DOI: 10.7270/Q21C20F9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM231167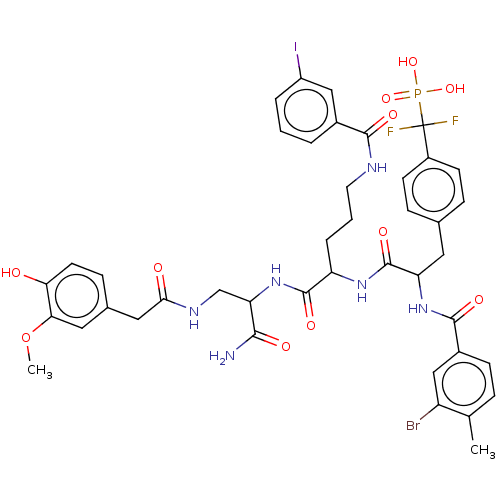 (US9340574, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in 3,3-dimethylglutarate buffer (50 mM 3,3-dimethylglutarate, pH 7.0, 1 ... | US Patent US9340574 (2016) BindingDB Entry DOI: 10.7270/Q2NV9H4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50607111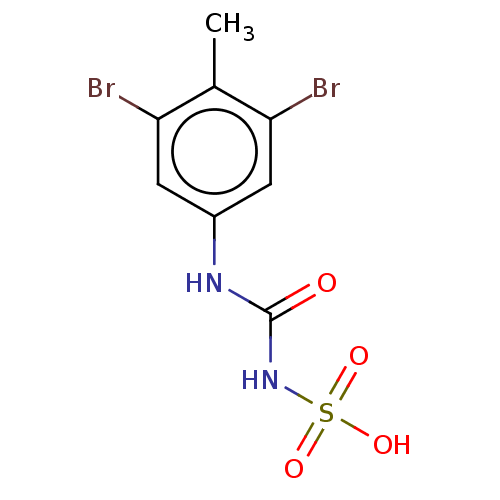 (CHEMBL5218807) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01143 BindingDB Entry DOI: 10.7270/Q2BK1HGF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50054344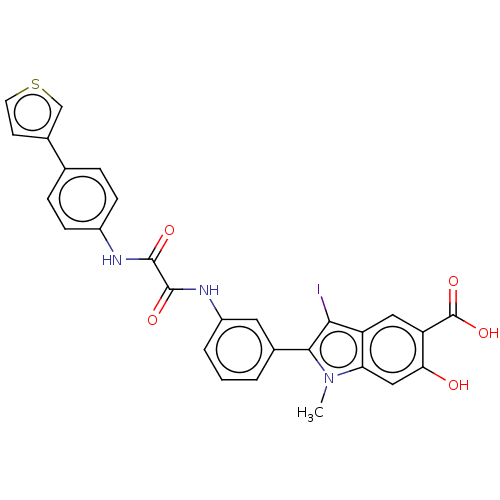 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description For selectivity studies, the PTPs, including LYP, mPTPA, SHP1-D1C, PTP1B, LMPTP, VHR, Laforin and PTPα-D1D2 were expressed and purified from E. ... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50054344 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPepsilon (unknown origin) | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50436357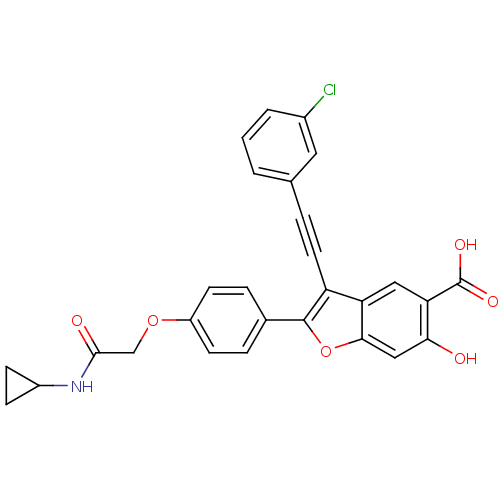 (CHEMBL2396719) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant PTPepsilon (unknown origin) using pNPP as substrate by spectrophotometric analysis | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50436358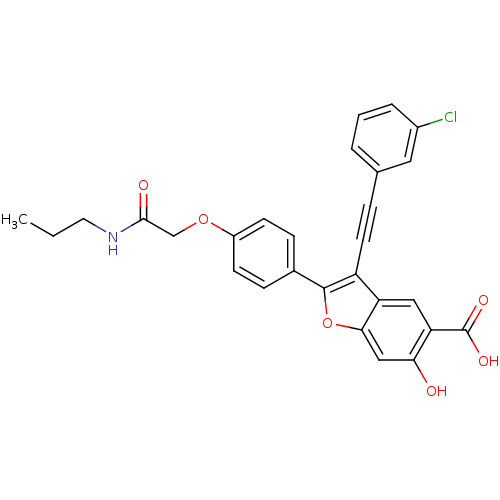 (CHEMBL2396718) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant PTPepsilon (unknown origin) using pNPP as substrate by spectrophotometric analysis | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50544431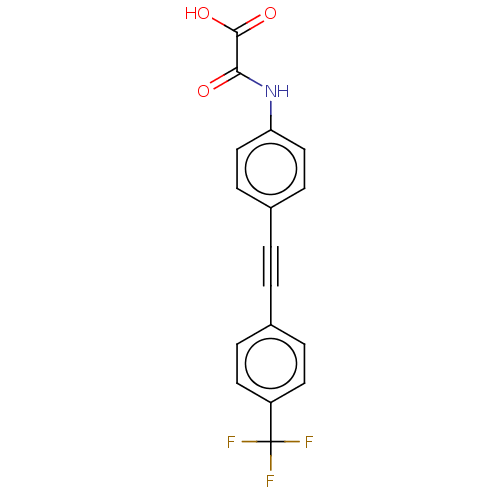 (CHEMBL4637459 | US11192850, Entry 4k) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of PTPE (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vis sp... | J Med Chem 63: 9212-9227 (2020) Article DOI: 10.1021/acs.jmedchem.0c00302 BindingDB Entry DOI: 10.7270/Q2QV3R3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50544440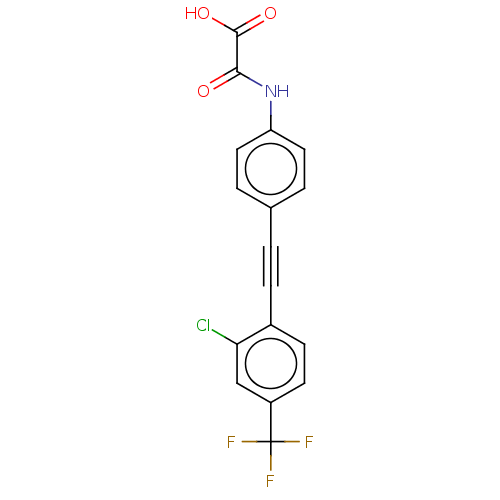 (CHEMBL4647367 | US11192850, Entry 4t) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of PTPE (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vis sp... | J Med Chem 63: 9212-9227 (2020) Article DOI: 10.1021/acs.jmedchem.0c00302 BindingDB Entry DOI: 10.7270/Q2QV3R3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50222205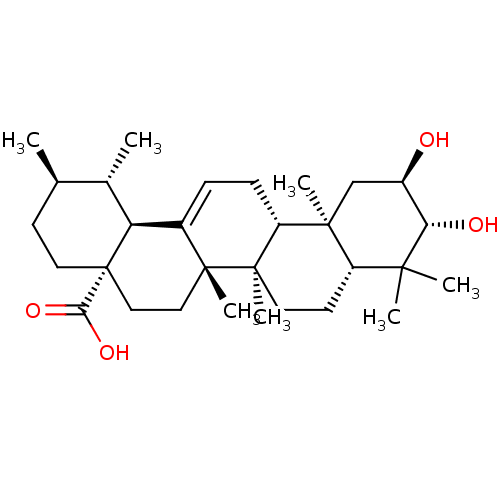 ((1S,2R,4aS,6aS,6bR,8aR,10R,11R,12aR,12bR,14bS)-10,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPepsilon | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50246580 ((S)-2-((4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-10-hyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPepsilon | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50246620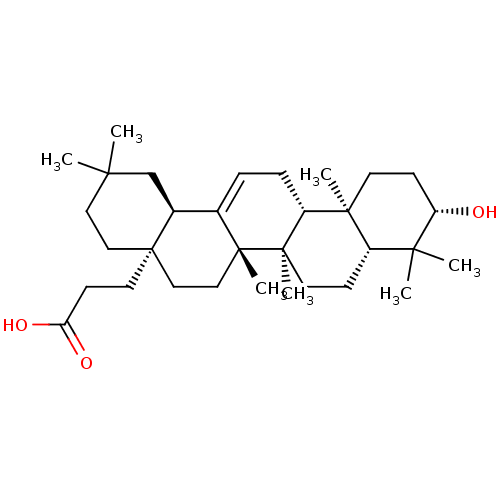 (3-((4aR,6aS,6bR,8aR,10S,12aR,12bR,14bR)-10-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPepsilon | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50246621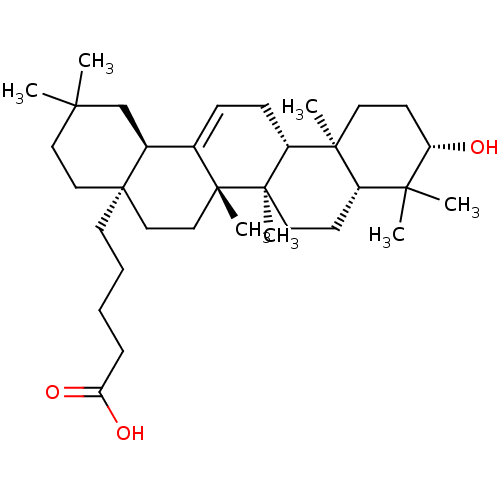 (5-((4aR,6aS,6bR,8aR,10S,12aR,12bR,14bR)-10-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPepsilon | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50246668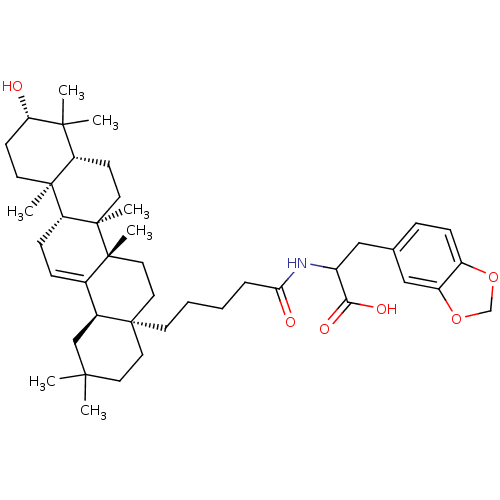 (3-(benzo[d][1,3]dioxol-5-yl)-2-(5-((4aR,6aS,6bR,8a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPepsilon | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50194130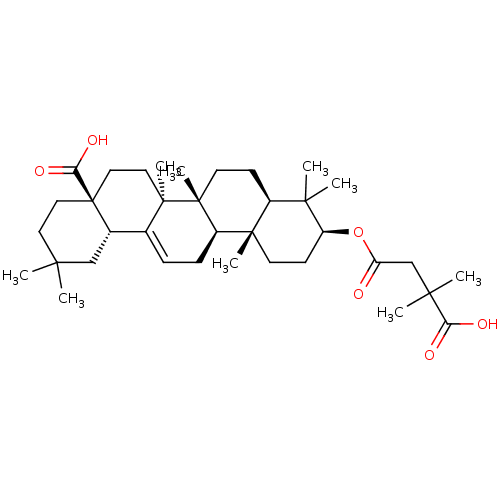 ((4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-10-(3-carboxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPepsilon | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50246744 ((4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-10-(4-carboxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPepsilon | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50246745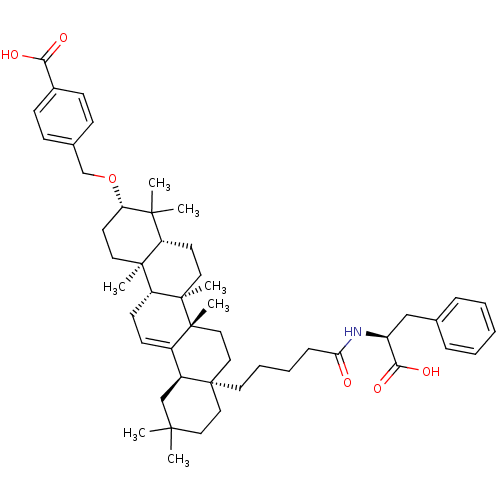 (3-(4-Carboxy-benzyloxy)-28-[4-butyric ((S)-1-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPepsilon | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPepsilon | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50079577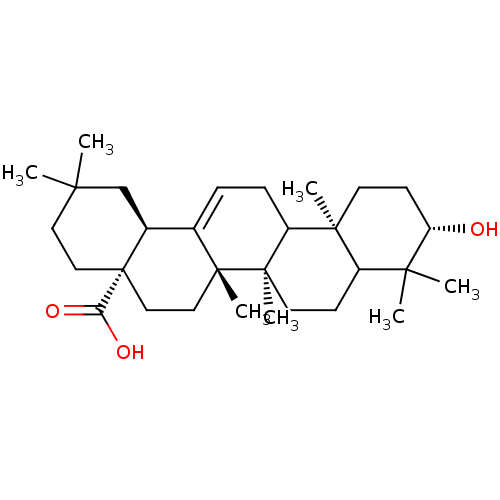 ((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPepsilon | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50246653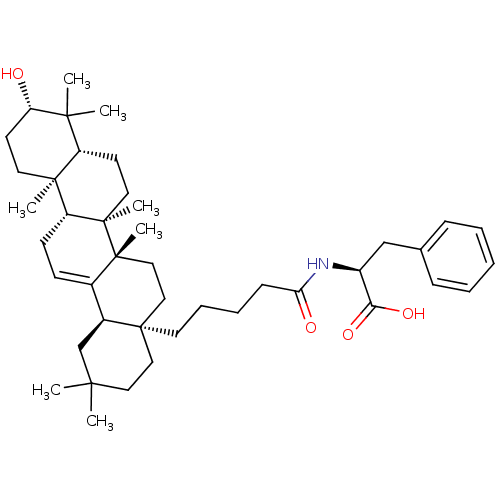 ((S)-2-(5-((4aR,6aS,6bR,8aR,10S,12aR,12bR,14bR)-10-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPepsilon | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50087856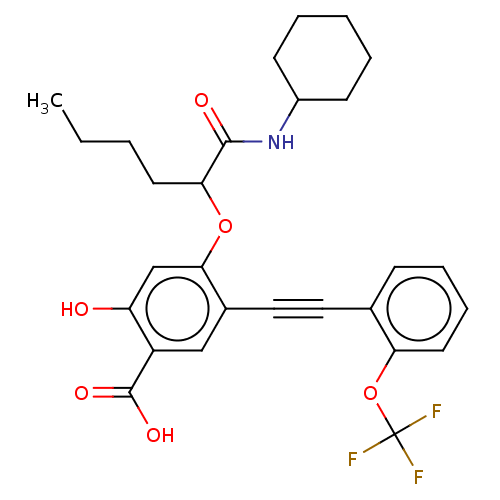 (CHEMBL3426913) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Curated by ChEMBL | Assay Description Inhibition of PTPepsilon (unknown origin) using pNPP as substrate at pH 7 at 25 degC by spectrophotometric analysis | Bioorg Med Chem 23: 2798-809 (2015) Article DOI: 10.1016/j.bmc.2015.03.066 BindingDB Entry DOI: 10.7270/Q2VD7168 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50112356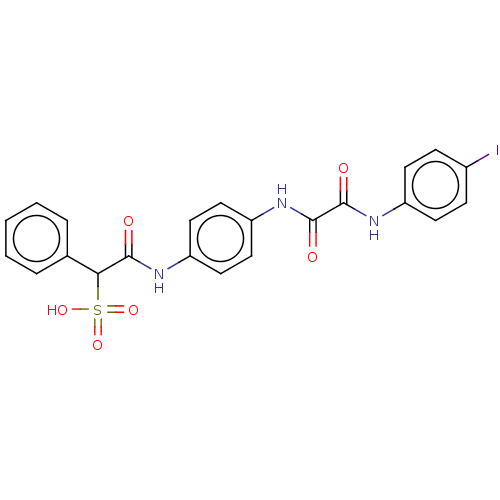 (CHEMBL3609373) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PTPepsilon using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50112357 (CHEMBL3609375) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PTPepsilon using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50112358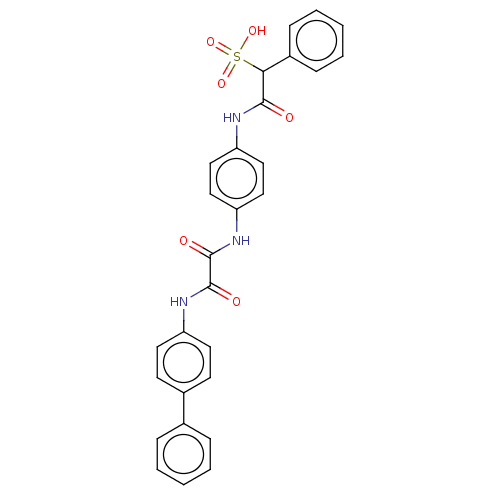 (CHEMBL3609374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PTPepsilon using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50544427 (CHEMBL4632818 | US11192850, Entry 4g) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of PTPE (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vis sp... | J Med Chem 63: 9212-9227 (2020) Article DOI: 10.1021/acs.jmedchem.0c00302 BindingDB Entry DOI: 10.7270/Q2QV3R3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50607110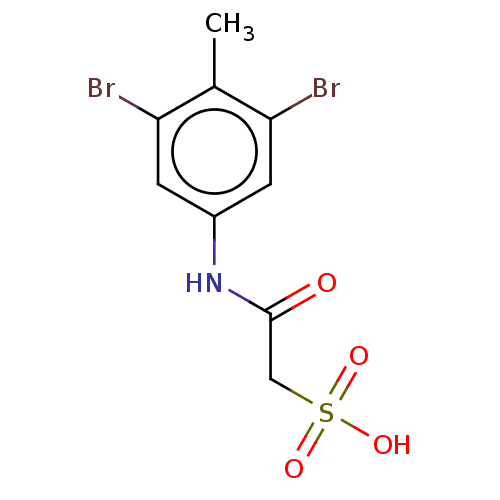 (CHEMBL5219519) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01143 BindingDB Entry DOI: 10.7270/Q2BK1HGF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50263036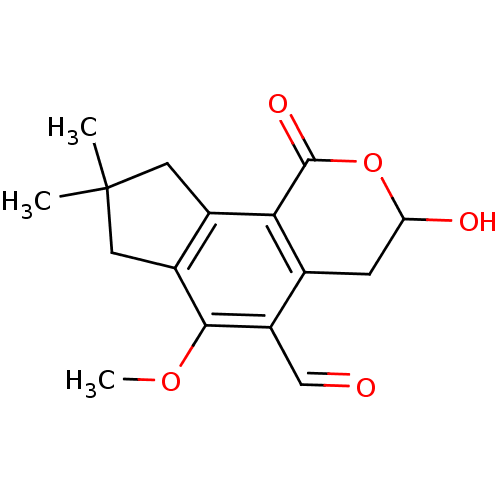 (3-Hydroxy-6-methoxy-8,8-dimethyl-1-oxo-1,3,4,7,8,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of PTPep (unknown origin) using DiFMUP as substrate incubated for 30 mins followed by substrate addition at pH 6.5 by standard phosphatase... | J Nat Prod 82: 3386-3393 (2019) Article DOI: 10.1021/acs.jnatprod.9b00663 BindingDB Entry DOI: 10.7270/Q25H7KRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50262987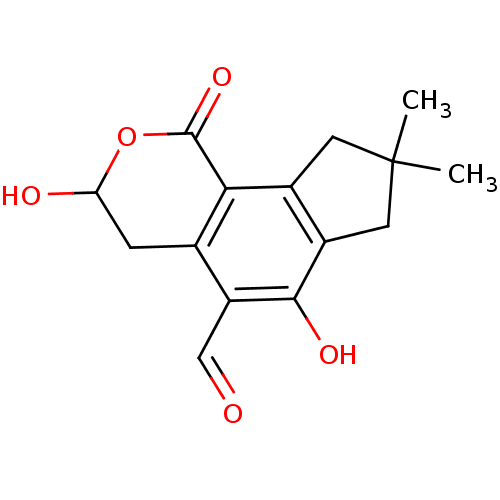 (CHEMBL506661 | Illudalic acid) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of PTPep (unknown origin) using DiFMUP as substrate incubated for 30 mins followed by substrate addition at pH 6.5 by standard phosphatase... | J Nat Prod 82: 3386-3393 (2019) Article DOI: 10.1021/acs.jnatprod.9b00663 BindingDB Entry DOI: 10.7270/Q25H7KRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50262987 (CHEMBL506661 | Illudalic acid) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of PTPep (unknown origin) using DiFMUP as substrate incubated for 30 mins followed by substrate addition at pH 6.5 by standard phosphatase... | J Nat Prod 82: 3386-3393 (2019) Article DOI: 10.1021/acs.jnatprod.9b00663 BindingDB Entry DOI: 10.7270/Q25H7KRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50558461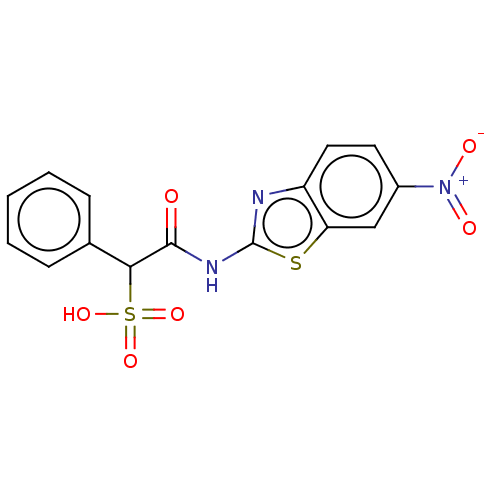 (CHEMBL4760367) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged PTPepsilon (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00993 BindingDB Entry DOI: 10.7270/Q2DN48Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50558485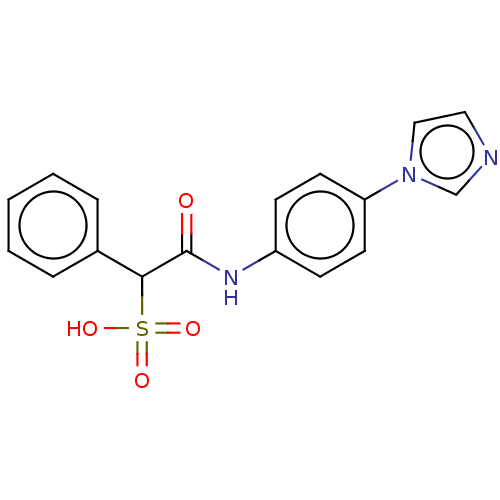 (CHEMBL4800195) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged PTPepsilon (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00993 BindingDB Entry DOI: 10.7270/Q2DN48Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50263036 (3-Hydroxy-6-methoxy-8,8-dimethyl-1-oxo-1,3,4,7,8,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of PTPep (unknown origin) using DiFMUP as substrate incubated for 30 mins followed by substrate addition at pH 6.5 by standard phosphatase... | J Nat Prod 82: 3386-3393 (2019) Article DOI: 10.1021/acs.jnatprod.9b00663 BindingDB Entry DOI: 10.7270/Q25H7KRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50558484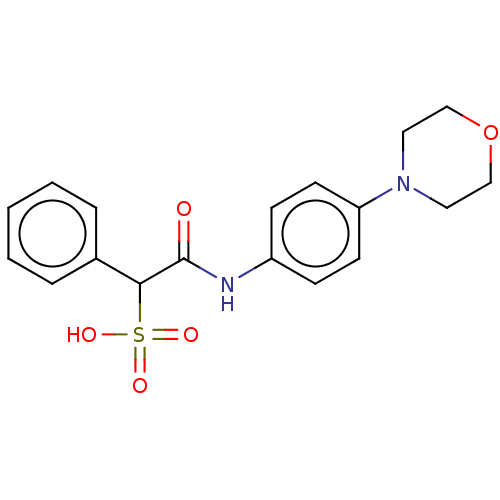 (CHEMBL4748824) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged PTPepsilon (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00993 BindingDB Entry DOI: 10.7270/Q2DN48Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50558483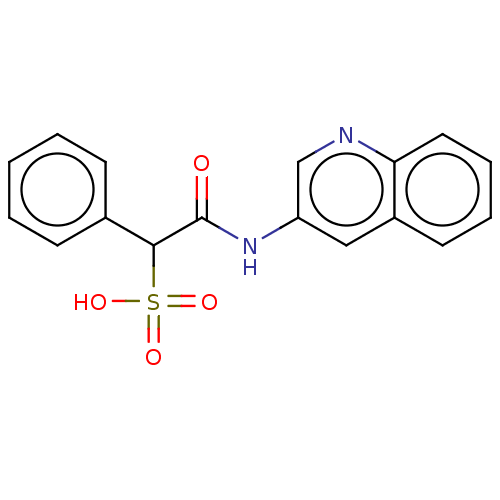 (CHEMBL4778085) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged PTPepsilon (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00993 BindingDB Entry DOI: 10.7270/Q2DN48Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50558482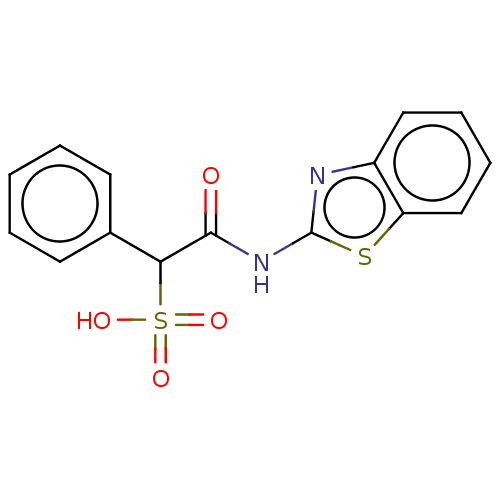 (CHEMBL4742123) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged PTPepsilon (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00993 BindingDB Entry DOI: 10.7270/Q2DN48Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50558481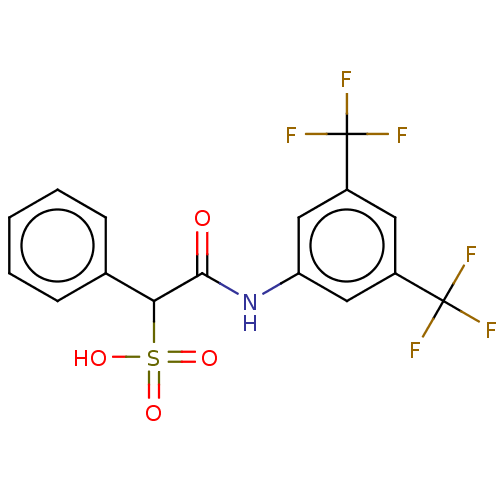 (CHEMBL4783639) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged PTPepsilon (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00993 BindingDB Entry DOI: 10.7270/Q2DN48Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50420258 (CEFSULODIN) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PTPepsilon using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50558488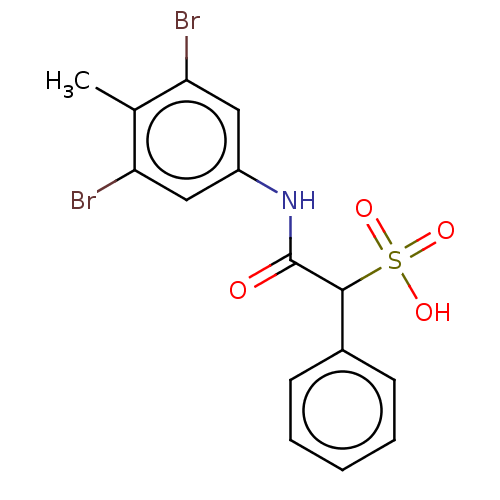 (CHEMBL4794972) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01143 BindingDB Entry DOI: 10.7270/Q2BK1HGF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50558488 (CHEMBL4794972) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged PTPepsilon (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00993 BindingDB Entry DOI: 10.7270/Q2DN48Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50558486 (CHEMBL4797766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged PTPepsilon (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00993 BindingDB Entry DOI: 10.7270/Q2DN48Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50558487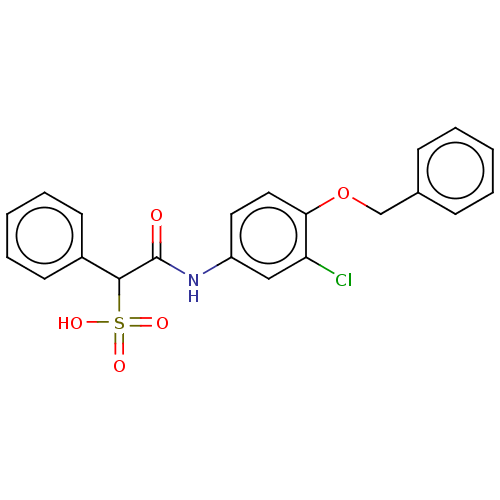 (CHEMBL4786243) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged PTPepsilon (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00993 BindingDB Entry DOI: 10.7270/Q2DN48Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50498446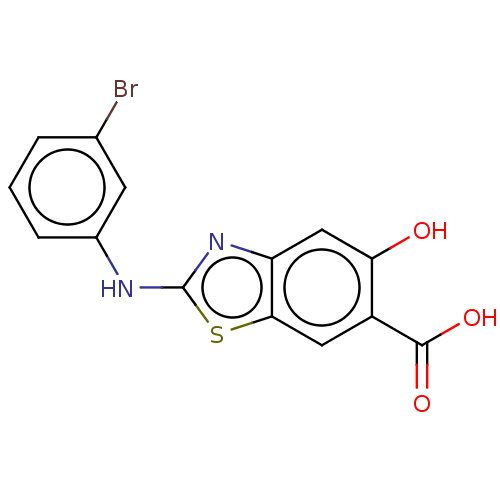 (CHEMBL3596431) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human PTPepsilon using pNPP substrate by spectrophotometry | Medchemcomm 5: 1496-1499 (2014) Article DOI: 10.1039/c4md00099d BindingDB Entry DOI: 10.7270/Q25D8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50188777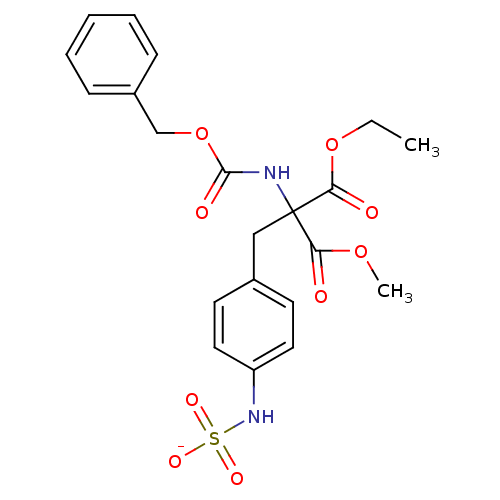 (CHEMBL213452 | ammonium N-[4-(2-{[(benzyloxy)carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTP epsilon | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50188784 (CHEMBL385251 | ammonium N-{4-[3-methoxy-2-(methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTP epsilon | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50188779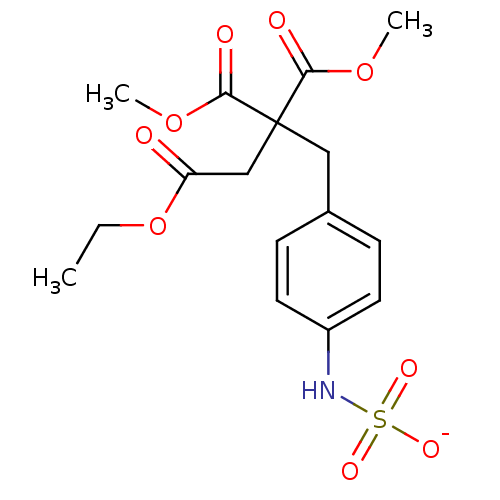 (CHEMBL378423 | ammonium N-{4-[4-ethoxy-2,2-bis(met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HPTP epsilon | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (Homo sapiens (Human)) | BDBM50071058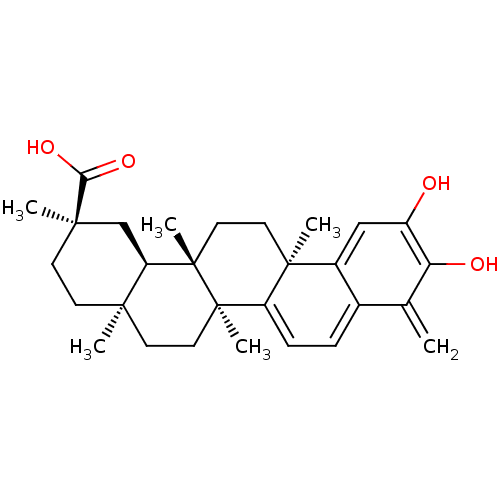 ((2R,4aS,6aS,12bR,14aS,14bR)-10-Hydroxy-2,4a,6a,9,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz Zentrum M�nchen Curated by ChEMBL | Assay Description Inhibition of human PTPRE (107 to 707 residues) expressed in Escherichia coli strain BL21(DE3) using DiFMUP as substrate preincubated for 10 mins fol... | J Med Chem 61: 11144-11157 (2018) Article DOI: 10.1021/acs.jmedchem.8b01224 BindingDB Entry DOI: 10.7270/Q2G44TN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||